Abstract
Aims:
The aim of this study is to impart radiopacity to platelet-rich fibrin (PRF) using two different bioactive agents (nano-hydroxyapatite [nHA] and dentin chips [DC]) and to assess the cell proliferation rate induced by the modified PRF.
Subjects and Materials:
PRF was modified with 50wt% of nHA (G bone-SHAG31, Surgiwear Company) and 50wt% of DC. The five samples of each group (Group 1 – dentin disc, Group 2 – PRF, Group 3 – PRF + 50wt% nHA, and Group 4-PRF + 50wt% DC) were digitally radiographed together with 8-mm aluminum stepwedge using DIGORA software (for Windows 2.9.113.490). The aluminum equivalent of radiopacity of the samples was compared with the dentin disc (control). Further cytotoxicity (on L929 mouse fibroblast cell lines) among the groups was assessed using methyl thiazolyl tetrazolium assay.
Statistical Analysis Used:
One-way ANOVA with posthoc Tukey-honestly significant difference tests were applied to assess the significance among the various groups.
Results:
The mean aluminum equivalent radiopacity among each group showed statistically significant results with P < 0.05. Group 3 (PRF + 50wt% nHA) can achieve an aluminum equivalent radiopacity (1.51 ± 0.089) better than Group 4 (0.97 ± 0.22 mmAl). The cell viability was >73% for all groups.
Conclusions:
This study found that the addition of bioactive radiopacifiers into PRF was able to impart radiopacity and these biomaterials were proved to be noncytotoxic.
Key words: Dentin chips, L929 mouse fibroblast cell lines, methyl thiazolyl tetrazolium assay, nano-hydroxyapatite, optical density, platelet-rich fibrin
INTRODUCTION
The biomaterials play a major role in regenerative dentistry, which are used to fill osseous defects and accelerate wound healing, and helps in regeneration of hard and soft tissue. In a quest to find a single-biomaterial, which promotes fast healing and maximum tissue regeneration, studies have been conducted using platelet concentrates. The complexity of preparation and the risk of cross-infection due to the use of bovine thrombin in Platelet rich plasma (PRP) led to the development of newer generation of platelet concentrates, called Platelet rich fibrin.[1] PRF consists of a strictly autologous fibrin matrix rich in platelets, leukocyte, cytokines, and various growth factors in a tetramolecular structure which is formed through the natural and progressive polymerization.[2] This concept was described in France by Choukroun in 2001. It has found its role in various clinical applications such as pulp capping, apexogenesis, revascularization, and bone regeneration. Studies have shown that PRF can be used as a scaffolding material in an infected necrotic immature tooth for pulpal regeneration and tooth revitalization.[3] The combination of PRF membrane as a matrix and mineral trioxide aggregate in apexification procedures has been proven to be an effective alternative for creating artificial root-end barriers and to induce faster periapical healing in cases with large periapical lesions.[4]
When placing the biomaterial into the root canal, it has to be in contact with live tissue or blood at the apical interface to allow for cell migration into the scaffold.[5] Moreover, for any root-end filling, while reviewing an immediate postoperative radiograph, it is important to ensure that the material is: (1) within the root canal; (2) well packed without the defects; (3) discernible from dentin; (4) discernible from other filling material used and, (5) distinguishable from superimposed bone trabeculae.[6] However, clinical challenge-associated with using PRF in the endodontic procedure is that they lack radiopaque properties, which are not discernable on radiographs. Hence, precise placement of material over the site is compromised, especially during apexification procedure and in the cases of regenerative procedures for open apex teeth. To encounter this issue, we bring forth with a revitalizing concept of adding radiopacifier and to make it a traceable material.
This article aims to compare the aluminum equivalent of radiopacity and cell proliferation rate obtained for the PRF after the incorporation of bioactive radiopacifier materials-nHA and DC.
SUBJECTS AND METHODS
The study was approved by the authorities of the Institutional Ethical Committee (R.C.NO: 0420/DE/2016) and followed the guidelines of National Research Committee (Indian Council of Medical Research).
Human blood collection and preparation of platelet-rich fibrin
Human whole-blood samples from healthy volunteers were collected in 10 ml glass test tubes without anticoagulant and immediately centrifuged at 3000 rpm for 10 min at room temperature (23°C) [Figure 1a]. After the centrifugation process, the PRF clot formed in between the topmost serum layer (platelet-poor plasma) and bottom-most red blood cell layer was carefully isolated.[7]
Figure 1.
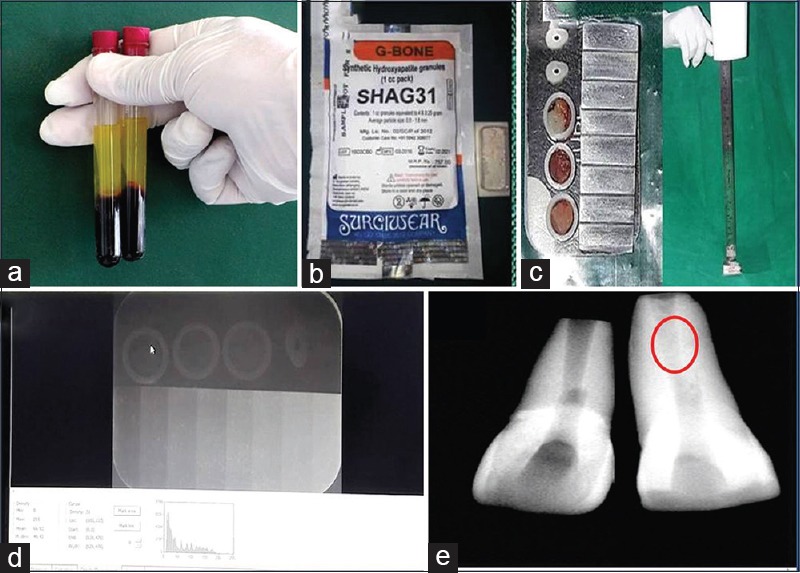
(a) Platelet-rich fibrin, (b) nano-hydroxyapatite (G Bone, SHAG31 - Surgiwear company) (c) samples radiographed along with 8-mm aluminum step wedge, (d) radiopacity measured using DIGORA for windows 2.9.113.490, (e) Preoperative radiograph of 11 (left side) with an open apex. Postoperative (right-side labeled with red circle) radiograph shows increased amount of radiopacity obtained within root canal when platelet-rich fibrin + 50wt% nanohydroxyapatite used as apexification material
Preparation of platelet-rich fibrin extract
The PRF was then frozen at 80°C for 1 h to separate the trapped growth factors and cytokines from the fibrin mesh. The separated liquid (extract) was harvested without the fibrin fibers using centrifugation at 1200 rpm for 10 min and constituted the PRF extract.[8]
Nanohydroxyapatite
Commercially available synthetic granular nano- hydroxyapatite (G Bone, SHAG31-Surgiwear company) is ground to powder and stored in Eppendorf tubes [Figure 1b].
Dentin chips
Freshly extracted third molars were collected from individuals aged 20 to 30 years and kept in 0.5% sodium hypochlorite for few minutes to remove soft tissue. Further stored in phosphate buffer saline containing thymol for a week at room temperature. The teeth included were intact, noncarious and those extracted for prophylactic reasons. Before further preparation, the teeth were rinsed and autoclaved (121°C, 15 min).[9,10] DC were prepared from the tooth using an autoclaved diamond coated disc in a low-speed handpiece without water spray and collected on an autoclaved glass slide and finally stored in an Eppendorf tubes.
Preparation of dentin disc
The dentin discs were obtained by cutting the roots of noncarious freshly extracted human maxillary canine teeth in 1-mm thick sections with a water-cooled carborundum disk.[11]
Incorporation of bioactive agents into platelet-rich fibrin
The PRF obtained was weighed using a digital weighing machine. Into the weighed PRF, 50wt% of nHA and 50wt% of DC were mixed separately. The sample is standardized using Teflon rings.
Group 1 – Control- Dentin discs (n = 3)
Group 2 – PRF (n = 5)
Group 3 – PRF modified with 50wt% nHA (nHA) (n = 5)
Group 4 – PRF modified with 50wt% DC (n = 5).
Procedure
The samples were digitally radiographed together with 8-mm aluminum stepwedge using Sordex DIGORA optime (KaVo Kerr, Mumbai, Maharashtra, India) for Windows 2.9.113.490 [Figure lc and d]. Five readings of density values were obtained from each sample, and the arithmetic mean of these repetitions was calculated. The dental X-ray unit was set at 50 Kvp, 10 mA, and exposure time of 0.3 s, with a focus-film distance of 30 cm. The density of stepwedge steps was measured, and a graph of radiographic density versus the thickness of aluminum was constructed. The aluminum equivalency values for the mean radiographic density readings of each sample were calculated using the conversion formula.[11]
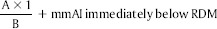
where,
A = radiographic density of the material (RDM) – radiographic density of the aluminum stepwedge increment immediately below RDM.
B = radiographic density of the aluminum stepwedge increment immediately above RDM – the radiographic density of the aluminum stepwedge increment immediately below RDM, 1 = 1 mm increment of the aluminum stepwedge.
The difference in radiopacity was appreciated when kept in an open apex tooth invitro, which mimicked the clinical situation [Figure 1e].
Assessment of proliferative activity by methyl thiazolyl tetrazolium assay
The cells (L929 mouse fibroblast cell lines) were seeded in 96-well plates at the density of 5 × 103 cells/well and were incubated in a CO2 incubator at 37°C and 5% CO2 under controlled humidified atmosphere overnight to allow them to attach to the plate.[8] For adherent cells, carefully aspirate the media. For suspension cells, spin the 96 well plate at 1000 rpm, 4°C for 5 min in a microplate-compatible centrifuge and carefully aspirate the media. Then, the cells were exposed to different concentrations (1 ng/ml, 10 ng/ml, 100 ng/ml, 1 μg/ml, 10 μg/ml, 100 μg/ml) of Group B (PRF), Group C (PRF + nHA), and Group D (PRF + DC) for 48 h. The dimethylsulfoxide (Group A) was kept as control. At the end of 48 h, 200 μl of serum-free media and 10 μl of Methyl thiazolyl tetrazolium (MTT) solution were added into each well and further incubated for 37°C for 3 h. At the end of the incubation period, the contents of the plates were discarded by simple decantation and the plates were dried overnight at room temperature. The purple-colored formazan crystals formed were dissolved in 150 μl of MTT solvent by shaking at 400 rpm for 15 min at room temperature in a thermoshaker. Occasionally, pipetting of the liquid may be required to fully dissolve the MTT formazan. The intensity of the color developed was absorbed at 570 nm in a multimode microplate reader. The amount of absorbance is proportional to cell number. The percentage growth/viability of cells was calculated using the following formula:

RESULTS
The values were recorded for each group and values were analyzed statistically by one-way analysis of variance (for intragroup analysis) followed by multiple comparison Tukey HSD test (for intergroup analysis). All statistical analysis were performed using IBM SPSS Statistics for Windows, Version 23.0. (Armonk, NY: IBM Corp). The confidence interval is taken as 95% for the analysis (P < 0.05).
The Group 3 (PRF + 50wt% nHA) was able to achieve significant amount of aluminum equivalent of radiopacity (1.51 ± 0.089 mmAl) than Group 4 – PRF + 50wt% DC (0.97 ± 0.22 mmAl) [Table 1]. Furthermore, it is observed that aluminum equivalent of radiopacity for Group 3 (PRF + 50wt% nHA) was almost half the density of Group 1 – Dentin disc (2.66 ± 0.02 mmAl) [Graph 1]. The MTT assay for cytotoxicity showed that samples, Group B (PRF), Group C (PRF + nHA), and Group D (PRF + DC) maintained the cell viability >73% [Figure 2a–d] and Graph 2].
Table 1.
Represents mean±standard deviation of samples of each group and corresponding aluminium equivalent of radiopacity obtained
| Sample | Radiopacity value (mean±SD) | Aluminium equivalent (mm eq Al) | F | P |
|---|---|---|---|---|
| Group 1 (dentin disc) | 51.71±0.48 | 2.643 | 3927.819 | <0.001** |
| Group 2 (PRF) | 7.75±0.59 | 0.2652 | ||
| Group 3 (PRF+nHA) | 41.89±1.39 | 1.516 | ||
| Group 4 (PRF+DC) | 32.60±1.05 | 0.968 |
**Highly significant (P<0.001). PRF: Platelet-rich fibrin, nHA: Nanohydroxyapatite, DC: Dentin chips, SD: Standard deviation
Graph 1.
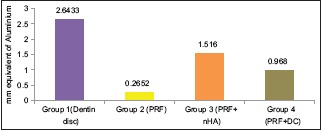
Represents mean aluminum equivalent of radiopacity of each group
Figure 2.
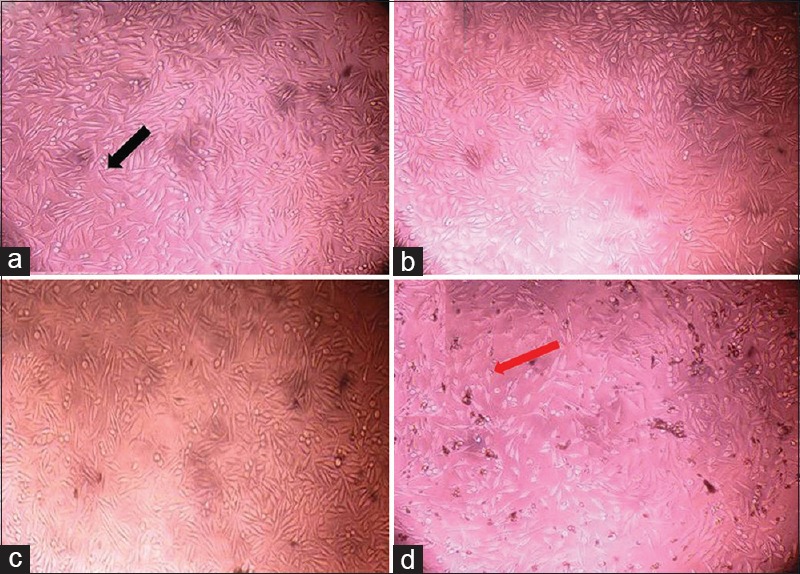
(a) Group A – control, (b) Group B – platelet-rich fibrin, (c) Group C – platelet-rich fibrin + nanohydroxyapatite, (d) Group D – platelet-rich fibrin + dentin chips. Black arrow indicates the cells with normal fibroblastic/spindle-shaped morphology and red arrow indicates the cells with lost morphology, indicates the cytotoxicity
Graph 2.
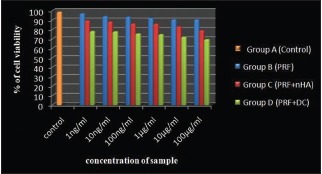
Represents comparison of percentage of cell viability within each concentration of sample between groups
DISCUSSION
Over the past decade, autogenous biomaterial like PRF, have gained the popularity for its inherent property in promoting regeneration of immature teeth. The potential theory behind the success of the use of PRF for regeneration of open apex could be attributed to a study conducted by Huang et al., who concluded that the PRF causes proliferation of human dental pulp cells and increases the protein expression of these dental pulp cells to differentiate into odontoblasts like cells.[12]
PRF appears radiolucent in radiographs hence precise placement toward the apical area of the root is compromised. There are various commercially available radiopacifiers such as bismuth oxide, iodoform, zirconium oxide, and barium sulfate used in dental restorative material, root-end filling material, and root canal sealers to impart radiopacity. However these radiopacifiers are being metals, it remained as a question to use them along with a bio-scaffold (PRF), which might have a possible interference with its biocompatibility and physical properties. Coomaraswamy et al. demonstrated that the addition of bismuth oxide to Portland cement lead to higher rates of solubility and degradation of the material.[13] Iodoform and barium sulfates, when used as radiopacifier, resulted in severe foreign-body reactions.[6] In addition, these radiopacifiers reported to have slow resorption rates which lead to residual radiopacity.[14] Residual radiopacity conceal the radiographic interpretation of healing.
Studies have showed that PRF in conjunction with bone grafts promoted wound healing, bone growth and maturation, graft stabilization, hemostasis and also improved the handling properties of graft materials.[15] Nano-hydroxyapatite which is an osteoconductive allograft material has been proven their role in proliferation and differentiation of mesenchymal cells into osteoblast cells. Elgendy et al.[16] compared clinical and radiographic outcomes of nanocrystalline hydroxyapatite with or without PRF membranes in the treatment of periodontal intrabony defects and concluded nHA is a suitable bone substitute in the periodontal treatment of intrabony defect. These when incorporated into a scaffold material, help in forming a favorable hard-tissue scaffolds with the proper mechanical strength, hydrophilicity, and excellent osteoconductivity.[16]
DC, on the other hand, have been applied in some in vitro and in vivo studies as an apical filling material in the root canal or graft material for filling the osseous defect.[9] This material is not commercially available, and for its preparation, human teeth were autoclaved and crushed into chips. The presence of dentin promotes the formation of a calcified tissue similar to ossein and accelerates healing while inhibiting inflammatory reaction.[17]
These propitious qualities of nano-hydroxyapatite and DC have made the material of choice in the present study to enhance the radiopacity of PRF.
Clinically, the combination of mineralized, rigid graft material, with a semi-fluid, nonrigid agent kept as multiple small pieces inside the canal using sterile scissors and is gently compacted using hand pluggers to form an apical barrier at the level of the apex.
There are various methods to determine the radiopacity of dental material and bone graft material. Tagger and Katz developed a method for analysis of the radiopacity of endodontic sealers using standardized samples radiographed next to an aluminum stepwedge.[18] The radiographs are digitized, and the specimens radiopacity is compared to that of the aluminum stepwedge using computer software. The comparative evaluation of digitized radiographic images using image-analysis software has been shown to determine the radiopacity of the materials in a simple and easily reproducible manner with reliable outcomes.
In this study, we used digital image analysis (Sordex DIGORA optime (KaVo Kerr, Mumbai, Maharashtra, India) for Windows 2.9.113.490.)which makes a direct capture of the radiographic image using a sensor that is sensitized by the X-rays, permitting a direct reading of image density with no need of previous digitization of the radiographs. The gray value of any spot indicated by the pointing device is immediately shown in pixels on the screen. The pixels already have their determined gray shades, directly providing the values at a scale 0–255, through the program.[19]
Image contrast and sharpness of the material radiographed can be varied by the type and amount of radiopacifier used.[20,21] On literature search, it was found that 1–40 wt% of radiopacifiers used in root-end filling material,[13,11] bone graft material,[20] and in root canal sealers.[18] In this study when we tried mixing the same amount of radiopacifier with PRF, failed to show a significant radiopacity. This might be due to the low density of the radiopacifiers used (nano-hydroxyapatite and DC) compared to metal radiopacifiers.[22]
The Organization for Standards, International Organization for Standardization (ISO) 6876:2001 establishes 3-mm Al as the minimum radiopacity for root canal sealers.[19] According to the American National Standards Institute/American Dental Association specification No. 571, radiopacity of root canal sealers should be at least 2 mm Al, more radiopaque than bone or dentin.[23] In this study aluminum equivalent of density was found to be higher in Group 3 (1.51 ± 0.089 mmAl) than Group 4 (0.97 ± 0.22) which was statistically significant (P = 0.001).
We were able to achieve an appreciable amount of radiopacity in Group 3 (PRF + 50wt% nHA) compared to Group 4 (PRF + 50wt% DC). However, it is important to confirm that crucial amount of radiopacifiers used above is not compromising the proliferative rate and viability of resident cells. Further MTT assay was conducted on L929 continuous cell lines to assess the cytotoxicity of material.
MTT Assay is simple, accurate, and gives reproducible results. The key component is 3-45-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide or MTT. This product is yellowish in solution. Mitochondrial dehydrogenases of viable cells cleave the tetrazolium ring, leading to the formation of purple crystals, which are insoluble in aqueous solutions. The crystals are redissolved in acidified isopropanol, and the resulting purple solution is measured spectrophotometrically.[24] It is indirect cytotoxicity, conducted with the adaptation of the ISO 10993-5 standard test method.[25] The material prepared into extracts and were used in culture media.
PRF was found to increase cell proliferation as a mitogen. In Group B, (PRF) 90% of cells were viable. Group C (PRF + nHA) and Group D (PRF + DC) has >73% viable cells. According to the ISO standard 10993-5, samples are considered cytotoxic when the cell viability reduction is larger than 30%.[25] The mechanism responsible for cell proliferation by PRF might be the due release of growth factors such as platelet-derived growth factor and transforming growth factor beta.[2] Furthermore, the cell lines maintained the original morphology in each dilution in four groups respectively.
CONCLUSIONS
The addition of bioactive radiopacifiers (nHA and DC) to PRF will provide a promising future for regenerative endodontic procedures by making it as traceable material, and this could help the clinician in precise placement of PRF. To prove the accessorized efficaciousness of this modified PRF on resident stem cells, further studies such as odontoblastic differentiation capability and mineralization potential are needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Agrawal M, Agrawal V. Platelet rich fibrin and its applications in dentistry – A review article. Natl J Med Dent Res. 2014;2:51–8. [Google Scholar]
- 2.Borie E, Oliví DG, Orsi IA, Garlet K, Weber B, Beltrán V, et al. Platelet-rich fibrin application in dentistry: A literature review. Int J Clin Exp Med. 2015;8:7922–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra N, Narang I, Mittal N. Platelet-rich fibrin-mediated revitalization of immature necrotic tooth. Contemp Clin Dent. 2013;4:412–5. doi: 10.4103/0976-237X.118379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khetarpal A, Chaudhry S, Talwar S, Verma M. Endodontic management of open apex using MTA and platelet – Rich fibrin membrane barrier: A newer matrix concept. J Clin Exp Dent. 2013;5:e291–4. doi: 10.4317/jced.51178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galler KM, Widbiller M. Perspectives for cell-homing approaches to engineer dental pulp. J Endod. 2017;43:S40–5. doi: 10.1016/j.joen.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Bortoluzzi EA, Guerreiro-Tanomaru JM, Tanomaru-Filho M, Duarte MA. Radiographic effect of different radiopacifiers on a potential retrograde filling material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:628–32. doi: 10.1016/j.tripleo.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 7.Huang FM, Yang SF, Zhao JH, Chang YC. Platelet-rich fibrin influences on proliferation and migration of human gingival fibroblasts. Int J Experiment Dent Sci. 2016;5:83–8. [Google Scholar]
- 8.Kim JH, Woo SM, Choi NK, Kim WJ, Kim SM, Jung JY, et al. Effect of platelet-rich fibrin on odontoblastic differentiation in human dental pulp cells exposed to lipopolysaccharide. J Endod. 2017;43:433–8. doi: 10.1016/j.joen.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Lymperi S, Taraslia V, Tsatsoulis IN, Samara A, Velentzas AD, Agrafioti A, et al. Dental stem cell migration on pulp ceiling cavities filled with MTA, dentin chips, or bio-oss. Biomed Res Int. 2015;2015:189872. doi: 10.1155/2015/189872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodis HE, Marshall GW, Jr, White JM. The effects of storage after extraction of the teeth on human dentine permeability in vitro. Arch Oral Biol. 1991;36:561–6. doi: 10.1016/0003-9969(91)90105-4. [DOI] [PubMed] [Google Scholar]
- 11.Húngaro Duarte MA, de Oliveira El Kadre GD, Vivan RR, Guerreiro Tanomaru JM, Tanomaru Filho M, de Moraes IG, et al. Radiopacity of portland cement associated with different radiopacifying agents. J Endod. 2009;35:737–40. doi: 10.1016/j.joen.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Huang FM, Yang SF, Zhao JH, Chang YC. Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. J Endod. 2010;36:1628–32. doi: 10.1016/j.joen.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Coomaraswamy KS, Lumley PJ, Hofmann MP. Effect of bismuth oxide radioopacifier content on the material properties of an endodontic portland cement-based (MTA-like) system. J Endod. 2007;33:295–8. doi: 10.1016/j.joen.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Orucoglu H, Cobankara FK. Effect of unintentionally extruded calcium hydroxide paste including barium sulfate as a radiopaquing agent in treatment of teeth with periapical lesions: Report of a case. J Endod. 2008;34:888–91. doi: 10.1016/j.joen.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Naik B, Karunakar P, Jayadev M, Marshal VR. Role of platelet rich fibrin in wound healing: A critical review. J Conserv Dent. 2013;16:284–93. doi: 10.4103/0972-0707.114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elgendy EA, Abo Shady TE. Clinical and radiographic evaluation of nanocrystalline hydroxyapatite with or without platelet-rich fibrin membrane in the treatment of periodontal intrabony defects. J Indian Soc Periodontol. 2015;19:61–5. doi: 10.4103/0972-124X.148639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saeed KW, Gataa IS, Garib BT. Fine calcified human dentin particles grafts in experimental bone defects in rabbit femur accelerate bone healing and maturation. Int J Dent Sci Res. 2015;2:8–13. [Google Scholar]
- 18.Tagger M, Katz A. Radiopacity of endodontic sealers: Development of a new method for direct measurement. J Endod. 2003;29:751–5. doi: 10.1097/00004770-200311000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Salzedas LM, Louzada MJ, de Oliveira Filho AB. Radiopacity of restorative materials using digital images. J Appl Oral Sci. 2006;14:147–52. doi: 10.1590/S1678-77572006000200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pekkan G, Aktas A, Pekkan K. Comparative radiopacity of bone graft materials. J Craniomaxillofac Surg. 2012;40:e1–4. doi: 10.1016/j.jcms.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Mendes MS, Resende LD, Pinto CA, Raldi DP, Cardoso FG, Habitante SM, et al. Radiopacity of mineral trioxide aggregate with and without inclusion of silver nanoparticles. J Contemp Dent Pract. 2017;18:448–51. doi: 10.5005/jp-journals-10024-2063. [DOI] [PubMed] [Google Scholar]
- 22.Yousefi AM, Oudadesse H, Akbarzadeh R, Wers E, Lucas-Girot A. Physical and biological characteristics of nanohydroxyapatite and bioactive glasses used for bone tissue engineering. Nanotechnol Rev. 2014;3:527–52. [Google Scholar]
- 23.Tanomaru-Filho M, da Silva GF, Duarte MA, Gonçalves M, Tanomaru JM. Radiopacity evaluation of root-end filling materials by digitization of images. J Appl Oral Sci. 2008;16:376–9. doi: 10.1590/S1678-77572008000600004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trubiani O, Caputi S, Di Iorio D, D'Amario M, Paludi M, Giancola R, et al. The cytotoxic effects of resin-based sealers on dental pulp stem cells. Int Endod J. 2010;43:646–53. doi: 10.1111/j.1365-2591.2010.01720.x. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Zhou J, Xu Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed Rep. 2015;3:617–20. doi: 10.3892/br.2015.481. [DOI] [PMC free article] [PubMed] [Google Scholar]


