Abstract
Context:
Relatively scanty literature on autochthonic African Negroid hair morphology provokes research interest for anthropological, forensic, and cosmetic purposes.
Aims:
This study aimed to contribute basic morphological information on Ghanaian African hairs.
Settings and Design:
The study was done in selected second-cycle schools in Accra, Ghana, using convenient sampling.
Subjects and Methods:
Hairs were obtained by pluck method, from 30 males and 30 females aged 15–20 years. Ghanaian African autochthony was established if individuals had two generations of indigenous Ghanaian parentage. Scalp, eyebrow, axilla, and pubic hairs were image captured using a digital light microscope eyepiece connected to a computer. Diameters of hair strands were measured; types of the medulla and the form and shape of the hair roots were studied.
Statistical Analysis Used:
ANOVA test (SPSS Version 17.0) was used to compare the means of quantitative hair features among the sexes and the four regions of the body studied.
Results:
Pubic hair shaft was thickest (respective male and female diameters were 100.21 μm, 88.40 μm) and eyebrow hair was thinnest (53.97 and 46.69-μm diameters in males and females, respectively). Axillary and scalp hairs were the closest in diameters with 76.21 and 72.02 μm, respectively, in males and 73.07 and 71.15 μm, respectively, in females. Continuous type medulla was predominant in all hairs, with a trend of percentage occurrence in descending order from the pubic, axilla, eyebrow, and scalp in both sexes.
Conclusions:
Bodily regional differences in diameter of hair shaft and medullary presence were affirmed.
Key words: Hair strand, human pilosity, skin appendage, width dimension
INTRODUCTION
Hairs are exclusive to and distinguish mammals from other vertebrates (namely, birds, reptiles, amphibians, and fishes). Exclusivity of hair to mammalian was given as reason for their being named as “hair animals.”[1,2] The mammalian order primates include humans who are unique in having relatively sparse terminal body hairs with obvious regional variation in morphology, density, and growth patterns. Out of the 193 species of primates, only humans are not covered with visibly dense terminal body hair; hence, the term “the naked ape” was coined to describe humans.[3,4] Among humans, racial, sexual, and age differences in distribution and development of body hair are notable, besides structural and color differences.[1,5,6] Voluminous literature exists on human hair dating back from 1910.[2] Invariably, human hair studies have been justified for use by the comparative anatomist, medicolegal expert, plastic surgeon, endocrinologist, cosmetologist, students of human races, and experts of individualization and forensic science.[4,6,7,8,9] Combining macroscopic and microscopic criteria of length, thickness, and size of root and tip to determine the minimum number of body hair regions that have morphological differences to warrant being studied separately, eleven regions were distinguished.[9] These were (i) beard and moustache, (ii) hypogastric, (iii) thoracic, (iv) forearm and leg, (v) upper arm and leg, (vi) gluteal, (vii) lumbosacral, (viii) lower back, (ix) upper back, (x) mid-phalangeal region, and (xi) ear.
Most of the plethora of published human hair studies are of Caucasoid origin, although reports on Negroid exist.[10,11,12,13] Moreover, accessible databases on human hair, contain information on Caucasians, Asians, and African–Americans[10] attesting paucity of data on indigenous Negroid Africans. Our laboratory has previously published on facial hairs in Ghanaian Africans,[14] and the present study contributes further to fill the information gap on hairs of indigenous Ghanaian Africans.
SUBJECTS AND METHODS
Ethical approval was granted by the Ethical and Protocol Review Committee of the University of Ghana, Accra, Ghana. The study design was observational and involved both descriptive and quantitative characteristics of hairs. Using convenient sampling, volunteers, aged 15–20 years, were selected from three selected schools in Accra, Ghana. One key challenge for the present study was the reality that various styling procedures make virgin indigenous Ghanaian African scalp hair rare among adults. To overcome this problem, volunteers were solicited from senior high schools where regulations proscribe scalp hair treatment. After permission was obtained from the institutional heads, students who volunteered were also requested by the school authorities to obtain endorsement from their parents to participate in the study. The study participants were screened for eligibility after a questionnaire was administered to determine each study participant's family ancestry and whether they had relaxed, platted, or weaved their scalp hair in the previous 2 months.
Inclusion criteria
It included volunteers whose parents and two generations of grandparents were all autochthonic Negroid Ghanaians. Volunteers signed a consent form (endorsed by their parents) to participate in the study. Their scalp hair did not have visual signs of cosmetic treatment (e.g., relaxed or stretched by chemicals, platted, or weaved), affirmed by their response to relevant questionnaire.
Exclusion criteria
Adolescents whose parents and/or the first- or second-generation grandparents were not native Negroid Ghanaians were excluded from the study. Similarly, anybody with any visual evidence or self-declared hair stylings such as weaving/twisting/platting or treatment with chemicals or heat was excluded.
Acquisition of hair samples
Hairs were obtained by the pluck method using a pair of cosmetologist's tweezers from 30 females and 30 males. Hair shafts about 3–5 mm long were plucked from four body areas with distinct morphological types of hair, namely the scalp, eyebrow, axilla, and pubic regions of each volunteer as described by Valcovic.[15] For each volunteer, five hair strands plucked from each of the four body regions were kept in four labeled envelopes. Confidentiality of the participants was ensured using a code that was known only to the lead investigator. To safeguard privacy, volunteers were trained to pluck their own pubic hairs and submit in a labeled envelope.
Preparation of hair for the study
The hair strands in this study were not cleaned with acetone or xylene or alcohol as commonly practiced by forensic scientists.[16] This was because the Ghanaian hair appeared to be very sensitive to acetone or xylene and when it was used to wash the hair, the hairs became very brittle and broke easily. Variable concentrations were tried without overcoming this problem. Hence, the hairs were studied without solvent cleaning.
Laboratory analysis
Hairs mounted on standard microscopy slides (AllPro, Middlesex, England) were examined under a binocular microscope (Leica Galen III, Serial Number 1314.U). One eyepiece of the microscope was replaced with a digital microscope eyepiece (Premiere ULEAD, Serial Number MA88) with universal serial bus connection to a Dell Inspiron 6000 Laptop Computer. After capturing the images of the hair, while still on the glass slides, and storing them on the computer, the images were exported to Microsoft Publisher 2003 Software for the diameter of the hair (overall width of hair shaft) and the medulla (within the hair shaft) to be measured. The publisher ruler was calibrated using a Micrometer Stage Graticule (L4078) (Agar Scientific Laboratories, Graticules Ltd., Tonbridge Kent England). The diameter of each hair strand was determined at three equidistant points 1 mm apart on each hair strand, covering a total length of 2 mm. The diameters obtained were tabulated according to the sex and regional origins and their means computed. ANOVA test (SPSS Version 17.0 Chicago, USA: SPSS Inc.) was used to compare the means of quantitative hair features among the sexes and the four regions of the body studied. Results were compiled in tabular and graphical forms. In addition to variables measured, hairs were also described qualitatively based on previously published features.[11,14,15,17] These included: the hair pigment or color, the hair root shape/type, hair texture as compared to hairs studied by other scientists, and frequency of ovoid bodies. The descriptions obtained for each hair strand were tabulated according to the sex of the participant and the region of the body where it was taken from.
RESULTS
Hair strand and medullary diameters
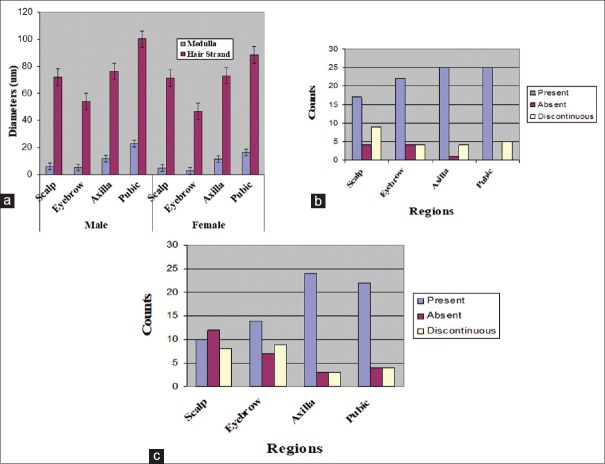
Hair strand and medullary diameters are presented in Figure 1a. Two-way ANOVA yielded statistically significant regional variations with respect to sex (P < 0.05). Pubic hair shaft was thickest in both male and female participants (100.21 and 88.40 μm, respectively) [Table 1]. Eyebrow hair-shaft diameter was the thinnest with shaft diameters of 53.97 and 46.69-μm in males and females, respectively [Table 1]. When present, medullary diameter of hairs mirrored shaft diameter in all variables studied in both sexes [Table 1] with pubic hairs having the widest mean medullary diameter and eyebrow hair, the narrowest. There was no significant difference between the diameters of the medulla of the hair among the sexes; this was shown in the overlap of their confidence intervals [Table 1]. Axillary and scalp hairs were similar in terms of shaft diameter of 76.21 and 72.02 μm, respectively, in males and 73.07 and 71.15 μm, respectively, in females.
Figure 1.
Bar charts showing diameter of hair shaft and features of the medulla. (a) Shows the diameters of hair shaft in both male and female hair from different regions. (b) Shows the features of medulla from different regions in males, whereas (c) shows features of the medulla in females
Table 1.
Mean diameters (µm) of hair strand and medulla in males and females
| Body regions | Mean hair strand diameter (µm) | Mean medullary diameter (µm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Scalp | 72.02 | 65.53-78.51 | 71.15 | 62.62-79.68 | 5.91 | 2.62-9.20 | 4.86 | 1.19-8.53 |
| Eyebrow | 53.97 | 49.13-58.81 | 46.69 | 42.24-51.14 | 5.12 | 2.43-7.81 | 2.64 | 0.84-4.44 |
| Axilla | 76.21 | 69.43-82.99 | 73.07 | 65.47-80.67 | 11.9 | 8.82-14.98 | 11.37 | 9.21-13.53 |
| Pubic | 100.21 | 88.86-111.56 | 88.44 | 78.35-98.53 | 23.0 | 18.47-27.53 | 16.34 | 12.26-20.42 |
95% CI – 95% confidence interval
Features of the medulla
Images of hair strands and the type of medulla are shown in Figure 2. Hair strands showed either discontinuous or continuous medulla. The continuous type of the medulla [Figure 2d] was predominant in all hairs, with the trend of percentage occurrence in descending order from the pubic, axilla, eyebrow, and scalp in both sexes [Table 2].
Figure 2.
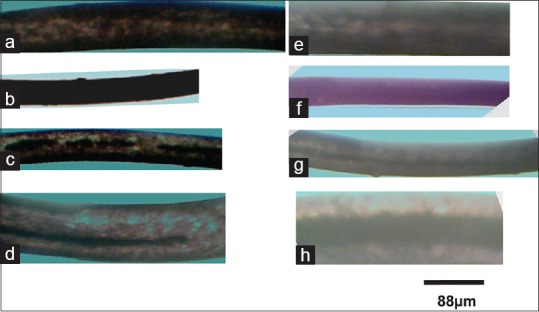
Micrographs showing hair strands and medulla (continuous, discontinuous, or absent) of male and female participants. (a) Female scalp hair with absent medulla, (b) female eyebrow hair with indistinguishable medulla, (c) female axillary hair with discontinuous medulla, (d) female pubic hair with continuous medulla deficient toward the tip, and (e-h) shows male scalp, eyebrow, axillary, and pubic strands. Artificial color has been added to improve contrast
Table 2.
Medullary counts according to the region of the body and sex of the participant
| Sex | Male | Female | ||||
|---|---|---|---|---|---|---|
| Variable | Continuous (%) | Absent | Discontinuous | Continuous (%) | Absent | Discontinuous |
| Scalp | 17 (57) | 4 | 9 | 10 (33) | 12 | 8 |
| Eyebrow | 22 (730 | 4 | 4 | 14 (47) | 7 | 9 |
| Axilla | 25 (83) | 1 | 4 | 24 (80) | 3 | 3 |
| Pubic | 25 (83) | 0 | 5 | 22 (73) | 4 | 4 |
The medulla varied in occurrence in the hair samples throughout the regions and the sex of participants [Figure 1b – female and c – male]. Some regions had more hairs with the medulla than others with a similar observation also among the sexes [Table 2]. For example, hair strands from the scalp and eyebrows in males had higher counts with the medulla compared to female hair strands.
The ratio of the diameter of the medulla to diameter of the hair strand (medullary ratio) presented in [Table 3] was not significantly different among the regions and between the sexes (P > 0.05 for two-way ANOVA).
Table 3.
Regional mean medullary ratios among sexes
| Sex | Male |
Female |
|---|---|---|
| Regions | Means of ratios | |
| Scalp | 0.21 (SE: 0.05) | 0.24 (SE: 0.07) |
| Eyebrow | 0.24 (SE: 0.04) | 0.21 (SE: 0.03) |
| Axilla | 0.20 (SE: 0.02) | 0.19 (SE: 0.01) |
| Pubic | 0.24 (SE: 0.03) | 0.24 (SE: 0.03) |
SE – Standard error
Shape of hair roots
Sample images of the roots of hairs from males and females are shown in Figure 3. There were different shapes including cylindrical, club, flattened, convex, and rod-like; with the club shape being the most predominant.
Figure 3.
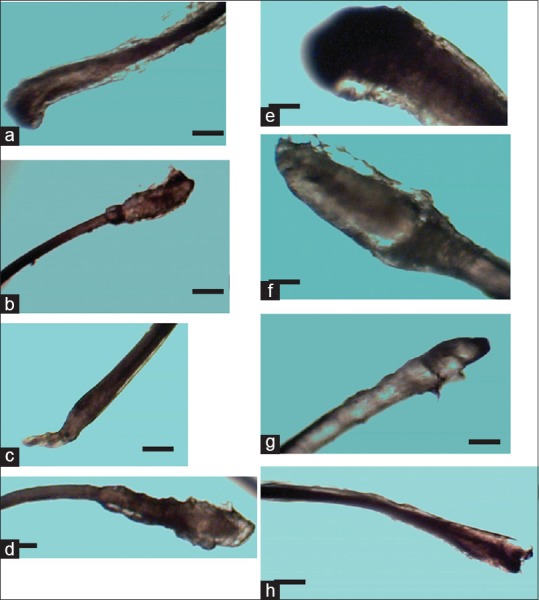
Micrographs various shapes of hair roots from different regions of females (a-d) and males (e-h). Scalp hairs from both females (a) and males (e) showed club-shape, male eyebrow roots were rod-like (b), male eyebrows (f) and female axillary roots (c) were cylindrical. Female (d) and male (h) pubic roots were convex and snake-like, respectively. The scale bar is 72 μm for a–d, g, and h. Scale bar for e and f is 150 μm
DISCUSSION
Diametric measurements
Studies undertaken by Ball et al.[18] on the morphological characteristics of hair of Egyptian mummies compared with that from other races, (excluding Negroid Africans) showed that there were significant morphometric differences between hairs taken from racial types and from different sexes within the racial types. Therefore, according to the authors, when hair is the tool for forensic identification and the race is unknown, gender is best discriminated using only size parameters of hair. This conclusion was not conformed to by data in the present study because mean hair strand diameters and mean medullary diameters of both males and females were not significantly different. For this reason, where the sex is concerned, the origin of the hair was not determinative using only size diameters.
Diameters of hair strands and medulla from the different regions showed highly significant differences. Consequently, the regional origin of a hair in the present study could be identified using diametric dimensions where sex is unknown. Negroid hairs have elliptical cross-section and possess major and minor diameters.[19] Optical limitation of the light microscopy used in this study did not allow discrimination between the major and minor axes of the studied hairs. This represents a limitation in this study.
Unlike the Caucasian hair, where axillary and pubic hairs are closest in size characteristics,[20] the axillary and the scalp hairs are rather closest in diameters in the present study. The Ghanaian scalp hair diameter was smaller than the average scalp hair diameter of other races, given to be 80 μm.[21] In the present study, the pubic hair was the thickest and the eyebrows the thinnest. The same trend applied to their medullary measurements. These trends in diameter were similar in both male and female hairs. However, it has been shown that the eyebrows of Caucasian males were always thicker in diameter than the scalp hair.[20] In Ghanaian Negroid hairs, the trend of diameter increased from the eyebrows to axillary, scalp, and pubic areas.
The medulla, when present in human hairs, is amorphous in appearance and when the mounting medium does not enter the medulla, this later appears opaque (black)[22] that explains its black appearance [Figure 2c and d] and [Figure 2g and h] with [Figure 2h] having a relatively thicker medulla as compared to the first ones.
The medullary ratio is generally less than one-third for human hair.[23] The Ghanaian hairs in the present study had medullary ratios of <0.25. The medulla is an open meshwork that usually forms during the vigorous growth of thick hairs.[24] This is the reason why it was present mostly in relatively thicker hairs. As the diameter of the hair fiber decreased, the medulla became interrupted by lengths of the cortex, and finer hairs had fragmented medulla. Consistent with this observation, discontinuity of the medulla was found as the hair diameter decreased, till they became absent in thinner hairs such as the eyebrows. According to Ling,[25] this type of medulla, which is found mostly in human hair, camel and, cow hairs, is called the nonlatticed form; it is narrower and denser in structure. The discontinuity of the medulla of this study showed a slight difference from other findings. The scalp hair medulla is mostly discontinuous when present;[23] however, in the present study, the scalp hair medulla was mostly continuous. Using an electron microscope, however, a new classification has been proposed as thick or thin medulla according to their optical patterns.[26] A “fragmented” or discontinuous medulla is one with a thick medulla intercalated with thin medulla. This could probably explain the absent or discontinuous medulla in some hairs.
Significance of descriptive features
These descriptions demonstrate regional aspects of hair from different body sites in indigenous Ghanaians. In general, most scalp hairs were of moderate pigmentation, moderately brownish to dark, whereas the eyebrow hairs had pigmentation that was mostly so very dark that the medulla was difficult to observe. However, the axillary and the pubic hairs were mostly brownish with the pubic hair being very light brownish making its medulla very easily observable and measurable. These observations were similar in both sexes. Moreover, scalp hairs had a combination of both brownish and black color in a hair.
The roots of all the hairs studied in both sexes had a variety of shapes. Although the club shape that is common to most human hairs, as mentioned by Reynolds,[27] was also commonly observed in this study, variations did occur. Aside club-shaped roots, there were also rod-like, cylindrical, flattened, snake-like, and convex shapes. The described types of roots may have been affected by plucking methods, especially in pubic hairs which volunteers self-plucked.
CONCLUSIONS
Findings from this study indicate that Ghanaian Negroid hair differs in a number of morphological features from hair of other races described in the literature.[10,18] The hairs were mostly brownish in color, except the very dark eyebrows. Diameter of hair could not be used to distinguish between the sexes; however, the four regions studied differed significantly in hair diameters. The medulla followed the same trend of its hair shaft both in sexual and regional comparisons. These provide a new baseline data which could be useful for future work in the area of hair morphology.
Financial support and sponsorship
This study was financially supported by the College of Health Sciences, University of Ghana.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Assistance by technicians in the Department of Anatomy, UGMS, and Noguchi Memorial Institute for medical research is greatly appreciated.
REFERENCES
- 1.Buffoli B, Rinaldi F, Labanca M, Sorbellini E, Trink A, Guanziroli E, et al. The human hair: From anatomy to physiology. Int J Dermatol. 2014;53:331–41. doi: 10.1111/ijd.12362. [DOI] [PubMed] [Google Scholar]
- 2.Noback CR. Morphology and phylogeny of hair. Ann N Y Acad Sci. 1951;53:476–92. doi: 10.1111/j.1749-6632.1951.tb31950.x. [DOI] [PubMed] [Google Scholar]
- 3.Rantala M. Evolution of nakedness in Homo sapiens. Journal of Zoology. 2007;273:1–7. [Google Scholar]
- 4.Kamberov YG, Guhan SM, DeMarchis A, Jiang J, Wright SS, Morgan BA, et al. Comparative evidence for the independent evolution of hair and sweat gland traits in primates. J Hum Evol. 2018;125:99–105. doi: 10.1016/j.jhevol.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De la Mettrie R, Saint-Léger D, Loussouarn G, Garcel A, Porter C, Langaney A, et al. Shape variability and classification of human hair: A worldwide approach. Hum Biol. 2007;79:265–81. doi: 10.1353/hub.2007.0045. [DOI] [PubMed] [Google Scholar]
- 6.Mistry S, Chatterjee M, Ghosh JR, Chakrabarti NK, Bandyopadhyay AR. Variations of scalp, pubic and axillary hair. Anthropol Anz. 2012;69:117–25. doi: 10.1127/0003-5548/2011/0119. [DOI] [PubMed] [Google Scholar]
- 7.Adeola HA, Van Wyk JC, Arowolo A, Ngwanya RM, Mkentane K, Khumalo NP, et al. Emerging diagnostic and therapeutic potentials of human hair proteomics. Proteomics Clin Appl. 2018;12:170048. doi: 10.1002/prca.201700048. [DOI] [PubMed] [Google Scholar]
- 8.Mkentane K, Van Wyk JC, Sishi N, Gumedze F, Ngoepe M, Davids LM, et al. Geometric classification of scalp hair for valid drug testing, 6 more reliable than 8 hair curl groups. PLoS One. 2017;12:e0172834. doi: 10.1371/journal.pone.0172834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garn SM. Types and distribution of the hair in man. Ann N Y Acad Sci. 1951;53:498–507. doi: 10.1111/j.1749-6632.1951.tb31952.x. [DOI] [PubMed] [Google Scholar]
- 10.Loussouarn G, Lozano I, Panhard S, Collaudin C, El Rawadi C, Genain G, et al. Diversity in human hair growth, diameter, colour and shape. An in vivo study on young adults from 24 different ethnic groups observed in the five continents. Eur J Dermatol. 2016;26:144–54. doi: 10.1684/ejd.2015.2726. [DOI] [PubMed] [Google Scholar]
- 11.Khumalo NP. African hair length: The picture is clearer. J Am Acad Dermatol. 2006;54:886–8. doi: 10.1016/j.jaad.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Quaresma MV, Martinez Velasco MA, Tosti A. Hair breakage in patients of african descent: Role of dermoscopy. Skin Appendage Disord. 2015;1:99–104. doi: 10.1159/000436981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khumalo NP. African hair morphology: Macrostructure to ultrastructure. Int J Dermatol. 2005;44(Suppl 1):10–2. doi: 10.1111/j.1365-4632.2005.02805.x. [DOI] [PubMed] [Google Scholar]
- 14.Aboagye B, Ahenkorah J, Hottor B, Addai F. Comparative characteristics of black and grey chest and selected facial hairs in negroid males. Internet J Biol Anthropol. 2014;7:14. [Google Scholar]
- 15.Valcovic V. Trace Elements Human Hair. New York, London: Rice University, Huston, Texas and Institute “Ruder Bošković” Zagreb; 1977. [Google Scholar]
- 16.Deedrick DW. Hairs, fibers, crimes and evidence. Forensic Sci Commun. 2000;2:2–5. [Google Scholar]
- 17.Loussouarn G. African hair growth parameters. Br J Dermatol. 2001;145:294–7. doi: 10.1046/j.1365-2133.2001.04350.x. [DOI] [PubMed] [Google Scholar]
- 18.Ball TB, Griggs CW, Kuchar M, Phillips R, Iskander N, Hess WM. Morphological characteristics of hair of Egyptian mummies compared with hair from U.S. caucasoids, mediterranean workers and Chinese. In: Birrell M, editor. Bulletin of the Australian Centre for Egyptology. Vol. 13. Brigham Young University, Utah: Australian Centre for Egyptology; 2002. [Google Scholar]
- 19.Franbourg A, Hallegot P, Baltenneck F, Toutain C, Leroy F. Current research on ethnic hair. J Am Acad Dermatol. 2003;48:S115–9. doi: 10.1067/mjd.2003.277. [DOI] [PubMed] [Google Scholar]
- 20.Hess WM, Seegmiller RE, Gardner JS, Allen JV, Barendregt S. Human hair morphology: A scanning electron microscopy study on a male caucasoid and a computerized classification of regional differences. Scanning Microsc. 1990;4:375–86. [PubMed] [Google Scholar]
- 21.Gaudette BD, Keeping ES. An attempt at determining probabilities in human scalp hair comparison. J Forensic Sci. 1974;19:599–606. [PubMed] [Google Scholar]
- 22.Ryder ML. Hair. The Institute of Biology's Studies in Biology. London: Edward Arnold; 1973. [Google Scholar]
- 23.Deedrick WD, Koch S. Microscopy of hair part 1: A practical guide and manual for human hairs. Forensic Science Communications. 2004:6. [Google Scholar]
- 24.Lyne AG, Short BF. Biology of the Skin and Hair Growth. Sydney: Angus and Robertson; 1965. [Google Scholar]
- 25.Ling JK. Pelage and molting in wild mammals with special reference to aquatic forms. Q Rev Biol. 1970;45:16–54. doi: 10.1086/406361. [DOI] [PubMed] [Google Scholar]
- 26.de Cássia Comis Wagner R, Kiyohara PK, Silveira M, Joekes I. Electron microscopic observations of human hair medulla. J Microsc. 2007;226:54–63. doi: 10.1111/j.1365-2818.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds EL. The appearance of adult patterns of body hair in man. Ann N Y Acad Sci. 1951;53:576–84. doi: 10.1111/j.1749-6632.1951.tb31959.x. [DOI] [PubMed] [Google Scholar]