Abstract
Context:
Candida species is a part of the normal mouth flora. Diabetes mellitus is a predisposing factor for the onset of oral candidiasis.
Aim:
The objective of this study was to estimate salivary glucose in patient with diabetes and healthy individuals, to determine total candidal counts as well as different candidal species in the saliva of patients with diabetes and nondiabetics.
Settings and Design:
A sample size of 80 patients was taken. Out of 80 patients, 30 patients were uncontrolled diabetes patients (Group I), 30 patients were controlled diabetes patients (Group II), and 20 patients were healthy individuals (Group III).
Subjects and Methods:
From all the salivary samples, salivary glucose estimation was done using the glucose oxidase-peroxidase method. Candidal colony-forming units (CFUs) were determined from all the salivary samples. CHROMagar Candida medium was used for the identification of various Candida species.
Statistical Analysis Used:
One-way ANOVA was used to test for differences between the means of the three groups. Pearson's correlation coefficient test was used to evaluate the relationships between the variables.
Results:
The mean salivary glucose levels were higher in Group I followed by Group II and Group III. The mean candidal CFU was significantly higher in Group I than in Group II and Group III.
Conclusion:
Candida glabrata was the predominant species found and more so in patients with diabetes and needs further study. Other common species isolated was C. albicans. Species identification can help in better treatment strategies and gain good control over the disease.
Keywords: Candida species, candidiasis, CHROMagar Candida medium, diabetes mellitus
Introduction
Diabetes mellitus is a predisposing factor to the fungal infections, especially those caused by Candida species is well-established.[1] Patients with diabetes are prone to infection; and oral candidiasis has been found to be more prevalent among patient with diabetes compared with nondiabetic controls.[2] In the majority, Candida is the part of the normal flora and is in the normal state kept under control by means of specific and nonspecific defense mechanisms and by the competition of the microbes in the normal flora.[3]
It has been reported by several studies that the prevalence of yeast carriage among patients with diabetes could reach up to 54%, and that Candida albicans could account for 25%–69% of the isolates.[2,4] In some studies, the oral carriage rate of Candida has been estimated at around 80%.[2] In diabetes mellitus, the candidal density has also been reported higher than in nondiabetic patients,[2,5] not confirmed by other investigators.[6,7] The disparity in results may be due to the difference in sampling technique.[8]
Yeasts which are part of the genus Candida consist of 150–200 species.[9] Several species of Candida can infect the oral mucosa, C. albicans is the most commonly encountered oral fungal agent and because of its greater level of pathogenicity and adherence properties, it may be highly infective. In 40%–65% of healthy adults , C. albicans is an oral commensal.[10] The non-C. albicans Candida (NCAC) species are a heterogenous group of organisms which are different from each other and from C. albicans.[11] Earlier, C. albicans was considered as the only species causing infection, and Candida parapsilosis, Candida tropicalis, and Candida guilliermondii were considered only as occasional pathogens.[11] The development of new medical therapies for cancer, the increase in invasive medical procedures, the emergence of human immunodeficiency virus and AIDS, and the wide-spread use of broad-spectrum antibiotics lead to the increased recovery of many other NCAC species causing mucosal infections.[11] Species such as C. inconspicua, C. lusitaniae, C. norvegensis, and C. rugosa have been isolated occasionally from patients.[12] C. albicans, C. tropicalis, Candida krusei, or Candida dubliniensis are the main causative factors.[3]
This study is aimed at estimating salivary glucose in diabetic and healthy individuals and the determination of total Candidal counts as well as different Candidal species in the saliva of patient with diabetes and nondiabetic individuals. Results obtained in the study might throw light on candidal prevalence in diabetic patients as compared to the healthy ones.
Subjects and Methods
Sample size of 80 patients was taken to conduct the study. The study comprised three groups. Group I consisted of 30 uncontrolled patients with diabetes. They were Type 2 diabetes patients with uncontrolled metabolic state. These patients were not taking any drugs other than those to control diabetes. Group II comprised 30 controlled patients with diabetes. They were Type 2 diabetic patients with their metabolic state under control. These patients were on oral hypoglycemic and not taking any other medication. Group III consisted of 20 healthy nondiabetic ones. These individuals were with no features of diabetes mellitus, and blood glucose levels were within normal limits.
The study was approved by the ethical committee of the People's College of Dental Sciences and Research Centre, Bhopal, with approval number PCDS/Acad/2011-12/1899. The study protocol was explained to patients and the informed consent was obtained from all the patients.
Unstimulated salivary samples were collected from all patients between 8 a.m. and 11 a.m. to avoid circadian variations. Whole saliva was collected by the spitting method for at least 5 min. The salivary samples were transported to the laboratory and were analyzed on the same day. Samples were centrifuged at 3000 rpm for 5–10 min and clear supernatants were processed immediately for estimation of glucose. Glucose was estimated in the supernatant saliva using the glucose oxidase-peroxidase method.[13,14,15] A volume of 1000 μL of reagent solution was pipetted into each test tube out of the three test tubes labeled “Blank,” “Standard,” and “Test”. A volume of 10 μL of the standard was added to the test tube labeled as 'Standard'. A volume of 10 μl of test sample was added to the “Test” of the test tube. These were mixed well and all the test tubes were kept in an incubator at 37°C for 10 min before aspiration. First, the reagent blank was aspirated in the semi-automated analyzer, followed by the standard solution for which the reading was noted, and finally, the test sample was aspirated and the reading was noted. The results were calculated, and the values were expressed as milligrams per deciliter (mg/dL).[15]
Expectorated saliva was collected in a sterile container. It was immediately concentrated by centrifuging for 10 min. Supernatant was discarded, and 0.001 μL inoculating loop was used to spread the sample on to Sabouraud's dextrose agar (SDA) plates supplemented with chloramphenicol (10 mg/mL). The loop is held between the thumb and index finger and passed at a 90° angle several times through the initial inoculum into the second quadrant of the plate (streak area 1). The plate is turned 90°, and the process is repeated, streaking into the third quadrant (streak area 2), and finally, after another 90° turn– into the fourth quadrant (streak area 3). The loop is flamed between quadrants unless the inoculum is light or the medium is selective or inhibitory. The plate was incubated overnight at 37°C. After incubation, colony-forming units (CFUs) were counted manually, and the number was multiplied by 1000 and expressed as CFU/mL. CHROMagar Candida, a differential and selective medium was used to permit the identification of C. albicans and other Candida species based on the colony color and characteristics.
Data entry, database management, and all statistical analysis were performed with the SPSS 20.0 trial version (Statistical Package for the Social Sciences, IBM Corporation, Bengaluru) software. One-way ANOVA was used to test for differences between the means of the three groups. Relationships between the variables were evaluated by the Pearson's correlation coefficient. A value of P < 0.05 was considered to be statistically significant.
Results
In Group I, out of 30 uncontrolled patients with diabetes, 27 (90%) carried Candida, with Candida glabrata in 56.6%, Candida albicans in 23.3%, and Candida tropicalis in 10% of patients. In Group II, 19 persons carried Candida in their oral cavity (63.3%). The most common species was C. glabrata in 40%, followed by Candida albicans in 20%, and Candida tropicalis in 3% cases. In Group III, 4 (20%) persons carried Candida in their oral cavity with C. glabrata as the most common (20%) [Tables 1 and 2].
Table 1.
Prevalence of candida among the three groups
| Group | Total | Growth, n (%) | No growth, n (%) |
|---|---|---|---|
| Uncontrolled patients with diabetes (Group I) | 30 | 27 (90) | 3 (10) |
| Controlled patients with diabetes (Group II) | 30 | 19 (63.3) | 11 (36.6) |
| Healthy controls (Group III) | 20 | 4 (20) | 16 (80) |
Table 2.
Speciation among the three groups by CHROMagar method
| Group | Total | Growth | Green (Candidaalbicans), n (%) | Blue (Candida tropicalis), n (%) | Pink (Candida krusei), n (%) | White/violet (Candida glabrata), n (%) |
|---|---|---|---|---|---|---|
| Group I | 30 | 27 | 7 (23.3) | 3 (10) | 0 | 17 (56.6) |
| Group II | 30 | 19 | 6 (20) | 1 (3.3) | 0 | 12 (40) |
| Group III | 20 | 4 | 0 | 0 | 0 | 4 (20) |
Salivary glucose levels were significantly higher in diabetic patients than in nondiabetic patients. The mean salivary glucose levels were the highest in Group I (21.93 ± 4.53 mg/dL) followed by Group II (4.73 ± 2.02 mg/dL) and Group III (1.38 ± 0.516 mg/dL) Significant differences (P < 0.05) in salivary glucose between all groups were observed.
The mean candidal CFU was significantly higher in Group I (62963 CFU/ml) than in Group II (12,368 CFU/ml) and Group III (29,000 CFU/ml)]. Comparison of candidal colony count among the three groups was found to be statistically significant (P < 0.05) [Figure 1].
Figure 1.
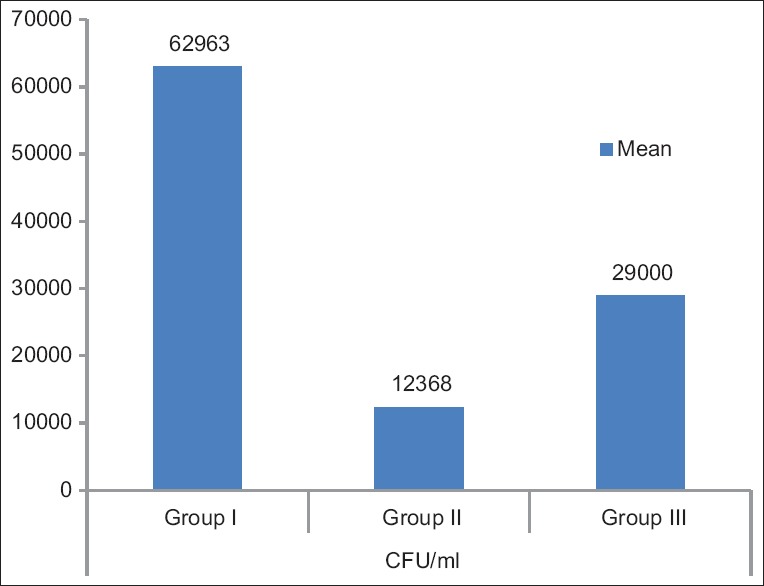
Comparison of colony-forming units of Candida among the three groups
In all groups, salivary glucose and candidal colony count were correlated by the Pearson's correlation coefficient test and no statistical significance was found in Group I and II; however, CFU of Candida showed a negative correlation with salivary glucose in Group III and was found to be statistically significant. (r = 0.637; P = 0.0003) [Figure 2].
Figure 2.
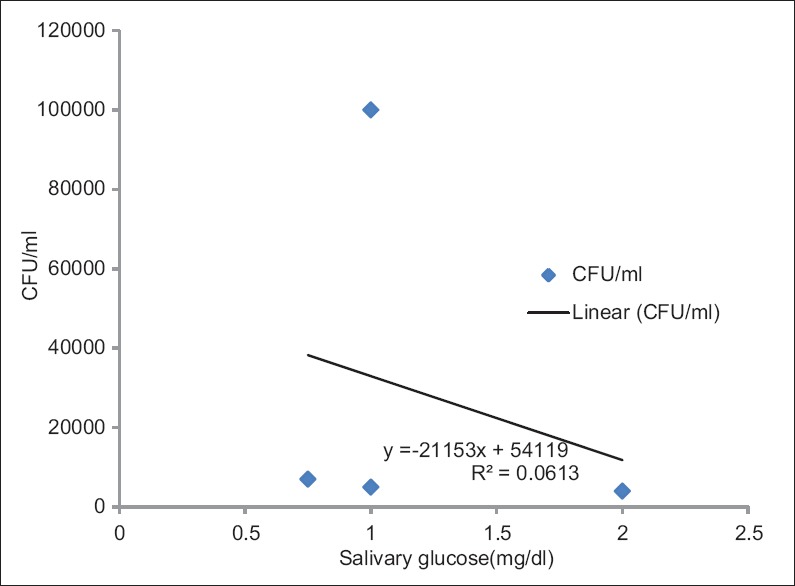
Correlation of colony-forming units of Candida and salivary glucose in nondiabetic healthy controls (Group III)
Discussion
In this study, glucose was detected in the saliva of nondiabetic patients, as reported by others.[16,17] In nondiabetic patients, the mean salivary glucose levels (1.380 ± 0.516 mg/dL) were higher in the present study compared to the other studies, which could be attributed to the carbohydrate-rich dietary pattern of the Indian population.
Salivary glucose levels were significantly higher in diabetic patients (Group I > Group II) than in healthy nondiabetics (Group III). Similar finding has been observed by others,[17] which suggests that salivary glucose levels likely follow a threshold mechanism. During or soon after birth, Candida species colonize mucosal surfaces of human beings and the risk of endogenous infection is ever present.[18] Patients with compromised host defenses are susceptible to ubiquitous fungi to which healthy people are exposed but are usually resistant.
In various studies, the carriage rate of Candida in the oral cavity was different. It could be due to different methods of sampling. In the oral cavity of diabetic patients, the carriage rate of Candida is claimed to be higher. Increased candidal density has been shown to be associated with increased concentration of salivary glucose.[19] Other investigators have also observed that increased Candida reflects increased salivary glucose levels.[17,19] In the present study, candidal colonies were isolated on SDA [Figure 3]. Colony-forming unit (CFU) is usually recorded, to obtain the clinical data to establish a clinical diagnosis of oral candidiasis.[19] In this study, the frequency of Candida colonization (62.5%) was similar to that observed in other studies (45%–70%).[20,21]
Figure 3.
Petri dish showing cream-colored candidal colonies (Sabouraud's dextrose agar)
The present study has confirmed that Candida is more prevalent in the oral cavity of patients with diabetes than nondiabetics as observed by earlier workers.[2,22] Carriage rate was high in patients with diabetes (76.6%) when compared to healthy persons in this study. Carriage rate in patients with diabetes in this study was a little higher than in some studies.[18,23,24] Our results, in regards to oral carriage rate of Candida in healthy individuals was in agreement with other studies.[18,24] Candidal CFUs were significantly higher in diabetic patients (Group I > Group II) compared with nondiabetic patients (Group III). It was similar to the findings of earlier studies.[25] C. albicans was a frequent species colonizing the mouth as expected [Figure 4]. There was a diversity of other species as well. C. glabrata was the most frequent non-albicans species (41.25%) [Figure 5]. This was followed by C. tropicalis. Candida albicans was common in the present study (20%) like in other studies.[23,24] Kadir et al. have found that oral carrier rate and density of Candida species were nonsignificantly higher in the patients with diabetes than in the nondiabetic control patients.[26]
Figure 4.
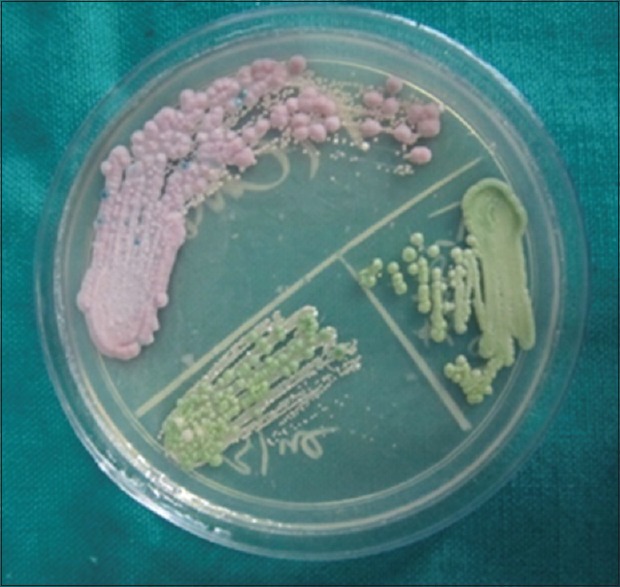
Petridish showing colonies of different colors: Candida glabrata as violet, Candida albicans as green (CHROMagar)
Figure 5.
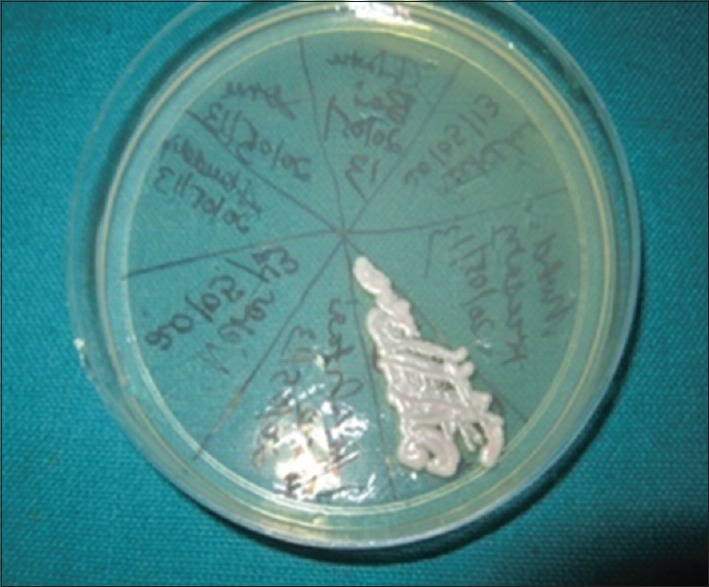
Petridish showing confluent growth of Candida glabrata (CHROMagar)
It has been demonstrated by Epstein et al. that carriers and patients with oral candidiasis can be reliably distinguished on the basis of quantitative cultures.[21] Epstein et al. established a correlation between signs and symptoms of candidiasis and high (>400 CFU/mL saliva)-colony counts.[21] Patients with clinical candidiasis harbor >400 CFU per mL of saliva. Between healthy and patients with diabetes such cutoff limits for CFU may serve as a useful clinical indicator.[19]
Jafari et al. found that Candida colonization was more prevalent in the oral cavity of Type 2 patients with diabetes than nondiabetics. The study did not observe any association between salivary glucose level and oral Candida colonization in Type 2 patients with diabetes. Higher colonization of Candida in diabetics with longer history of diabetes was seen. Results of this study indicated a higher oral Candida carriage in Type 2 diabetics and recommended more attention for controlling of diabetes.[27] The use of CHROMagar could allow mycology laboratories to rapidly identify Candida spp. in clinical samples (Ainscough and Kibbler, 1998). This capability will also enable clinicians to more rapidly make appropriate antifungal choices thus decreasing the patient morbidity and mortality.[28] Speciation of Candida was done based on the color exhibited by the colonies on CHROMagar. Green-colored colonies indicated C. albicans, blue-colored colonies indicated C. tropicalis, pink-colored colonies indicated C. krusei, and white/violet-colored colonies indicated C. glabrata [Figure 6]. Within the groups, a significant correlation between salivary glucose and candidal CFUs was present only in nondiabetic patients.
Figure 6.
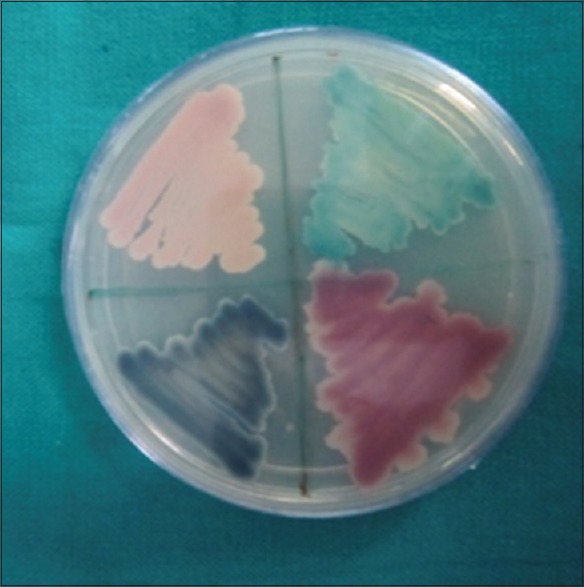
Petridish showing colonies of different colors: Candida glabrata as white/violet, Candida tropicalis as blue, and Candida albicans as green (CHROMagar)
High levels of salivary glucose increase candidal adherence to buccal epithelial cells.[29] In tissues, salivary glucose forms chemically reversible glycosylation products with proteins during hyperglycemic episodes, and this leads to accumulation of glycosylation products on buccal epithelial cells, which, in turn, may increase the number of available receptors for Candida.[30] Odds et al.[8] and Willis et al.[22] have found an increase in candidal density with increased concentration of salivary glucose. Another consideration is the ability of Candida to adhere to the oral epithelium. Adhesion of an organism to the host is a prerequisite for colonization and subsequent infection.[31] In patients with diabetes, oral epithelium favor adhesion and colonization of Candida unlike in nondiabetic patients. It is possible that there may be intrinsic qualitative changes on the cell surface receptors which modulate Candida adhesion in diabetic patients.[32] Other factors that play a role in DM are decreased candidacidal activity of neutrophils, particularly in the presence of glucose.[33]
Longitudinal studies should be developed to evaluate whether patients with high Candida colonization are prone to develop oral candidiasis and which are the clinical factors that predispose them to develop colonization by non albicans species, as well as their role in oral disease. In the present study it was found that, in diabetic patients, the increase in salivary glucose levels likely contributes to their increased candidal carriage and the potential for increased susceptibility to oral candidiasis. The findings of the study would be helpful in any further studies which, if done prospectively on a larger cohort, can be confirmatory.
Conclusion
Diabetes Mellitus (DM) is gaining the status of a potential epidemic. Increased oral carriage rate of Candida species have been reported in patients with Diabetes Mellitus.
In the present study, we have done review and discussed the clinical data in the literature on the relationship between diabetes and oral candidal carriage and infection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. U. S. Shukla, Department of Biostatistics, People's College of Medical Sciences and Research Centre, Bhopal for his statistical analysis of the data in this study.
References
- 1.Akpan A, Morgan R. Oral candidiasis. Postgrad Med J. 2002;78:455–9. doi: 10.1136/pmj.78.922.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamey PJ, Darwaza A, Fisher BM, Samaranayake LP, Macfarlane TW, Frier BM, et al. Secretor status, candidal carriage and candidal infection in patients with diabetes mellitus. J Oral Pathol. 1988;17:354–7. doi: 10.1111/j.1600-0714.1988.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 3.Rautemaa R, Rusanen P, Richardson M, Meurman JH. Optimal sampling site for mucosal candidosis in oral cancer patients is the labial sulcus. J Med Microbiol. 2006;55:1447–51. doi: 10.1099/jmm.0.46615-0. [DOI] [PubMed] [Google Scholar]
- 4.Aly FZ, Blackwell CC, MacKenzie DA, Weir DM. Identification of oral yeast species isolated from individuals with diabetes mellitus. Mycoses. 1995;38:107–10. doi: 10.1111/j.1439-0507.1995.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 5.Bai KY, Reddy CD, Abu-Talib SH. Oral candidal carriage in young insulin dependent diabetics. J Indian Soc Pedod Prev Dent. 1995;13:20–3. [PubMed] [Google Scholar]
- 6.Loiselle RJ, Barnes G, Bahn AN. The occurrence of Candida albicans in the oral cavity of diabetics. J Dent Res. 1964;43:903. [Google Scholar]
- 7.Peters RB, Bahn AN, Barens G. Candida albicans in the oral cavity of diabetics. J Dent Res. 1966;45:771. doi: 10.1177/00220345660450034601. [DOI] [PubMed] [Google Scholar]
- 8.Odds FC, Evans EG, Taylor MA, Wales JK. Prevalence of pathogenic yeasts and humoral antibodies to Candida in diabetic patients. J Clin Pathol. 1978;31:840–4. doi: 10.1136/jcp.31.9.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odds FC. Candida and Candidosis-a Review and Bibliography. 2nd ed. London: Baillière Tindall, W.B Saunders; 1988. [Google Scholar]
- 10.Zegarelli DJ. Fungal infections of the oral cavity. Otolaryngol Clin North Am. 1993;26:1069–89. [PubMed] [Google Scholar]
- 11.Moran GP, Sullivan DJ, Coleman DC. Emergence of non-Candida albicans Candida species as pathogens. In: Calderone RA, editor. Candida and Candidiasis. 4th ed. 2002. pp. 37–53. Ch. 4. [Google Scholar]
- 12.Richardson MD, Warnock DW. Fungal Infection: Diagnosis and Management. 3rd ed. Oxford: Blackwell Publishing; 2003. [Google Scholar]
- 13.Sathya PS, Bharani GO, Nagalingam M, Jayanthi M, Kanagavalli U. Potential of salivary protein as a biomarker in prognosis of diabetes mellitus. J Pharm Res. 2011;4:2228–9. [Google Scholar]
- 14.Sashikumar R, Kannan R. Salivary glucose levels and oral candidal carriage in type II diabetics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:706–11. doi: 10.1016/j.tripleo.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 15.Panchbhai AS, Degwekar SS, Bhowte RR. Estimation of salivary glucose, salivary amylase, salivary total protein and salivary flow rate in diabetics in India. J Oral Sci. 2010;52:359–68. doi: 10.2334/josnusd.52.359. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Aryeh H, Cohen M, Kanter Y, Szargel R, Laufer D. Salivary composition in diabetic patients. J Diabet Complications. 1988;2:96–9. doi: 10.1016/0891-6632(88)90011-6. [DOI] [PubMed] [Google Scholar]
- 17.Darwazeh AM, MacFarlane TW, McCuish A, Lamey PJ. Mixed salivary glucose levels and candidal carriage in patients with diabetes mellitus. J Oral Pathol Med. 1991;20:280–3. doi: 10.1111/j.1600-0714.1991.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 18.Fisher BM, Lamey PJ, Samaranayake LP, MacFarlane TW, Frier BM. Carriage of Candida species in the oral cavity in diabetic patients: Relationship to glycaemic control. J Oral Pathol. 1987;16:282–4. doi: 10.1111/j.1600-0714.1987.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 19.Kumar BV, Padshetty NS, Bai KY, Rao MS. Prevalence of Candida in the oral cavity of diabetic subjects. J Assoc Physicians India. 2005;53:599–602. [PubMed] [Google Scholar]
- 20.Cannon RD, Holmes AR, Mason AB, Monk BC. Oral Candida: Clearance, colonization, or candidiasis? J Dent Res. 1995;74:1152–61. doi: 10.1177/00220345950740050301. [DOI] [PubMed] [Google Scholar]
- 21.Epstein JB, Pearsall NN, Truelove EL. Quantitative relationship between Candida albicans in saliva and the clinical status of human subjects. J Clin Microbiol. 1980;12:475–6. doi: 10.1128/jcm.12.3.475-476.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willis AM, Coulter WA, Fulton CR, Hayes JR, Bell PM, Lamey PJ, et al. Oral candidal carriage and infection in insulin-treated diabetic patients. Diabet Med. 1999;16:675–9. doi: 10.1046/j.1464-5491.1999.00134.x. [DOI] [PubMed] [Google Scholar]
- 23.Tapper-Jones LM, Aldred MJ, Walker DM, Hayes TM. Candidal infections and populations of Candida albicans in mouths of diabetics. J Clin Pathol. 1981;34:706–11. doi: 10.1136/jcp.34.7.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Attas SA, Amro SO. Candidal colonization, strain diversity, and antifungal susceptibility among adult diabetic patients. Ann Saudi Med. 2010;30:101–8. doi: 10.4103/0256-4947.60514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gugnani HC, Becker K, Fegeler W, Basu S, Chattopadhya D, Baveja U, et al. Oropharyngeal carriage of Candida species in HIV-infected patients in India. Mycoses. 2003;46:299–306. doi: 10.1046/j.1439-0507.2003.00896.x. [DOI] [PubMed] [Google Scholar]
- 26.Kadir T, Pisiriciler R, Akyüz S, Yarat A, Emekli N, Ipbüker A, et al. Mycological and cytological examination of oral candidal carriage in diabetic patients and non-diabetic control subjects: Thorough analysis of local aetiologic and systemic factors. J Oral Rehabil. 2002;29:452–7. doi: 10.1046/j.1365-2842.2002.00837.x. [DOI] [PubMed] [Google Scholar]
- 27.Jafari AA, Khanpayah E, Ahadian H. Comparison of the oral Candida carriage in type 2 diabetic and non diabetics. Jundishapur J Microbiol. 2013;6:8495. [Google Scholar]
- 28.Nejad BS, Rafiei A, Moosanejad F. Prevalence of Candida species in the oral cavity of patients with periodontitis. Afr J Biotechnol. 2011;10:2987–90. [Google Scholar]
- 29.Darwazeh AM, MacFarlane TW, Lamey PJ. The in vitro adhesion of Candida albicans to buccal epithelial cells (BEC) from diabetic and non-diabetic individuals after in vivo and in vitro application of nystatin. J Oral Pathol Med. 1997;26:233–6. doi: 10.1111/j.1600-0714.1997.tb01229.x. [DOI] [PubMed] [Google Scholar]
- 30.Soysa NS, Samaranayake LP, Ellepola AN. Diabetes mellitus as a contributory factor in oral candidosis. Diabet Med. 2005;23:455–9. doi: 10.1111/j.1464-5491.2005.01701.x. [DOI] [PubMed] [Google Scholar]
- 31.Gibbons RJ, Houte JV. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- 32.Samaranayake LP. Host factors and oral candidosis. In: Samaranayake LP, Mac Farlane TW, editors. Oral Candidosis. London: Butterworth; 1990. p. 75. [Google Scholar]
- 33.Ranganathan K, Narasimhan P, Vidya KM, Gunaseelan R, Kumarasamy N, Solomon S, et al. Oral Candida species in healthy and HIV-infected subjects in Chennai, South India. Trop Med Health. 2008;36:101–6. [Google Scholar]


