Abstract
Background:
Diabetes can increase the generation of free radicals and can be harmfully effective in spermatogenesis. Crocin is a carotenoid and is accountable for the red color of saffron. Crocin has shown numerous pharmacological actions such as antioxidant roles and radical scavenging. The aim of this study was to determine the effects of crocin on sperm parameters and the diameter of seminiferous tubules in diabetic rats.
Materials and Methods:
In this experimental study, diabetic rats were induced by Streptozotocin (STZ) (60 mg/kg). Sixty-four rats were equally divided into the following eight groups; (1) normal control group, (2–4) crocin groups, receiving various doses of crocin (12.5, 25, and 50 mg/kg), (5) diabetic control group, and (6–8) diabetic groups, receiving STZ plus crocin (12.5, 25, and 50 mg/kg) injected intraperitoneally once a day for 28 consecutive days. The sperm count, motility, morphology, viability, spermatogenesis index (SI), and the diameter of seminiferous tubules were examined and compared.
Results:
The results demonstrated that count, motility, viability, normal sperm morphology, SI, and the diameter of seminiferous tubules decreased significantly in the diabetic control group compared to the normal control group (P < 0.05). However, in the diabetic groups, count, motility, normal morphology, viability, SI, and the diameter of seminiferous tubules enhanced significantly in total doses compared to those of the diabetic control group (P < 0.05).
Conclusion:
It seems that, as a strong antioxidant, crocin could compensate for the toxicity induced through STZ and raise the quality of some sperm parameters.
Keywords: Crocin, diabetes, seminiferous tubules, sperm parameters
Introduction
Sexual dysfunction is one of the main secondary complications in diabetic males.[1] Numerous factors can disturb spermatogenesis processes and reduce sperm‘s quality and quantity.[2] Some diseases such as diabetes affect spermatogenesis. Diabetes mellitus is a chronic disorder of carbohydrate, lipid, and protein metabolism, which is considered through hyperglycemia. Diabetes can involve male reproductive function at several stages.[3] Diabetes affects male reproductive function through increasing oxidative stress and oxidative injury in the testis.[4] Oxidative stress has been labeled as a significant factor in several diseases such as diabetes and has improved the production of free radicals.[5] Free radicals, membrane lipid peroxidation, and protein oxidation increase in diabetic patients.[6] An extreme production of reactive oxygen species (ROS) can be damaging to the sperm cells.[7] Hyperglycemia can lead to increase pro-inflammatory cytokines in the testis. On the other hand, a chronic inflammation increases the production of free radicals and ROS.[8] The spermatozoa plasma membrane contains great amounts of unsaturated fatty acids. Therefore, it is susceptible to peroxidative injury.[9] The lipid peroxidation destroys the structure of lipid matrix of spermatozoa membranes and disturbs sperm‘s motility.[10] It seems that the consumption of antioxidants can partially prevent the complications of diabetes.[11] Antioxidants have important effects on spermatogenesis.[12] Crocin is a carotenoid, which is commercially gained from the dried trifid stigma of the culinary spice Crocus sativus L. (saffron) and is responsible for the red color of saffron.[13] Since long ago, in traditional medicine, saffron has been used to treat infertility, impotence, and sexual potential stimulant.[14] Crocin was shown to have numerous pharmacological actions such as antioxidant, anticancer, radical scavenging, and genoprotective effects.[15] Since diabetes has many effects on reproductive activities in men and no study has ever investigated the effects of the crocin on diabetes-induced impairments in sperm parameters and seminiferous tubules, the present study was carried out to evaluate the effects of crocin on disorders of sperm parameters in diabetic rats.
Materials and Methods
Animals
In this experimental study, 64 male Wistar rats (8 weeks old) with the weight range of 220–250 g were purchased from Razi Institute of Iran. Before the onset of the study, they were kept in an animal house for 1 week under controlled laboratory conditions at temperature 22°C ± 2°C and humidity (50%–60%). Besides, they were kept for 12 h in the lighting and 12 h in the darkness with free access to water and food in order to be improved to the new environment. To keep the rats, standard cages in a medical school where eight mice were put in each cage were used.[2]
Chemicals
Crocin (digentiobiosyl 8, 8’-diapocarotene-8, 8’-oate; C44H64O24) powder was purchased (Merck, Germany).[13] The powder was diluted through normal saline (0.9%) to make diverse doses. A streptozotocin (STZ) (S0130-500MG, Sigma-Aldrich, CO, St. Louis, USA) at 60 mg/kg was used.[10]
Diabetic induction
To induce diabetes in the animals, the STZ (Sigma) was administered intraperitoneally (IP) (60 mg/kg). Three days after administration, blood samples were taken from the rats’ cauda to ensure that they were diabetic. The rats with the blood glucose of more than 300 mg/dl were considered as diabetic and were maintained on a 12-h alternating light to dark cycle. The blood glucose was measured three times: (a) after the injection of STZ in order to ensure that the rats were diabetic, (b) 3 weeks after the initial injection, and (c) 6 weeks after the first injection. Furthermore, the serum glucose level was measured before STZ administration (90 mg/dl).[16]
Experimental design
The rats were divided into eight groups in the following way: (1) the normal control group, received normal saline containing 100-mM sodium citrate and were fed with standard rodent diet and tap water throughout the study, (2–4) the crocin groups received crocin (12.5, 25, and 50 mg/kg, respectively), (5) the diabetic control group received a single intraperitoneal administration of STZ and was fed with standard rodent diet and tap water throughout the study, and (6–8) the diabetic groups received a single intraperitoneal administration of STZ and were fed with standard rodent diet and tap water supplemented with 12.5, 25, and 50 mg/kg crocin 5 days after the administration. The experiment was carried out within 4 weeks. Then, crocin was injected IP.[14]
Sperm collection
One month after the 1st day of the treatment, the rats were sacrificed through an intraperitoneal injection of ketamine (40 mg/kg), and laparotomy was conducted. Then, testes and epididymis were collected. The cauda part of the epididymis was used to assess sperm parameters, and the right testis was handled for histological exercise. In each animal, both cauda epididymis were located, minced, and protected in a warm Petri dish containing 10-ml Hank‘s balanced salt solution at 37°C. The spermatozoas were permitted to disperse into the buffer. After 20 min, the cauda of epididymis was removed, and the suspension was mildly shaken in order to be homogenized and was examined under a light microscope at a magnification of ×400.[9]
Sperm count
For calculating the sperm, 500 μL of the sperm suspension was diluted (dilution of 1:10) with formaldehyde fixative (10% formalin in phosphate buffered saline) (Sigma, USA). Nearly, 10 μL from the diluted solution was moved into an hemocytometer. Hemocytometer was located in a moist chamber (Petri dish) with a dampened filter paper and was kept there for 7 min. Hemocytometer was placed on the microscope stage. Then, the settled sperms were counted (four small corners were counted and center squares were situated in the great center square of the counting chamber) and evaluated per 250 small squares of a hemocytometer using a ×40 objective.[10]
Normal sperm morphology techniques
The normal sperm morphology was assessed through the analysis of the sperm smears made from the left cauda epididymis. An aliquot of the sample was used to make the smears and estimate the spermatozoa deformities. Eosin/nigrosin stain was used to estimate normal spermatozoa morphology. In order to test, one drop of eosin/nigrosin was added to the suspension and mixed mildly. The slides were then viewed below a light microscope at ×400. A total of 300 spermatozoa were analyzed on each slide (3000 cells in each group) to identify the irregularities of the head and tail.[7]
Sperm viability method
Viability was measured through eosin Y staining (5% in saline). Forty microliter samples of a fresh sperm suspension were placed on a crystal slide, mixed with 10 μL eosin and detected below a light microscope (×00). Live sperms remained unstained following the staining process, while those showing pink or red coloration were classified as dead. At least 200 sperm were randomly counted from each sample in 10 fields of visualization, and the percentage of the live sperms was noted.[17]
Sperm high motility
The ratio of sperm motility was assessed visually through a light microscope (Olympus Co., Tokyo, Japan) at ×400. Using this technique, one drop of sperm suspension was located on a glass slide and enclosed with a lamella. The number of the sperms with rapid progressive forward movement was calculated in frequent microscopic fields of vision and the percentages of high motile sperms were attained. High motility estimations were acquired from 10 diverse fields in each sample. The mean of 10 successive estimates was used as the last high motility score.[14]
Measurement of the germinal layer of seminiferous tubules
After the testes fixation through neutral-buffered formalin 10%, the histological processes including dehydrating, clearing, and embedding were carried out. Five-micron thick sections were prepared and H and E staining method was used. More than 20 sections were equipped with the respective blocks. The germinal layer of seminiferous tubules thickness was measured using a Motic camera and software (Moticam 2000, Spain).[2]
Spermatogenesis index
Testis tubules were evaluated for their modified spermatogenesis index (SI) through Johnson‘s score. Based on Johnson‘s score, a grade from 1 to 10 was given to each tubule cross-section ranging from no cells to complete spermatogenesis.[18]
Statistical analysis
The quantitative data were presented as mean ± standard deviation. One-way analysis of variance followed by least significant difference and Tukey post hoc tests were used to determine the statistical significance among the groups through SPSS package (Version 18, SPSS Inc., USA). The Kruskal–Wallis test was used to examine data normality and homogeneity of variances, considering a significance level of 0.05.
Results
Sperm count and morphology normality
The sperm count and morphology increased significantly in the diabetic control group compared to those in the normal control group (P < 0.05). No significant variations were detected in all of the crocin groups compared to the normal control group (P > 0.05). However, sperm count and normal morphology increased significantly in all diabetic groups compared to the diabetic control group (P < 0.05) [Figures 1 and 2].
Figure 1.
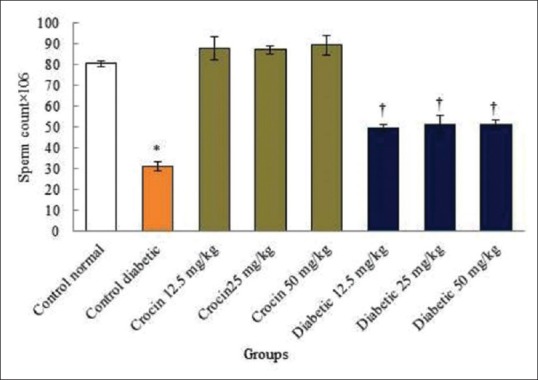
Effects of diabetes and crocin on sperm count. *Significant decrease in the diabetic control group compared to the normal control group (P < 0.05). †Significant increase in all diabetic groups compared to the diabetic control group (P < 0.05)
Figure 2.
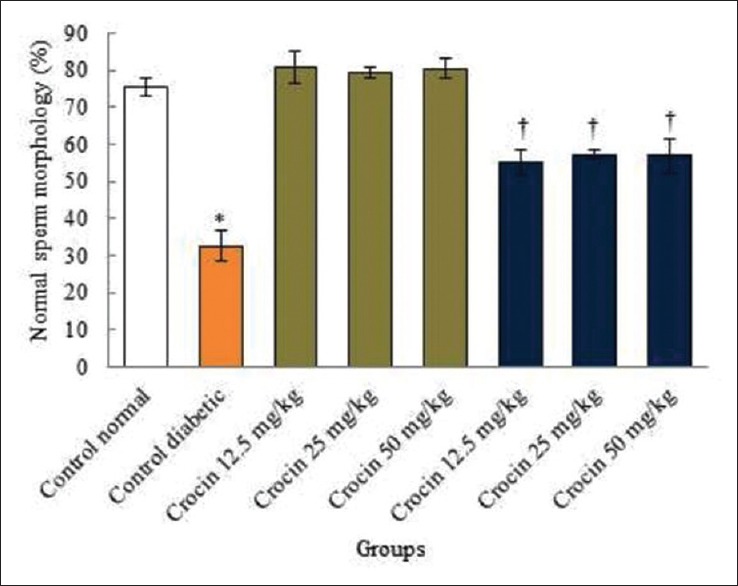
Comparison of the normal sperm morphology in the diabetic control, normal, and diabetic groups in rats. Significant decrease in the diabetic control group compared to the normal control group (P < 0.05). †Significant increase in all diabetic groups compared to the diabetic control group (P < 0.05)
Sperm viability and motility
Diabetes caused a significant reduction in the sperm viability and motility compared to the normal control group (P < 0.05). No significant changes were observed in all crocin groups compared to the normal control group (P > 0.05). In addition, in all diabetic groups, sperm viability and high motility displayed significant increase compared to the diabetic control group (P < 0.05) [Figures 3 and 4].
Figure 3.
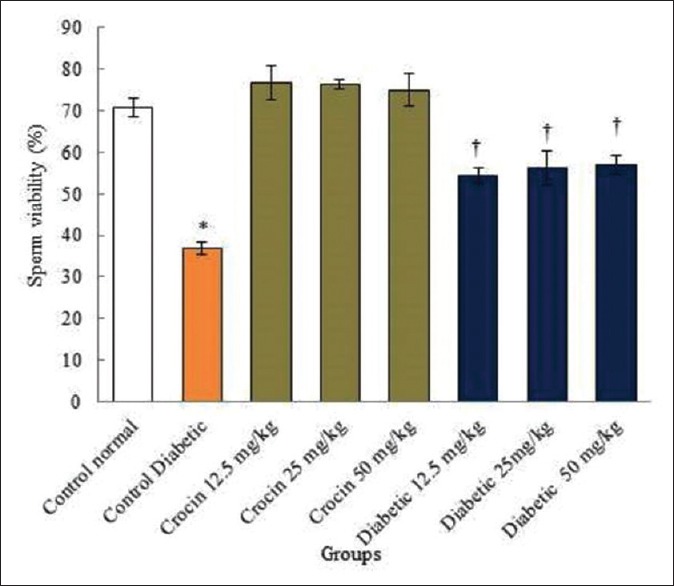
The correlation analysis between control diabetic, normal, and diabetic groups in rats and sperm viability. *Significant decrease in the diabetic control group compared to the normal control group (P < 0.05). †Significant increase in all diabetic groups compared to the diabetic control group (P < 0.05)
Figure 4.
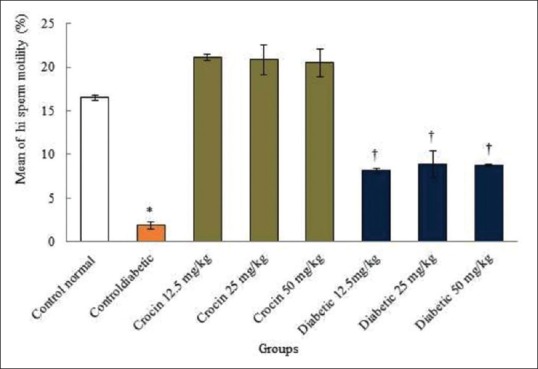
The comparison of the mean of sperm high motility among the treatment groups. *Significant decrease in the diabetic control group compared to the normal control group (P < 0.05). †Significant increase in all diabetic groups compared to the diabetic control group (P < 0.05)
Seminiferous tubules
Diabetes caused a significant reduction in the germinal layer of seminiferous tubules diameter compared to the normal control group (P < 0.05). In fact, no significant changes were observed in all crocin groups compared to the normal control group (P > 0.05). Germinal layer of seminiferous tubules diameter in all diabetic groups increased significantly compared to the diabetic control group (P < 0.05) [Figures 5 and 6].
Figure 5.
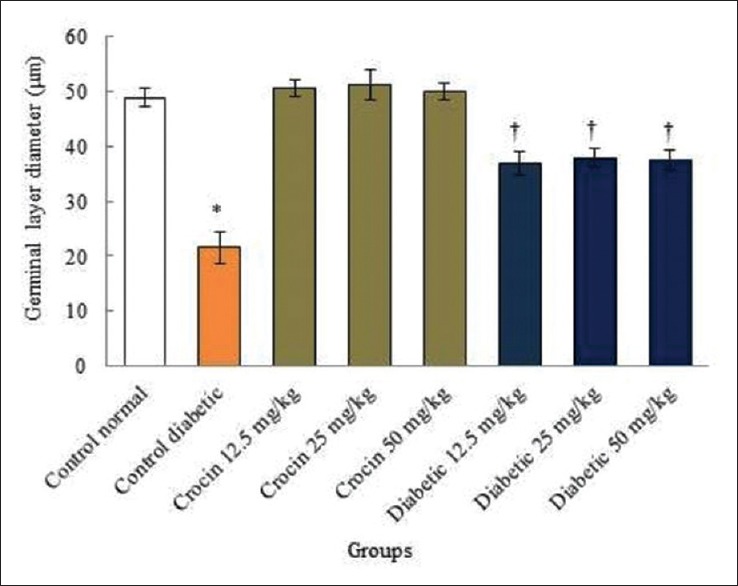
The comparison of germinal layer diameter of seminiferous tubules in the treatments groups of animals. *Significant decrease in the diabetic control group compared to the normal control (saline) group (P < 0.05). †Significant in all diabetic groups compared to the diabetic control group (P < 0.05)
Figure 6.
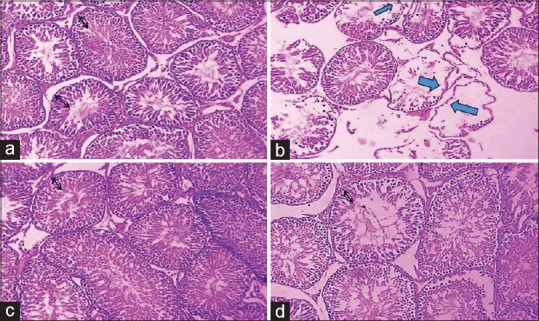
The effect of streptozotocin, crocin, and streptozotocin plus crocin on the germinal layer of seminiferous tubules (×40). Normal seminiferous tubules structure and germinal layer thickness in the normal control group (a) and crocin (150 mg/kg) (c) were seen. In the diabetic control group (b) decreased in germinal layer thickness and the sperm cell was seen (blue arrows). In the diabetic (50 mg/kg) group (d) a normal seminiferous tubules structure was seen. The black arrows in figures show the normal germinal layer thickness
Spermatogenesis index
Diabetes caused a significant decrease in the SI compared to the normal control group (P < 0.05). No significant changes were observed in all crocin groups compared to the normal control group (P > 0.05). In addition, SI in all diabetic groups displayed significant increase compared to the diabetic control group (P < 0.05) [Figure 7].
Figure 7.
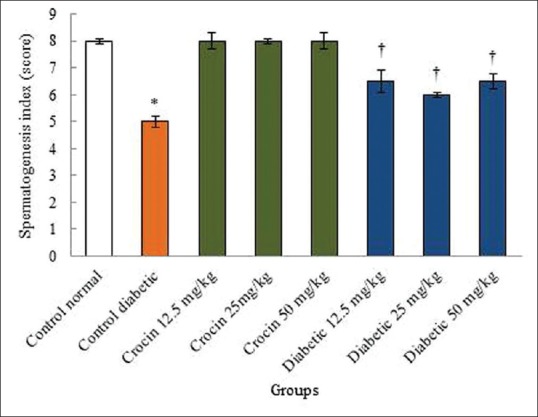
The effects of diabetes, crocin, and diabetes plus crocin on spermatogenesis index. *Significant decrease in the diabetic control group compared to the normal control (saline) group (P < 0.05). †Significant increase in all diabetic groups compared to the diabetic control group (P < 0.05)
Discussion
Diabetes causes sexual complaints. In more specific terms, most of diabetic patient‘s involvement reduced their sexual want and fertility power. The most important reason of infertility in males is their incapability to sufficiently produce healthy and dynamic sperms.[2] Pharmaceutical plants have been used to treat diabetes around the world; however, their effects have been rarely investigated.[16] Thus, considering the properties of crocin and the lack of studies on the effects of these carotenoid and negative properties of diabetes on males’ reproductive system, the present study evaluated the effects of the crocin on the disorders of sperm parameters and testis tissue induced by STZ in male rats. The results of the sperm count, motility, viability, and normal morphology in diverse study groups indicated a significant decrease in the above-mentioned parameters in the diabetic group. Similarly, a significant increase was detected in sperm count, motility, viability, and normal morphology in all crocin groups compared to the diabetic control group. Spermatogenesis is a multifaceted procedure which can be affected by frequent aspects in reduced fertility in males.[7] Oxidative stress is one of those reasons, which is induced in the case of accumulation of ROS due to an inequality among antioxidant systems.[19] It seems that blood glucose increased the oxidative stress in the body through an disproportion among oxidation and revitalization and amplified mitochondrial superoxides production.[20] ROS can affect DNA and RNA synthesis in sperm cells.[9] Therefore, it was assumed that crocin raises the count and motility of sperms in the treated groups through improving the antioxidant protection of the body.[21] This was in agreement with the findings of Salahshoor et al. who indicated that stimulation of diabetes in rats reduced significantly their sperm count, viability, motility, and normal morphology comported to the control group.[2] Seemingly, oxidative stress also acts in sexual germ cells and interrupts their division and differentiation.[22] Through increasing the manufacture of free radicals, hyperglycemia modifies the sperm parameters and destroys the integrity of DNA.[23] Furthermore, the plants containing antioxidants approve the motility and morphological capacities of sperms and improve sperm‘s quality.[17] In the present study, the improvement of some sperm parameters can be attributed to the antioxidant properties of crocin.[14] It seems that the sperms miss a large volume of their cytoplasm due to the antioxidant deficiency during spermatogenesis and become more sensitive to the increased ROS. These results revealed that crocin can perform as an antioxidant and advance the sperm quality through increasing the expression of antioxidant genes. Therefore, it seems necessary to use an antioxidant with plant origin such as crocin without side effects and with reinforcing effects.[13] In line with the results of the present study, the findings of Asadi et al. indicated that saffron improves significantly the epididymal sperm parameters that are exposed to cadmium.[24] Oxidative stress can cause faulty gamma with different chromatin (remodel), which becomes susceptible to the attack of free radicals, and can decrease the number of spermatogonia, spermatocytes, spermatids, and spermatozoa.[25] In agreement with the results of the present study, Niknam and Mahmoudi described that the induction of diabetes increased significantly sperm abnormality and reduced sperm count and motility as well as the diameter of seminiferous tubules and sperm cells in male rat.[26] It seems that crocin, as one of the main compounds of saffron, recovers sexual function in animal models. Crocin can similarly increase testosterone level and play an essential role in improving sperm quality.[27] Our results are consistent with the findings of Salahshoor et al. which showed that the administration of crocin increased the sperm motility and count and decreased abnormal sperms, compared to the nicotine control group.[14] The results of the present study indicated that diabetes decreased the diameter of germinal layer of seminiferous tubules. It should be noted that basement membrane‘s thickness in seminiferous tubules plays a significant role in reducing spermatogenesis. Diabetes can increase basement membrane‘s thickness and reduce the internal diameter of seminiferous tubules.[28] Rajasekaran et al. reported that increased blood glucose caused a growth in basement membrane‘s thickness which is in line with the findings of the present study.[29] It seems that the antioxidant properties of crocin, neutralize the decreasing effects of diabetes on germinal layer diameter.[2] Moreover, the results of the present study indicated that diabetes decreased the SI. Similarly, a significant increase was detected in SI in all crocin and diabetic groups compared to the diabetic control group. Therefore, the SI shifted from level 8 (few spermatozoa) to 5.5 (no spermatozoa and many spermatocytes) during the treatment with STZ and the administration of crocin increased the spermatozoa. Our results are supported by the findings of Khayatnouri et al. which showed that the administration of saffron increased the SI in rats.[30]
Conclusion
The results of this study showed that STZ, as an inducer of diabetes, can produce abnormalities in the testicular tissue and sperm parameters. Besides, as an antioxidant, crocin could increase the quality of some parts of the spermatozoa such as raising sperm viability, count, normal morphology, and high motility and recover germinal layer of seminiferous tubule in diabetic rats. The antioxidant effects of crocin may be the main reason for its optimistic impact on spermatic parameters. Thus, crocin could be valuable in treating infertile diabetic men and partially improving male fertility properties. However, further studies are essential to explain its exact mechanism of action.
Financial support and sponsorship
The study was financially supported by the Research Council of Kermanshah University of Medical Sciences (No: 97499).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are grateful to the Research Council of Kermanshah University of Medical Sciences (No: 97499) for their financial support.
References
- 1.Condorelli RA, La Vignera S, Mongioì LM, Alamo A, Calogero AE. Diabetes mellitus and infertility: Different pathophysiological effects in type 1 and type 2 on sperm function. Front Endocrinol (Lausanne) 2018;9:268. doi: 10.3389/fendo.2018.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salahshoor MR, Haghjoo M, Roshankhah S, Makalani F, Jalili C. Effect of thymoquinone on reproductive parameter in morphine-treated male mice. Adv Biomed Res. 2018;7:18. doi: 10.4103/abr.abr_69_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanter M, Aktas C, Erboga M. Protective effects of quercetin against apoptosis and oxidative stress in streptozotocin-induced diabetic rat testis. Food Chem Toxicol. 2012;50:719–25. doi: 10.1016/j.fct.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 4.Amaral S, Moreno AJ, Santos MS, Seiça R, Ramalho-Santos J. Effects of hyperglycemia on sperm and testicular cells of Goto-Kakizaki and streptozotocin-treated rat models for diabetes. Theriogenology. 2006;66:2056–67. doi: 10.1016/j.theriogenology.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Khaki A, Nouri M, Fathiazad F, Ahmadi-Ashtiani HR, Rastgar H, Rezazadeh SH. Protective effects of quercetin on spermatogenesis in streptozotocin-induced diabetic rat. J Med Plants. 2009;4:57–64. [Google Scholar]
- 6.Pitocco D, Zaccardi F, Di Stasio E, Romitelli F, Santini SA, Zuppi C, et al. Oxidative stress, nitric oxide, and diabetes. Rev Diabet Stud. 2010;7:15–25. doi: 10.1900/RDS.2010.7.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roshankhah SH, Salahshoor MR, Aryanfar S, Jalili F, Sohrabi M, Jalili C. Effects of curcumin on sperm parameters abnormalities induced by morphine in rat. J Med Biomed Sci. 2017;6:1–10. [Google Scholar]
- 8.Lanjhiyana S, Garabadu D, Ahirwar D, Bigoniya P, Rana AC, Patra KC, et al. Antihyperglycemic potential of Aloe vera gel in experimental animal model. Ann Biol Res. 2011;2:17–31. [Google Scholar]
- 9.Jalili C, Ahmadi SH, Roshankhah SH, Salahshoor MR. Preventing effect of genistein on reproductive parameter and serum nitric oxide levels in morphine-treated mice. Int J Reprod Biomed. 2016;14:95–102. [PMC free article] [PubMed] [Google Scholar]
- 10.Jalili C, Kamani M, Roshankhah S, Sadeghi H, Salahshoor MR. Effect of falcaria vulgaris extracts on sperm parameters in diabetic rats. Andrologia. 2018;50:e13130. doi: 10.1111/and.13130. [DOI] [PubMed] [Google Scholar]
- 11.Choi SW, Ho CK. Antioxidant properties of drugs used in type 2 diabetes management: Could they contribute to, confound or conceal effects of antioxidant therapy? Redox Rep. 2018;23:1–24. doi: 10.1080/13510002.2017.1324381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jalili C, Salahshoor MR, Jalili F, Kakaberaei S, Akrami A, Sohrabi M, et al. Therapeutic effect of resveratrol on morphine-induced damage in male reproductive system of mice by reducing nitric oxide serum level. Int J Morphol. 2017;35:1342–7. [Google Scholar]
- 13.Roshankhah SH, Salahshoor MR, Jalili F, Karimi F, Sohrabi M, Jalili C. Crocin effects on the nicotine-induce ovary injuries in female rat. Int J Life Sci Pharm. 2017;7:1–8. [Google Scholar]
- 14.Salahshoor MR, Khazaei M, Jalili C, Keivan M. Crocin improves damage induced by nicotine on A number of reproductive parameters in male mice. Int J Fertil Steril. 2016;10:71–8. doi: 10.22074/ijfs.2016.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salahshoor MR, Roshankhah SH, Jafarzadeh AR, Shabanizadeh Darehdori A, Jalili C. The protective effect of crocin on pancreatic disorders resulting from nicotine prescription in male balb/c mice. Pharmacophore. 2017;8:e–117371. [Google Scholar]
- 16.Ghorbani R, Mokhtari T, Khazaei M, Salahshoor MR, Jalili C, Bakhtiari M. The Effect of walnut on the weight, blood glucose and sex hormones of diabetic male rats. Int J Morphol. 2014;32:858–63. [Google Scholar]
- 17.Jalili C, Salahshoor MR, Khani F, Roshankhah SH. protective effect of curcumin against nicotine-induced damage on reproductive parameters in male mice. Int J Morphol. 2014;32:869–74. [Google Scholar]
- 18.Johnsen SG. Testicular biopsy score count – A method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–5. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 19.Salahshoor MR, Roshankhah S, Hosseni P, Jalili C. Genistein improves liver damage in male mice exposed to morphine. Chin Med J (Engl) 2018;131:1598–604. doi: 10.4103/0366-6999.235117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellogg AP, Pop-Busui R. Peripheral nerve dysfunction in experimental diabetes is mediated by cyclooxygenase-2 and oxidative stress. Antioxid Redox Signal. 2005;7:1521–9. doi: 10.1089/ars.2005.7.1521. [DOI] [PubMed] [Google Scholar]
- 21.Singla RK, Bhat VG. Crocin: An overview. Indo Glob J Pharm Sci. 2011;1:281–6. [Google Scholar]
- 22.Mahdavi R, Faramarzi E, Seyedrezazadeh E, Mohammad-Zadeh M, Pourmoghaddam M. Evaluation of oxidative stress, antioxidant status and serum Vitamin C levels in cancer patients. Biol Trace Elem Res. 2009;130:1–6. doi: 10.1007/s12011-008-8309-2. [DOI] [PubMed] [Google Scholar]
- 23.Karimi J, Goodarzi MT, Tavilani H, Khodadadi I, Amiri I. Increased receptor for advanced glycation end products in spermatozoa of diabetic men and its association with sperm nuclear DNA fragmentation. Andrologia. 2012;44 Suppl(1):280–6. doi: 10.1111/j.1439-0272.2011.01178.x. [DOI] [PubMed] [Google Scholar]
- 24.Asadi MH, Zafari F, Sarveazad A, Abbasi M, Safa M, Koruji M, et al. Saffron improves epididymal sperm parameters in rats exposed to cadmium. Nephrourol Mon. 2014;6:e12125. doi: 10.5812/numonthly.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aitken RJ, Curry BJ. Redox regulation of human sperm function: From the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid Redox Signal. 2011;14:367–81. doi: 10.1089/ars.2010.3186. [DOI] [PubMed] [Google Scholar]
- 26.Niknam Z, Mahmoudi R. The effect of Aloe vera extract on the sperm quality in male diabetic rats. Bull Environ Pharmacol Life Sci. 2014;3:223–8. [Google Scholar]
- 27.Khayatnouri M, Safavi SE, Safarmashaei S, Babazadeh D, Mikailpourardabili B. The effect of saffron orally administration on spermatogenesis index in rat. Adv Environ Biol. 2011;5:1514–21. [Google Scholar]
- 28.Guneli E, Tugyan K, Ozturk H, Gumustekin M, Cilaker S, Uysal N, et al. Effect of melatonin on testicular damage in streptozotocin-induced diabetes rats. Eur Surg Res. 2008;40:354–60. doi: 10.1159/000118032. [DOI] [PubMed] [Google Scholar]
- 29.Rajasekaran S, Sivagnanam K, Subramanian S. Antioxidant effect of Aloe vera gel extract in streptozotocin-induced diabetes in rats. Pharmacol Rep. 2005;57:90–6. [PubMed] [Google Scholar]
- 30.Khayatnouri M, Safavi SE, Safarmashaei S, Babazadeh D, Mikailpourardabili B. The effect of saffron orally administration on spermatogenesis index in rat. Adv Environ Biol. 2011;1:1514–22. [Google Scholar]


