Abstract
Background:
Fluvoxamine, a well-known selective serotonin reuptake inhibitor, is used for the management of mental disorders and various types of chronic pain. In our previous study, we found the inhibitory effect of fluvoxamine on inflammatory mediator‘s expression. In the line of the indicated study, we sought to evaluate the effect of fluvoxamine on the expression of some inflammatory mediators such as cyclooxygenase-2 (COX-2).
Materials and Methods:
An in vitro model system of lipopolysaccharide-stimulated human U937 macrophages was used. The expression of COX-2 protein was measured by flow cytometry.
Results:
The expression of COX-2 significantly decreased by fluvoxamine in U937 macrophages.
Conclusion:
The results of the present study provide further evidence for the anti-inflammatory effect of fluvoxamine. This effect appears to be mediated by the downregulation of inflammatory genes. Further studies are needed to evaluate the complex cellular and molecular mechanisms of fluvoxamine.
Keywords: Cyclooxygenases-2, fluvoxamine, inflammation, macrophage
Introduction
Depression is a multifaceted disorder with diverse causes, associated with the risk of severe medical illnesses. Many studies have shown that depression is mediated by proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-1.[1,2] Attenuation of peripheral inflammation is indicated as one of the possible mechanisms of antidepressant action which are used for the treatment of major depressive disorder. Antidepressants block the reuptake of serotonin and/or norepinephrine into the presynaptic nerve terminals, resulting in enhanced synaptic monoamine levels. Many studies have shown that antidepressants from several classes decrease the production of proinflammatory cytokines.[3,4]
Inflammation is a biological response with multiple factors which act in a complex network. The entrance of leukocytes including neutrophils and macrophages into the site of inflammation is critical for the inflammatory process. Macrophages are important inflammatory cells involved in the initiation of inflammatory responses and play critical roles in the development of acute inflammation by secreting various proinflammatory mediators, including TNF-α, IL-6, cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS).[5]
Prostaglandin E2 (PGE2), which is synthesized from arachidonic acid by COX-2, plays a major role as a mediator of the inflammatory responses.[6] Inhibition of PGE2 by blocking the expression of COX-2 in macrophages is a strategy in the development of anti-inflammatory agents. It seems that macrophages, both in the periphery and in the brain, are activated in depression and release proinflammatory cytokines that are responsible for activating the cyclooxygenase and increasing the inflammatory process.[7]
Lipopolysaccharide (LPS) is a potent activator of macrophages which produce inflammatory mediators such as COX-2.[8]
Selective serotonin reuptake inhibitors (SSRIs) are broadly used in the treatment of major depression, panic disorder, obsessive–compulsive disorder, and eating disorders. In comparison to other types of antidepressants, SSRIs have fewer side effects and better tolerated. Studies related to their anti-inflammatory activities are limited and to some extent are controversial.[9]
Therefore, in the present study, we aimed to evaluate the effect of fluvoxamine, one of the SSRIs, on the expression of COX-2 protein using an in vitro model system of LPS-stimulated human U937 macrophages that has been a widely characterized model of the mammalian cellular response to various inflammatory stimuli.
Materials and Methods
Chemicals
Human monocytic cells (U937) were purchased from the Pasteur Institute (Tehran, Iran). RPMI 1640 cell culture medium, fetal bovine serum (FBS), trypsin-ethylenediaminetetraacetic acid, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide were obtained from Gibco (USA). Phorbol myristate acetate (PMA), LPS from Escherichia coli 055:B5, and dimethyl sulfoxide were obtained from Sigma-Aldrich (USA). Fluvoxamine was donated by Iran Daru Pharmaceutical Co., Tehran, Iran, and was dissolved in phosphate-buffered saline (PBS) for cells. COX-2 antibody was purchased from Santa Cruz Co.
Human U937 macrophage cell culture
The human monocyte cell line U937 was grown in complete RPMI 1640 medium supplemented with 10% (v/v) FBS at 37°C in a humidified atmosphere of 95% air and 5% CO2. Antibiotics, penicillin (100 U/mL) and streptomycin (100 μg/mL), were added to the cell culture during the growth phase, but removed before experimental manipulation. To differentiate the cells into adherent macrophages, they were seeded at a density of 5 × 105 cells/well and incubated for 48 h in the presence of PMA at the final concentration of 100 nM into the cellular medium. The cells were then washed and incubated in normal growth medium for additional 24 h before the addition of LPS (1 μg/ml). Different concentration of fluvoxamine from 10−8 M to 10−6 M was added to the medium 1 h before the addition of LPS (1 μg/ml). The cells with LPS alone and control cells (without LPS and component) were also included in the study. The cells were used for the assessment of COX-2 protein by flow cytometry.
Intracellular staining for flow cytometry
After incubation with LPS and fluvoxamine, for intracellular staining, 100 μl of human U937 macrophages (1 × 106) was transferred to polystyrene tubes (BD Biosciences, Labware, Falcon). The cells were fixed in 0.01% formaldehyde. Then, they were incubated with Tween 20 (0.5% v/v in PBS) in dark at room temperature for 15 min. Tween 20 disrupts membranes and enables antibody (COX-2) to go through pores without dissolving plasma membrane. For staining cells, they (1 × 106) were incubated with 1 μg of COX-2 antibody for 30 min. Then, they were washed and prepared in PBS solution for flow cytometry. Antibody was conjugated to fluorescein, which was detected with FL1 detector. The samples were analyzed on a BD FACS cytometer equipped with a standard argon laser 488-nm excitation and with 530/30 band-pass filter for FL1 for the detection of fluorescein isothiocyanate. To exclude cell debris and clumps, the samples were gated on forward scatter versus side scatter. Fluorescence of 10,000 cells was quantified from histogram plots using the mean fluorescence intensity (MFI Geometric). Fold change was calculated by dividing the MFI of the treated sample (MFI treated) by that of the untreated sample (MFI untreated).
Statistical analysis
All experiments were performed in triplicate. Statistically significant differences between treated and untreated cells were determined using independent t-test. All the data are expressed as mean ± standard deviation, and P < 0.05 was considered statistically significant.
Results
Differentiation of monocytes to macrophages
Monocyte was incubated with PMA for 48 h. Monocyte appeared suspended and round. After incubation with PMA, they differentiated to macrophages and look adherent and stretched [Figure 1].
Figure 1.
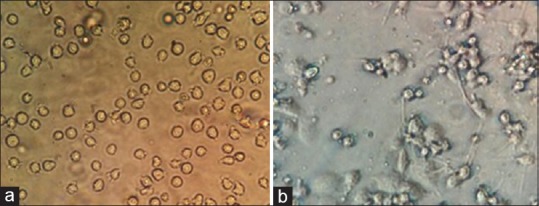
Differentiation of monocytes (a) to macrophages (b)
Inhibition of lipopolysaccharide-stimulated cyclooxygenase-2 expression by fluvoxamine in macrophages
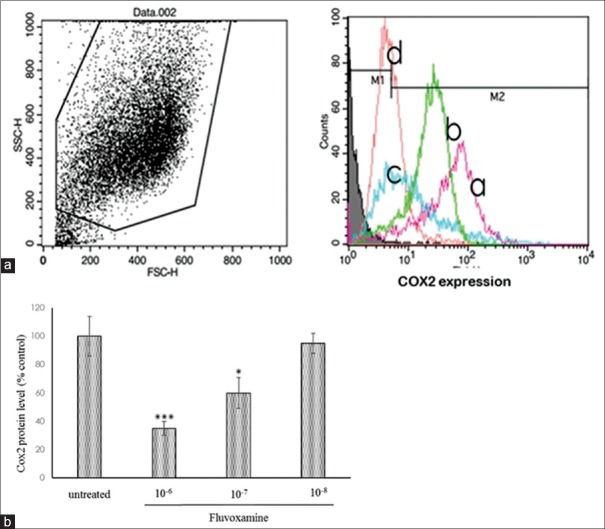
We evaluated the effect of fluvoxamine on the expression of COX-2 in U937 macrophages stimulated with LPS. As demonstrated in Figure 2a, the expression of COX-2 protein decreased in 65% of macrophages at the 10−6 M concentration and 40% of cells in the concentration of 10−7 M of fluvoxamine. However, in 10−8 M, the expression of COX-2 did not change significantly in LPS-stimulated macrophages [Figure 2b].
Figure 2.
Effect of fluvoxamine on cyclooxygenase-2 expression in lipopolysaccharide-induced U937 macrophages. (a) The cells pretreated with the indicated concentrations of fluvoxamine (10−6 M: d), (10−7 M: c), and (10−8 M: b) for 1 h, and then, the cells were activated with lipopolysaccharide (1 μg/ml) (a: untreated cells). After 24 h, expression of cyclooxygenase-2 was measured by flow cytometry. (b) Fluvoxamine considerably decreases the expression of cyclooxygenase-2 in 10−6 M and 10−7 M concentration. Mean ± standard deviation values of experiments are shown. *P < 0.05, ***P < 0.001 compared with control group
Discussion
The present study was performed to investigate the potential anti-inflammatory effects of fluvoxamine and to elucidate the molecular mechanism(s) involved. The findings of this study evidently showed that fluvoxamine suppressed the expression of COX-2 in U937 macrophages. Fluvoxamine exhibits strong effects as a SSRI. Several reports showed that human peripheral blood mononuclear cells as well as central nervous system possess serotonin and norepinephrine transporter and might be directly affected by antidepressants.[10,11,12] Moreover, serotonin and noradrenaline are released from lymphocytes and monocytes[13] and can prompt immunomodulatory properties through receptors that are present on immune cells.
Therefore, we investigated the influence of fluvoxamine on LPS-induced monocytes to evaluate if the anti-inflammatory effect of fluvoxamine can be mediated through macrophages. We showed that fluvoxamine attenuated the expression of COX-2 in macrophages. In our previous study, we indicated that the mRNA expression of COX-2 was significantly decreased by fluvoxamine in endothelial cells, macrophages, and also in carrageenan-induced paw edema in rat. To confirm the prior study, we illustrated that COX-2 protein was also decreased by fluvoxamine in macrophages.[14] COX-2 is catalyzing the production of PGE2 from arachidonic acid.[15] In line with our results, Taler et al. reported that the expression of COX-2 decreased with SSRIs on human T-lymphocytes.[16] Moreover, amitriptyline and fluoxetine were shown to attenuate the production of proinflammatory cytokine-induced PGE2 and nitric oxide by cultured human synovial cells.[17] Furthermore, Roumestan et al. described that desipramine as an serotonin–norepinephrine reuptake inhibitor (SNRI) by affecting gene expression in peripheral cell types including monocytes can decrease the inflammatory mediators.[18]
In summary, the data show that fluvoxamine may reduce the inflammatory responses of monocytes; the mechanism could include a cascade of gene expression secondary to effects on the serotonin transporter that is expressed on the surface of monocytes and lymphocytes.
Several studies have demonstrated that some antidepressants increase intracellular concentrations of cyclic adenosine monophosphate (cAMP) through activation of monoamine receptors such as serotonin and noradrenaline receptors.[19] Another in vitro study proposed that the anti-inflammatory effects of various antidepressants on microglia are at least partially mediated by the cAMP-dependent protein kinase A (PKA) pathway.[20] In some cell types, it has been shown that cAMP/PKA pathway inhibits the nuclear factor-kappa-B (NF-κB) activity,[21] and its activation is identified to induce the expression of iNOS and various proinflammatory cytokines in human monocytes.[22] Here, we stimulated macrophages by LPS. Binding of LPS to toll-like receptor 4 activates two principal signaling pathways, which results in activation of the transcription factor, NF-κB, an important upstream modulator for COX-2 and iNOS expression.[23,24] Moreover, a number of studies showed that some antidepressants including SNRIs exert their anti-inflammatory effect by inhibition of NF-κB signaling pathway.[17,19] Based on these studies and our findings, we suggest that fluvoxamine evokes its suppressive effect on the expression of COX-2 by modulating NF-κB pathway. To our knowledge, this is the first study to evaluate the anti-inflammatory effect of fluvoxamine through the inhibition of COX-2 expression in an in vitro model.
Conclusion
The results of this study provide further evidence for the anti-inflammatory effect of fluvoxamine through inhibition of COX-2 expression based on in vivo findings. Further studies are needed to evaluate whether these effects are related to neurotransmitters such as norepinephrine and/or serotonin or not.
Financial support and sponsorship
This paper was financially supported by the Research Department of Isfahan University of Medical Sciences, Isfahan, I.R. Iran.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 2.Maes M, Song C, Lin AH, Bonaccorso S, Kenis G, De Jongh R, et al. Negative immunoregulatory effects of antidepressants: Inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacology. 1999;20:370–9. doi: 10.1016/S0893-133X(98)00088-8. [DOI] [PubMed] [Google Scholar]
- 3.Gobin V, Van Steendam K, Denys D, Deforce D. Selective serotonin reuptake inhibitors as a novel class of immunosuppressants. Int Immunopharmacol. 2014;20:148–56. doi: 10.1016/j.intimp.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 4.De Berardis D, Conti CM, Serroni N, Moschetta FS, Olivieri L, Carano A, et al. The effect of newer serotonin-noradrenalin antidepressants on cytokine production: A review of the current literature. Int J Immunopathol Pharmacol. 2010;23:417–22. doi: 10.1177/039463201002300204. [DOI] [PubMed] [Google Scholar]
- 5.Hussein SZ, Mohd Yusoff K, Makpol S, Mohd Yusof YA. Gelam honey inhibits the production of proinflammatory, mediators NO, PGE (2), TNF-α, and IL-6 in carrageenan-induced acute paw edema in rats. Evid Based Complement Alternat Med. 2012;2012:109636. doi: 10.1155/2012/109636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Invest. 2002;110:651–8. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonard BE. The immune system, depression and the action of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:767–80. doi: 10.1016/s0278-5846(01)00155-5. [DOI] [PubMed] [Google Scholar]
- 8.Kuroda E, Yamashita U. Mechanisms of enhanced macrophage-mediated prostaglandin E2 production and its suppressive role in th1 activation in Th2-dominant BALB/c mice. J Immunol. 2003;170:757–64. doi: 10.4049/jimmunol.170.2.757. [DOI] [PubMed] [Google Scholar]
- 9.Sadeghi H, Hajhashemi V, Minaiyan M, Movahedian A, Talebi A. A study on the mechanisms involving the anti-inflammatory effect of amitriptyline in carrageenan-induced paw edema in rats. Eur J Pharmacol. 2011;667:396–401. doi: 10.1016/j.ejphar.2011.05.053. [DOI] [PubMed] [Google Scholar]
- 10.Fazzino F, Montes C, Urbina M, Carreira I, Lima L. Serotonin transporter is differentially localized in subpopulations of lymphocytes of major depression patients. Effect of fluoxetine on proliferation. J Neuroimmunol. 2008;196:173–80. doi: 10.1016/j.jneuroim.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Faraj BA, Olkowski ZL, Jackson RT. Expression of a high-affinity serotonin transporter in human lymphocytes. Int J Immunopharmacol. 1994;16:561–7. doi: 10.1016/0192-0561(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 12.Urbina M, Pineda S, Piñango L, Carreira I, Lima L. [3H] Paroxetine binding to human peripheral lymphocyte membranes of patients with major depression before and after treatment with fluoxetine. Int J Immunopharmacol. 1999;21:631–46. doi: 10.1016/s0192-0561(99)00035-1. [DOI] [PubMed] [Google Scholar]
- 13.Tynan RJ, Weidenhofer J, Hinwood M, Cairns MJ, Day TA, Walker FR, et al. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav Immun. 2012;26:469–79. doi: 10.1016/j.bbi.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Rafiee L, Hajhashemi V, Javanmard SH. Fluvoxamine inhibits some inflammatory genes expression in LPS/stimulated human endothelial cells, U937 macrophages, and carrageenan-induced paw edema in rat. Iran J Basic Med Sci. 2016;19:977–84. [PMC free article] [PubMed] [Google Scholar]
- 15.DeWitt DL. Prostaglandin endoperoxide synthase: Regulation of enzyme expression. Biochim Biophys Acta. 1991;1083:121–34. doi: 10.1016/0005-2760(91)90032-d. [DOI] [PubMed] [Google Scholar]
- 16.Taler M, Gil-Ad I, Lomnitski L, Korov I, Baharav E, Bar M, et al. Immunomodulatory effect of selective serotonin reuptake inhibitors (SSRIs) on human T lymphocyte function and gene expression. Eur Neuropsychopharmacol. 2007;17:774–80. doi: 10.1016/j.euroneuro.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Yaron I, Shirazi I, Judovich R, Levartovsky D, Caspi D, Yaron M, et al. Fluoxetine and amitriptyline inhibit nitric oxide, prostaglandin E2, and hyaluronic acid production in human synovial cells and synovial tissue cultures. Arthritis Rheum. 1999;42:2561–8. doi: 10.1002/1529-0131(199912)42:12<2561::AID-ANR8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 18.Roumestan C, Michel A, Bichon F, Portet K, Detoc M, Henriquet C, et al. Anti-inflammatory properties of desipramine and fluoxetine. Respir Res. 2007;8:35. doi: 10.1186/1465-9921-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duman RS. Novel therapeutic approaches beyond the serotonin receptor. Biol Psychiatry. 1998;44:324–35. doi: 10.1016/s0006-3223(98)00031-6. [DOI] [PubMed] [Google Scholar]
- 20.Hashioka S, Klegeris A, Monji A, Kato T, Sawada M, McGeer PL, et al. Antidepressants inhibit interferon-gamma-induced microglial production of IL-6 and nitric oxide. Exp Neurol. 2007;206:33–42. doi: 10.1016/j.expneurol.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Delfino F, Walker WH. Hormonal regulation of the NF-kappaB signaling pathway. Mol Cell Endocrinol. 1999;157:1–9. doi: 10.1016/s0303-7207(99)00127-6. [DOI] [PubMed] [Google Scholar]
- 22.Ollivier V, Parry GC, Cobb RR, de Prost D, Mackman N. Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells. J Biol Chem. 1996;271:20828–35. doi: 10.1074/jbc.271.34.20828. [DOI] [PubMed] [Google Scholar]
- 23.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–15. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 24.Sharif O, Bolshakov VN, Raines S, Newham P, Perkins ND. Transcriptional profiling of the LPS induced NF-kappaB response in macrophages. BMC Immunol. 2007;8:1. doi: 10.1186/1471-2172-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]