Abstract
Aim:
The aim of the present review article is to establish a systematic review to describe the role of various salivary components such as pH, buffering capacity, proteins, electrolyte, antioxidant, enzymes, and minerals in occurrence and initiation of dental caries in participants with and without dental caries.
Methodology:
An electronic search was conducted in the PubMed, Scopus, Web of Science, and Google Scholar databases. The MESH terms (Medical Subject Headings) were “dental caries susceptibility,” “caries risk assessment, ”Salivary Biomarker,” “saliva,” “Proteins,” “electrolytes,” “biomarker,” “Anti-oxidant’s.” The database search was limited to the studies published between 2008 and 2018 and only human studies were included. Furthermore, the STROBE criteria checklist was used to analyze the studies which had to be included in the systematic review.
Results:
A total of 150 articles were retrieved through electronic database. After evaluating the title, abstract, and full text of these articles, only 11 were selected for the present systematic review. However, two articles were excluded because they were classified as high risk of bias according to the STROBE criteria. Out of 9 studies selected, 5 were classified as low-risk bias and 4 were classified as moderate-risk bias.
Conclusion:
Based on the results of the systematic review, out of 11 studies, 7 found to have a statistically significant difference between individuals with and without caries experience. Hence, it can be concluded that there is an association between various components of saliva and dental caries.
Key words: Biomarkers, dental caries, saliva, STROBE criteria
INTRODUCTION
Dental caries is a multifactorial microbial disease of the tooth and is one of the most common health problems. Saliva surrounds the hard and soft tissues of the oral cavity composed of organic and inorganic components. It contains various factor's required for host protection hence can be an important biomarker for diagnosis of dental caries. Clinicians can diagnose, prevent, and know the prognosis of the infectious process of dental caries using salivary kits which would facilitate evidence-based treatment decisions. Biomarker's are capable of providing information's as a body fluid about the physiologic state of the body. Reliable and reproducible biomarker can be called as a molecular signature and hence can be used in risk assessment, diagnosis, prognosis, and monitoring of disease.[1] Stimulated salivary flow rate is 7 ml/min and unstimulated salivary flow rate is 3 ml/min.[2] Dental caries shows conflicting results, thus evidence-based dentistry recommends a systematic review based on which association between various components of saliva can be standardized.[2] Hence, we can evaluate the physiology of saliva, antioxidant levels, protein, electrolyte alterations, and its correlation with dental caries.
Physiology of saliva depends on the salivary flow and viscosity. The parasympathetic and sympathetic nerve supply affects the neurohormonal signaling for salivary secretion. The salivary fluid is responsible for oral microbiome and film formation also the maintenance of oral environment.[3] Interaction between oral microbiota and saliva takes place in different mechanisms, that is, by clearance of microorganisms by binding to them, oral pellicles serves as a binding site for microbial adhesion, and enhancing microbial killing also by serving as microbial nutritional substrates.[4]
Secretion various ions in the salivary fluid maintain the viscoelastic nature of saliva along with the salivary proteins. Thus, it helps in aiding digestion, increases antimicrobial action, and facilitates taste and lubrication.[5] The salivary proteins, namely the proline-rich proteins, mucins, histatins, cystatins, and statherins, provide protection to the tooth surface also attract calcium ions and promote remineralization. Demineralization is retarded by the pellicle formed also decreases microbial adherence, thereby protecting the tooth surface from pH variation. Buffering action also is affected, thus neutralization of acids takes place.[6]
Thus, systematic review explains few studies that describe the role of these components of saliva and its role in occurrence and initiation of dental caries.
METHODOLOGY
Data
Observational studies which evaluated the relationship between various salivary components and dental caries.
Source
An electronic search was conducted in the PubMed, Scopus, Web of Science, and Google Scholar databases. The MESH terms (Medical Subject Headings) was “dental caries susceptibility,” “caries risk assessment,” “Salivary biomarkers,” “saliva,” “Proteins,” “electrolytes,” “biomarker,” “Anti-oxidant's.” The database search was limited to the studies published between 2008 and 2018 and only human studies were included.
Study selection
Inclusion criteria
The review article must be written in English language according to the PECOS – Population, Exposure, Comparator, Outcome, and Study design.[7]
Population (P): Healthy Individuals Who Are Not Under Any Medication Which Affects The Salivary Flow And Composition
Exposure (E): Salivary components as biomarker's in dental caries
Comparator (C): Participants with or without caries experience or individuals with high or low caries
Outcome (O): Dental caries in permanent dentition
Study Design (S): Clinical trials, case–control studies, in vitro studies, cross-sectional studies, or cohort studies published in scientific journals.
Exclusion criteria
Case Report's, Review Articles, Book Chapter's, Thesis, And Guidelines Were Not Taken Into Consideration.
Furthermore, according to the STROBE criteria, checklist was used to analyze the studies which had to be included in the systematic review. Twelve criteria were selected and studies which presented only 8 out of 12 criteria were selected as low-risk bias, 4–7 were considered as moderate-risk bias, and which had only 3 were selected as high-risk bias.
RESULTS
A total of 150 articles were retrieved through electronic database. After evaluating the title, abstract, and full text of these articles, only 11 were selected for the present systematic review [Figure 1]. However, two articles were excluded because they were classified as high risk of bias accoding to the STROBE criteria. Out of 9 studies selected, 5 were classified as low-risk bias and 4 were classified as moderate-risk bias [Table 1].
Figure 1.
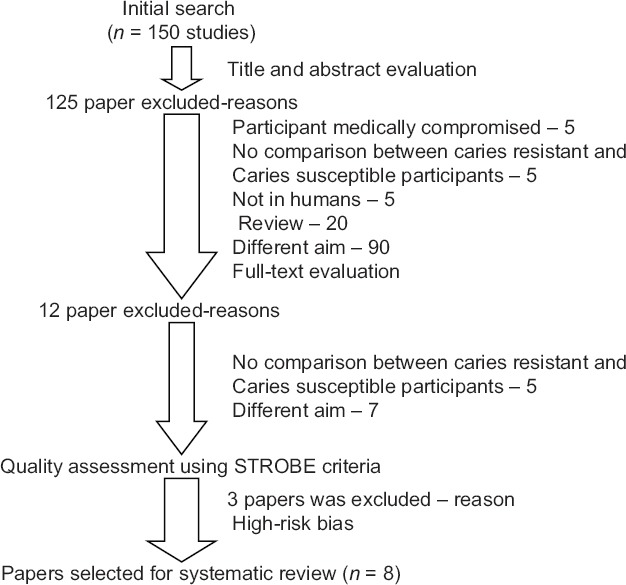
Stages of the study selection process
Table 1.
Quality assessment of the studies using STROBE criteria X-presence of criteria
| Criteria | Author, year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nascimento et al., 2009 | Kaur et al., 2012;; | Mithra et al., 2013 | Mithra et al., 2013 | Joana et al., 2013 | Pavitra et al., 2013 | Mithra et al., 2014 | Halina et al., 2014 | Teng-Yu et al., 2015 | Nireeksha et al., 2017 | Monica et al., 2018 | |
| Inclusion criteria | X | X | X | X | X | X | X | ||||
| Exclusion criteria | X | X | X | X | X | X | |||||
| No exposition of fluoride during tooth development | X | X | X | X | X | X | X | X | |||
| Dental caries diagnosis criteria | X | X | X | X | X | ||||||
| Radiographic exam | X | X | X | X | X | X | X | X | |||
| Experienced examiner calibrated examiner | X | X | X | X | X | X | X | ||||
| Salivary collection description | X | X | X | X | X | X | X | X | |||
| Salivary analysis description | X | X | X | X | X | X | X | X | X | ||
| Statistical analysis description | X | X | X | X | X | ||||||
| Paired groups | X | X | X | X | X | X | |||||
| Blinded study | X | X | X | X | X | X | |||||
| Risk of bias | Low | Moderate | Moderate | Moderate | High | High | Low | Low | Low | High | Low |
One of the selected study divided the sample into two groups 8 Individuals With DMFT 3 Were Considered As Control Group And 27 Individuals With DMFT >11 Were Case Control Group. However, for our systematic review, the groups with caries experience were selected (Halina et al.).
Joana et al. conducted the study to assess the dental caries experience and the other factors taken into consideration were sex, age, race, and ethnicity. However, the present systematic review concentrates on the influence of pH and buffering capacity on dental caries.
Based on the results of the study, 7 of them found to have a statistically significant difference between the participants and only one study by Joana et al. did not have any correlation between the pH, flow, buffering capacity, and dental caries [Table 2].
Table 2.
Description of individual study
| Authors, year, country | Sample size | Caries index | Type of saliva | Salivary parameters | Data analysis | Results |
|---|---|---|---|---|---|---|
| Nascimento et al., 2009, USA | 45 | DMFT | Whole unstimulated saliva | Arginine and urea | ANOVA | This study supports the theory that increased caries risk is associated with reduced alkali-generating capacity of the bacteria colonizing the oral cavity[8] |
| Kaur et al., 2012, India | 60 | DMFT | Stimulated and unstimulated | Flow rate, pH, buffering capacity, relative viscosity, calcium, phosphorus, and alkaline phosphatase levels | Chi-square statistical analysis | The results of the study showed that with an increase in salivary flow, pH, buffering capacity, there will be a decrease in caries incidence leading to good oral health and a reduced caries incidence. Increased salivary viscosity, salivary biochemical indicators such as calcium, phosphorus, and alkaline phosphatase also play their respective role in determining caries susceptibility of an individual[9] |
| Mithra et al., 2013, India | 100 | DMFT | Resting salivaStimulated saliva | Antioxidant level | ANOVA | There is a correlation between antioxidant level and dental caries as the antioxidant level increases the severity of dental caries also increases[10] |
| Mithra et al., 2013, India | 12,500 | DMFT | Unstimulated saliva | SOD activity, copper and zinc levels | Student t-test | SOD activity, as well as copper and zinc levels, increased in the caries-active group and the results were statistically significant[11] |
| Joana et al., 2013, Seattle | 1763 | Decayed, missing, or filled permanent teeth; visible cavitation; and visible interproximal enamel carious lesions | Stimulated and unstimulated | ConsistencyFlowpHBuffering capacity | Log-linear regression | The results of the study showed that there was no significant correlation between the salivary flow, pH and buffering capacity, and dental caries[2] |
| Pavitra et al., 2013, India | 39 | DMFT | Unstimulated | Total protein levels | Pearson’s correlation test | The total salivary proteins levels show a linear increase with the DMFT index[12] |
| Mithra et al., 2014, India | 80 | DMFT | Unstimulated | Salivary albumin levels | One-way ANOVAPearson’s correlation test | Increase in the levels of caries with decrease in the levels of albumin[13] |
| Halina et al., 2014 | 27 | DMFT | Unstimulated | MUC1MUC5B | Mann-Whitney nonparametric’ U test | The levels of MUC1 and MUC5B were increased in individuals with high dental caries[14] |
| Teng-Yu et al., 2015, China | 128 | DMFT | Unstimulated, whole saliva | PR3 | ANOVA | The protease PR3 is associated with the severity of dental caries, with low levels being associated with a greater severity of caries[15] |
| Nireeksha et al., 2017, India | 80 | DMFT | Unstimulated | PRPs | One-way ANOVA Post hoc Tukey’s test | Proline-rich protein bands were present in 95% of the caries-free group, whereas caries active group showed 65% proline-rich protein bands[16] |
| Monica et al., 2018 | 142 | Visual detection of dental caries based on the following scores: 0 - Absence of caries 1 - Change in enamel aspect 2 - Enamel breakdown but no dentin alteration 3 - Presence of underlying dark dentin 4 - Presence of a distinct cavity 5 - Presence of a large cavity |
Unstimulated | AlphaAmylase | Mann-Whitney test | The results showed that caries active children had higher levels of salivary enzyme which was statistically significant[17] |
SOD: Superoxide dismutase, MUC: Mucin, PR3: Proteinase 3, PRPs: Proline-rich proteins, DMFT: Decayed, Missing, and Filled Teeth
DISCUSSION
The study selected for the present systematic review was those that satisfied the STROBE criteria for quality assessment. Many studies were present which showed the correlation between biochemical markers and its association between dental caries, but only nine studies were selected for the analysis.
It has to be noted that various factors can influence the comparison between the selected studies, for example, the sample size ranged from 27 to 1763 between different studies. The age, gender, and the type of saliva also varied among various studies.
Dental caries is multifactorial disease of the teeth; hence, it is difficult to establish a single biochemical marker to predict the severity of the disease.[18]
The biochemical indicator taken into consideration in literature search was salivary proteins, antioxidant level, enzymes, and minerals.
The present article reviewed the various published literature to assess the biochemical components of saliva and their effect on dental caries. The results of the various studies found that if there is an increase in pH, buffering capacity, and flow, there is decreased caries incidence. Thick, sticky, and frothy saliva with an increased viscosity makes the tooth more susceptible to caries.[9]
Saliva acts as the nature's primary defense system for the oral cavity and is important for protecting the exposed tooth surfaces. Saliva has the capacity to reverse the demineralization of the exposed tooth surface by simple mechanical rinsing, antimicrobial activity, buffering capacity, calcium phosphate binding proteins, immune surveillance, and the secretion of antimicrobial peptides.(van Nieuw Amerongen et al., 2004).[19]
The most important factor in prevention of dental caries includes remineralization of the initial carious lesion which requires the presence of calcium, phosphate, and fluoride. The amount of calcium and phosphate in the saliva gets supersaturated with calcium and phosphate salts which have a protective influence on the dental hard tissues.
Salivary protein has been explained to have a double-edged role that plays a protective mechanism depending on their site, location, and action the microorganism colonizes and leads to the production of mucin which acts as a protective mechanism against caries development and protects the tooth from desiccation.[14]
C-reactive protein is a component of saliva which when increased is attributed to the immune response during caries progression and development of inflammation.
According to Nobbs et al. (2011), the proline-rich proteins act as a protective mechanism against dental caries. They attach to Streptococcus mutans through major adhesion antigen and this immunological reaction protects the tooth from dental caries.
Nireeksha et al (2017) explained that the proline-rich protein decreases the caries incidence by neutralizing the acid production by Streptococci.[16]
Salivary albumin acts as a marker for the severity of underlying disease and inflammation and has an inhibitory effect on dental caries by preventing the enamel demineralization by penetrating into the enamel pores (Hedge et al., (2014) and Monica M (2018).[13,17]
The most important microelements that are present include calcium, sodium, magnesium, zinc, and fluoride; these are of great importance for the mineralization and maturation of hard tooth tissue. Curzon has noted that zinc and calcium act as a antiplaque agent, whereas strontium and zinc enhance the remineralization in enamel. The increase in the copper content in the saliva can be attributed to the fact that during carious progression, there will be breakdown of the hydroxyapatite crystals of the enamel, thereby releasing copper ions from the tooth structure into (Green, 1970]. There was an increased caries incidence reported when rats were fed a zinc-deficient diet because zinc is implicated in biomineralization, where it stimulates bone growth and mineralization and influences osteoclast activity Sejdini M (2018).[20]
Superoxide dismutase (SOD) catalyzes the dismutation is a detoxifying antioxidant enzyme which acts against free radicals by catalyzing the dismutation of the superoxide ion into oxygen and hydrogen peroxide. Hence, when the SOD level increases in the saliva, there is an increase in caries activity in an individual.
The knowledge of composition of saliva is important for the function of their individual component and also for the growing interest in salivary-based diagnosis. The scope for future studies is the sensitive proteomics, genomic which have opened new avenues for the characterization of very small amounts of organic material. Besides, defined criteria are essential to avoid risk of bias due to the multifactorial etiology of dental caries.[11]
CONCLUSION
Based on the results of the systematic review out of 11 studies, 7 of them found to have a statistically significant difference between individuals with and without caries experience. Hence, it can be concluded that there is an association between various components of saliva and dental caries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hemadi AS, Huang R, Zhou Y, Zou J. Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int J Oral Sci. 2017;9:e1. doi: 10.1038/ijos.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunha-Cruz J, Scott J, Rothen M, Mancl L, Lawhorn T, Brossel K, et al. Salivary characteristics and dental caries: Evidence from general dental practices. J Am Dent Assoc. 2013;144:e31–40. doi: 10.14219/jada.archive.2013.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proctor GB. The physiology of salivary secretion. Periodontol 2000. 2016;70:11–25. doi: 10.1111/prd.12116. [DOI] [PubMed] [Google Scholar]
- 4.Scannapieco FA. Saliva-bacterium interactions in oral microbial ecology. Crit Rev Oral Biol Med. 1994;5:203–48. doi: 10.1177/10454411940050030201. [DOI] [PubMed] [Google Scholar]
- 5.Guy H. The secretion, components, and properties of saliva. Carpenter Annu Rev Food Sci Technol. 2013;4:267–76. doi: 10.1146/annurev-food-030212-182700. [DOI] [PubMed] [Google Scholar]
- 6.Van Nieuw Amerongen A, Bolscher JG, Veerman EC. Salivary proteins: Protective and diagnostic value in cariology? Caries Res. 2004;38:247–53. doi: 10.1159/000077762. [DOI] [PubMed] [Google Scholar]
- 7.Lips A, Antunes LS, Antunes LA, Pintor AV, Santos DA, Bachinski R, et al. Salivary protein polymorphisms and risk of dental caries: A systematic review. Braz Oral Res. 2017;31:e41. doi: 10.1590/1807-3107BOR-2017.vol31.0041. [DOI] [PubMed] [Google Scholar]
- 8.Nascimento MM, Gordan VV, Garvan CW, Browngardt CM, Burne RA. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol Immunol. 2009;24:89–95. doi: 10.1111/j.1399-302X.2008.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur A, Kwatra KS, Kamboj P. Evaluation of non-microbial salivary caries activity parameters and salivary biochemical indicators in predicting dental caries. J Indian Soc Pedod Prev Dent. 2012;30:212–7. doi: 10.4103/0970-4388.105013. [DOI] [PubMed] [Google Scholar]
- 10.Hegde MN, Hegde ND, Ashok A, Shetty S. Evaluation of total antioxidant capacity of saliva and serum in caries-free and caries-active adults: An in vivo study. Indian J Dent Res. 2013;24:164–7. doi: 10.4103/0970-9290.116670. [DOI] [PubMed] [Google Scholar]
- 11.Hegde MN, Hegde ND, Ashok A, Shetty S. Biochemical indicators of dental caries in saliva: An in vivo study. Caries Res. 2014;48:170–3. doi: 10.1159/000355580. [DOI] [PubMed] [Google Scholar]
- 12.Patankar R, Yadav M, Vibbhakar P. Salivary total protein levels and their correlation to dental caries. Int J Oral Maxillofac Pathol. 2013;4:13–6. [Google Scholar]
- 13.Hegde MN, Bhat R, Punja A. Correlation Between Dental Caries And Salivary Albumin In Adult Indian Population – An In Vivo Study. British Journal of Medicine & Medical Research. 2014;25:4238–44. [Google Scholar]
- 14.Gabryel-Porowska H, Gornowicz A, Bielawska A, Wójcicka A, Maciorkowska E, Grabowska SZ, et al. Mucin levels in saliva of adolescents with dental caries. Med Sci Monit. 2014;20:72–7. doi: 10.12659/MSM.889718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang TY, Zhou WJ, Du Y, Wu ST, Yuan WW, Yu Y, et al. Role of saliva proteinase 3 in dental caries. Int J Oral Sci. 2015;7:174–8. doi: 10.1038/ijos.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shetty N, Hegde MN, Kumari S, Ullal H, Kedilaya V. Salivary proteins as biomarkers in dental caries:In vivo study. Dent Oral Craniofac Res. 2017;3:1–7. [Google Scholar]
- 17.Monica M, Valad R, Stoica A. Analysis of salivary level of alpha-amylase as a risk factor for dental caries. Acta Medica Transilvanica March. 2018;23(1):93–5. [Google Scholar]
- 18.Laputkova G, Schwartzova V. Protein roles in oral health and as predictors of caries risk. Open Life Sci. 2018;13:174–200. doi: 10.1515/biol-2018-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Nieuw Amerongen A, Bolscher JGM, Veerman ECI. Salivary Proteins: Protective and Diagnostic Value In Cariology? Caries Res. 2004;38:247–53. doi: 10.1159/000077762. [DOI] [PubMed] [Google Scholar]
- 20.Sejdini M, Begzati A, Salihu S, Krasniqi S, Berisha N, Aliu N, et al. The role and impact of salivary Zn levels on dental caries. Int J Dent. 2018;2018:8137915. doi: 10.1155/2018/8137915. [DOI] [PMC free article] [PubMed] [Google Scholar]


