Abstract
Introduction:
The interaction between chlorhexidine (CHX) and sodium hypochlorite (NaOCl) yields a thick precipitate capable of occluding dentinal tubules. Previous studies are unclear as to the above-mentioned precipitate contains para-chloroaniline (PCA) or not. PCA is a known toxic and carcinogenic compound which may lead to methemoglobinemia in humans.
Aim:
This study aims to evaluate the precipitate formed on combination of different irrigants, weigh the amount of precipitate formed and to analyze the precipitate for PCA by using thin layer chromatography (TLC), high performance liquid chromatography (HPLC), column chromatography (CC), electron spray ionization mass spectrometry (ESI-MS), Ultraviolet (UV), and nuclear magnetic resonance (1H-NMR and C-13 NMR).
Materials and Methods:
Four different irrigants namely 2% CHX gluconate, 3% NaOCl, 5% neem and 5% tulsi were taken in different test tubes. Group 1, 2 and 3 included 1 ml 2% CHX combined with 1 ml each of 3% NaOCl, 5% neem and 5% tulsi. Group 4 and 5 comprised of 1 ml 3% NaOCl in combination with 1 ml 5% each of neem and tulsi. Finally, group 6 constituted 1 ml 5% neem mixed with 1 ml 5% tulsi. Each group was observed for 2 min for the formation of any precipitate, and the formed precipitate was weighed and analyzed using 1H-NMR and C-13 NMR, TLC, CC, HPLC, ESI-MS, and UV.
Statistical Analysis:
One-way ANOVA and Post hoc–Tukey test were used.
Results:
Presence of PCA was detected in group 1 (CHX + NaOCl), group 2 (CHX + neem) and group 3 (CHX + tusli) in all the sensitive methods employed.
Conclusion:
The presence of PCA in precipitate was confirmed by TLC, CC, HPLC, ESI-MS, and UV. Based on the results of the present study, we assume that components in CHX are responsible for precipitate formation which contains PCA as well. Extrusion of precipitate beyond the apex may cause periapical tissue damage and delay wound healing at the same time.
Key words: Chlorhexidine, electron spray ionization mass spectrometry, high-performance liquid chromatography, neem extract, nuclear magnetic resonance, sodium hypochlorite, thin layer chromatography, tulsi extract and ultraviolet
INTRODUCTION
No irrigating solution imparts all essential properties when used independently, making it mandatory to combine different irrigants to enhance antimicrobial efficacy and dissolution of organic and inorganic tissues.[1,2] Irrigants play a crucial role in debridement and disinfection of the root canal space.[3] Since microorganisms have been established as the sole entity responsible for initiating pulpal and periapical pathologies,[4] mechanical instrumentation alone may not be sufficient to remove bacteria and necrotic tissue from root canals owing to the complex anatomy.[5]
Sodium hypochlorite (NaOCl), the most commonly used antibacterial irrigant, in spite of its stupendous tissue dissolving properties, is said to be ineffective against specific bacteria at lower concentrations.[6] Chlorhexidine (CHX) on the other hand is a broad-spectrum antimicrobial agent, widely used in endodontics for root canal disinfection, is shown to be as effective as NaOCl in terms of antibacterial activity. However, CHX certainly does score over the latter especially against few NaOCl resistant strains owing to its substantivity. However, most importantly, CHX lacks the ability to dissolve organic matter which is regarded as a potential clinical disadvantage.[7]
Biomechanical instrumentation along with a combination of irrigants such as NaOCl and CHX has been suggested in the past to provide a two or three fold advantage, thereby contributing to complete disinfection of the root canal space.
However, it has been proved time and again that the use of CHX and NaOCl in combination is contraindicated within the root canal space as an instant reddish brown precipitate is produced through an acid-base reaction that envelopes the dentinal walls with a thickness ranging from 139 μm to 684 μm, capable of occluding dentinal tubules thereby preventing the penetration of intracanal medicament, thus compromising the seal of obturated root canals.[8] The amount of para-chloroaniline (PCA) found in the aforesaid precipitate is directly related to the concentration of NaOCl used.[9] Moreover, PCA is produced by the hydrolysis of CHX as a function of time, alkaline environment (high pH), and heat.[10,11]
The presence of the carcinogen found in precipitate has been a matter of debate over the past decade. While most published studies confirmed its presence,[12,13,14] a few have denied the same altogether.[15,16] Studies found that PCA, apart from being proven carcinogenic in animals, also led to methemoglobinemia in humans.[17,18] This is regardless of toxicity from PCA powder or the spontaneous breakdown of CHX. The International Agency for Research on Cancer (IARC, 2006) classified PCA as group 2B carcinogens.[19] Group 2B agents imply that there is limited evidence of carcinogenicity in humans and less than sufficient evidence of carcinogenicity in experimental animals.
Numerous reports concerning the toxic effects of chemically available irrigants prompted researchers to search for natural alternatives. Neem and tulsi are best known not only for their antibacterial property but also for their biocompatibility as well. An extensive search of the literature revealed the absence of any publication linking CHX and herbal extracts with the precipitate formation to PCA. Hence, the aim of this study was to evaluate the precipitate formed following interaction of CHX with sodium hypochlorite, neem, and tulsi, weigh the amount of precipitate formed and finally analyze each precipitate for PCA by employing nuclear magnetic resonance (NMR) (1H-NMR and C-13 NMR), electron spray ionization mass spectrometry (ESI-MS), Ultraviolet (UV), column chromatography (CC), thin layer chromatography (TLC), high performance liquid chromatography (HPLC).
Null hypothesis
No precipitate was formed in any group following interaction of irrigants. The precipitate did not contain PCA.
MATERIALS AND METHODS
Four different types of irrigants namely 2% CHX gluconate, 3% NaOCl, 5% neem and 5% tulsi were used in this study.[6]
Neem and tulsi extracts were supplied in the form of powder, readily soluble in distilled water. These extract dilutions were prepared in 5% concentration by mixing 5 g of powder in 100 ml of distilled water and kept in place for 24 h. The procured solutions were filtered using WhatManns filter paper No1. The four irrigants were then grouped into six groups of various combinations [Table 1]. Group 1, 2 and 3 included 1 ml 2% CHX combined with 1 ml 3% each of NaOCl (Positive control), 5% neem and 5% tulsi. Group 4 and 5 comprised of 1 ml 3% NaOCl in combination with 1 ml 5% each of neem and tulsi. Finally, group 6 constituted 1 ml 5% neem mixed with 1 ml 5% tulsi. Following which, all groups were observed for 2 min for precipitate formation [Figure 1]. The formed precipitate was filtered; air dried and weighed using weighing electronic balance. The data obtained were statistically analyzed using One-way ANOVA and Post hoc–Tukey test [Tables 2 and 3].
Table 1.
Groups
| Groups | Irrigants |
|---|---|
| Group 1 | 1 ml 2% CHX + 1 ml 3% NaOCl |
| Group 2 | 1 ml 2% CHX + 1 ml 5% neem |
| Group 3 | 1 ml 2% CHX + 1 ml 5% tulsi |
| Group 4 | 1 ml 3% NaOCl + 1 ml 5% neem |
| Group 5 | 1 ml 3% NaOCl + 1 ml 5% tulsi |
| Group 6 | 1 ml 5% Neem + 1 ml 5% tulsi |
CHX: Chlorhexidine, NaOCl: Sodium hypochlorite
Figure 1.

Precipitate formation
Table 2.
One way ANOVA
| Group | n | Mean (mg) | SD | SE |
|---|---|---|---|---|
| CHX + NaOCl | 10 | 3.9700 | 0.18886 | 0.05972 |
| CHX + Neem | 10 | 12.0000 | 0.29439 | 0.09309 |
| CHX + Tulsi | 10 | 29.9800 | 1.16981 | 0.36992 |
| Total | 30 | 15.3167 | 11.08124 | 2.02315 |
CHX: Chlorhexidine, NaOCl: Sodium hypochlorite, SD: Standard deviation, SE: Standard error
Table 3.
Post hoc tukey test
| Group | Group | Mean difference | SE | Significant |
|---|---|---|---|---|
| CHX + NaOCl | CHX + Neem | −8.03000* | 0.31525 | 0.000 |
| CHX + tulsi | −26.01000* | 0.31525 | 0.000 | |
| CHX + neem | CHX + NaOCl | 8.03000* | 0.31525 | 0.000 |
| CHX + tulsi | −17.98000* | 0.31525 | 0.000 | |
| CHX + tulsi | CHX + NaOCl | 26.01000* | 0.31525 | 0.000 |
| CHX + neem | 17.98000* | 0.31525 | 0.000 |
CHX: Chlorhexidine, NaOCl: Sodium hypochlorite, SE: Standard error
0.5 g each of dried solid mass of precipitate formed in groups 1, 2, and 3 were dissolved either in 25 ml of methanol or 25 mL of dimethyl sulfoxide (DMSO) and analyzed to detect presence of PCA by using NMR (1H-NMR and C-13 NMR), ESI-MS, UV, CC, TLC, HPLC. Commercially available 98% PCA (4-Chloroaniline; Sigma–Aldrich) in powder form was used in this study as the reference standard.
Nuclear magnetic resonance spectroscopy
The powdered form of the precipitate was dissolved in either methanol or DMSO and was subjected to 1H and 13C–NMR spectroscopy unit (Bruker Ultra Shield 500; Bruker, Billerica, MA).
Electron spray ionization mass spectrometry
Electron spray was carried out in ESI-MS equipment (Bruker Daltonics, Billerica, MA).
Ultraviolet
UV analysis was done using V-650 spectrophotometer (JASCO International CO., LTD, Japan).
High-performance liquid chromatography analysis
HPLC analysis was performed on a Thermo model Ultimate 3000 instrument equipped with a thermo variable wavelength detector to determine the peak purity and similarity test of PCA. HPLC grade solvents were pre-filtered using a Millipore system and analysis was performed on Agilent Eclipse plus C18 (4.6 mm × 150 mm, i.d. 5 μm) column. The mobile phase was acetonitrile: 1% phosphoric acid in water at a flow rate of 1.5 mL/min; the detection wavelength was 241 nm, close to the absorption maxima for both compounds. Column oven temperature was set to 30°C. The injection volume for standards and samples were 20 μL. For gradient elution: 0–10 min 100% water, 10 min 50% acetonitrile, 15 min 50% acetonitrile, 17 min 100% water, 20 min 100% water. Each analysis was repeated 3 times using 10 ppm solutions of sample and standard.
Thin layer chromatography analysis
A volume of 10 mg of precipitate was dissolved in 10 mL of ethyl acetate followed by 0.50 g of brown solid, dissolved in 25 mL of ethyl acetate which was then passed through the silica gel CC and elution with ethyl acetate: Hexane (1:1), evaporated to dryness using N2 evaporator and finally the residue was reconstituted with 2 mL of ethyl acetate prior to TLC analysis. Chromatography was performed using preactivated (110°C) silica gel 60 F254; TLC plates (either 20 cm × 20 cm) [Figure 2]. Automatic TLC applicator was used to transfer samples and standards onto the TLC plate. The application parameters were identical for all the analysis performed, and the delivery speed of the syringe was 10 μL. Each TLC plate was developed to a height of about 10 cm with a mobile phase of ethyl acetate: Hexane (1: 2, v/v) under laboratory conditions (25–35°C) and 40%–50% relative humidity. The developed plates were heated at 110°C for 25 min after drying to develop the color of the spots. For semi-quantitative determination, spots corresponding to 1 and 2 were scanned using a TLC Scanner at 254 nm (wavelength chosen to be appropriate for both 1–6). PCA was calculated by dividing the distance traveled by the compound and the distance traveled by the solvent.
Figure 2.

Thin layer chromatography
Statistical analysis
The data were systematically arranged using the Statistical Package for the Social Sciences version 23, IBM Corp, Armonk, New York, USA, for Windows. Descriptive measures included mean and standard deviation, minimum and maximum values. One-Way ANOVA was applied to compare mean differences between and within groups which formed precipitate. Post hoc-Tukey test was used to determine the highest amount of precipitate formed among the groups (P < 0.05).
RESULTS
Notably, the precipitate was not formed in groups 4, 5, and 6, i.e., the ones in which CHX was not incorporated. By contrast, groups 1, 2 and 3 in which CHX was present, a weighty reddish brown, dark green and green precipitate were elicited respectively with a statistically significant difference across the three groups (P < 0.05). More amount of precipitate was observed in group 3 (dark green precipitate) with a mean weight of 29.98 mg, followed by group 2 (green precipitate) with 12 mg and group 1 (reddish brown precipitate) with 3.97 mg.
The presence of PCA was detected in group 1 (CHX + NaOCl), group 2 (CHX + neem) and group 3 (CHX + tulsi) in all the sensitive methods employed.
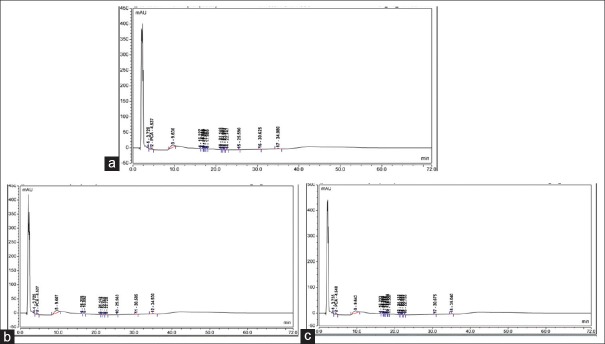
PCA was detected in CHX + NaOCl [Figure 3a], CHX + neem [Figure 3b] and CHX + tulsi [Figure 3c] as its retention time of 4.537, 4.527 and 4.548 min were found to be matching with standard PCA's retention time of 4.028 min. The retention time was measured using HPLC with UV detector. The results were analyzed semi quantitatively by TLC with CC and quantitatively by HPLC with UV detector [Table 4].
Figure 3.
(a) High performance liquid chromatography for chlorhexidine + sodium hypochlorite. (b) High performance liquid chromatography for chlorhexidine + neem. (c) High performance liquid chromatography for chlorhexidine + tulsi
Table 4.
Percentage dry weight of para-chloroaniline using thin layer chromatography and high performance liquid chromatography
| Sample name | Amount of compound determined (PCA) (percentage of dry weight) | |
|---|---|---|
| TLC (semi quantitative) | HPLC + UV | |
| CHX + NaOCl | 10 | 14.00 |
| Neem + CHX | 10 | 10.57 |
| Tulsi + CHX | 10 | 9.00 |
CHX: Chlorhexidine, NaOCl: Sodium hypochlorite, TLC: Thin layer chromatography, PCA: Para-chloroaniline, HPLC: High performance liquid chromatography, UV: Ultraviolet
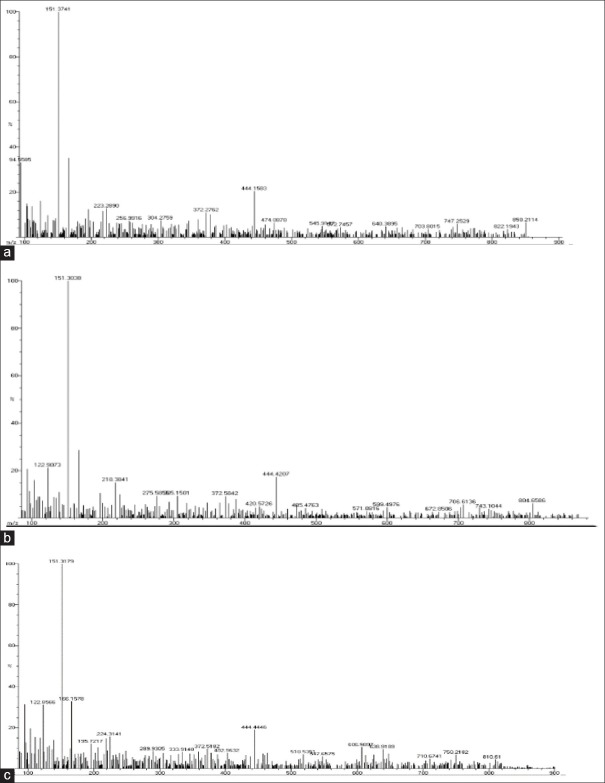
In ESI-MS, peak values for standard PCA were found to be 125,149 and 167 m/z which were also coinciding with peak values of the three precipitate samples: CHX + NaOCl (123.90) [Figure 4a], CHX + Neem (122.90) [Figure 4b], CHX + tulsi (166.15) [Figure 4c]. The minor difference was probably due to the addition of protons.
Figure 4.
(a) Electron spray ionization mass spectrometry for chlorhexidine + sodium hypochlorite. (b) Electron spray ionization mass spectrometry for chlorhexidine + neem. (c) Electron spray ionization mass spectrometry for chlorhexidine + tulsi
UV analysis showed that peak values of CHX + NaOCl (318) [Figure 5a], CHX + neem (263) [Figure 5b], CHX + tulsi (264) [Figure 5c] were coinciding with standard PCA peak values of 320 and 260.
Figure 5.
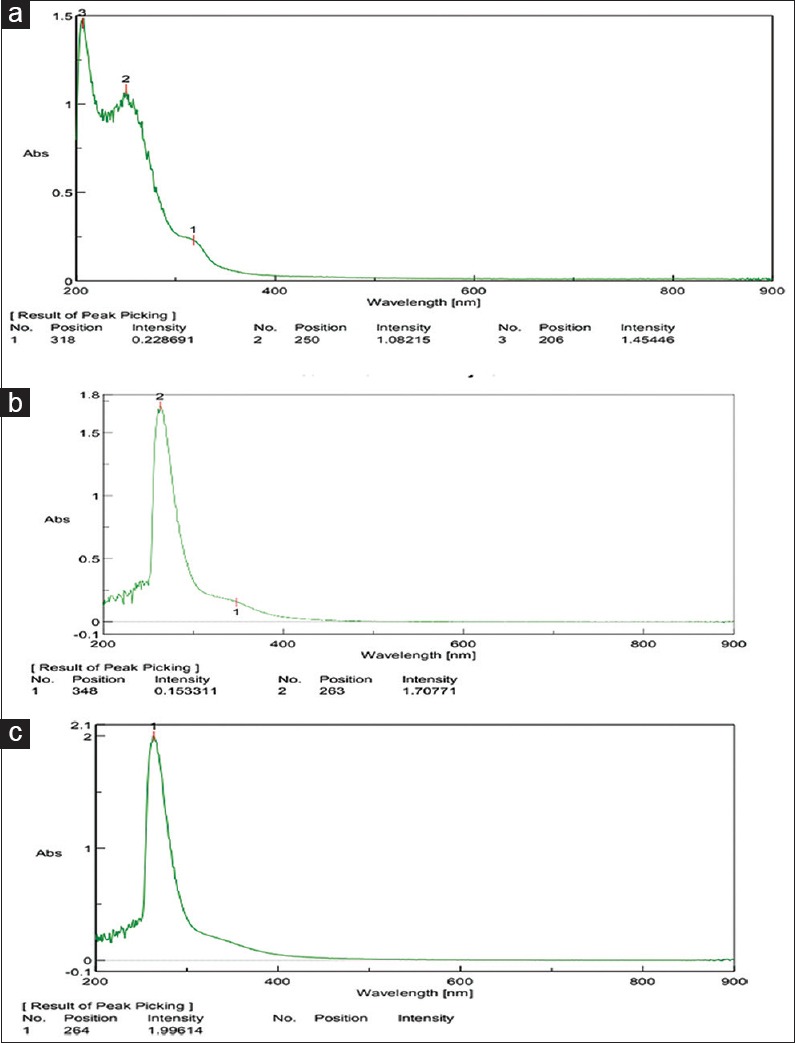
(a) Ultraviolet for chlorhexidine + sodium hypochlorite. (b) Ultraviolet for chlorhexidine + neem. (c) Ultraviolet for chlorhexidine + tulsi
Both 1H and 13C-NMR results showed that peak signals of standard PCA were different from the three precipitate samples. Standard PCA yielded two doublet signals at 7.00 and 6.55 ppm which were different from signals recorded between 7.30 and 7.55 ppm in CHX + NaOCl, CHX + Neem and CHX + tulsi [Figure 6].
Figure 6.
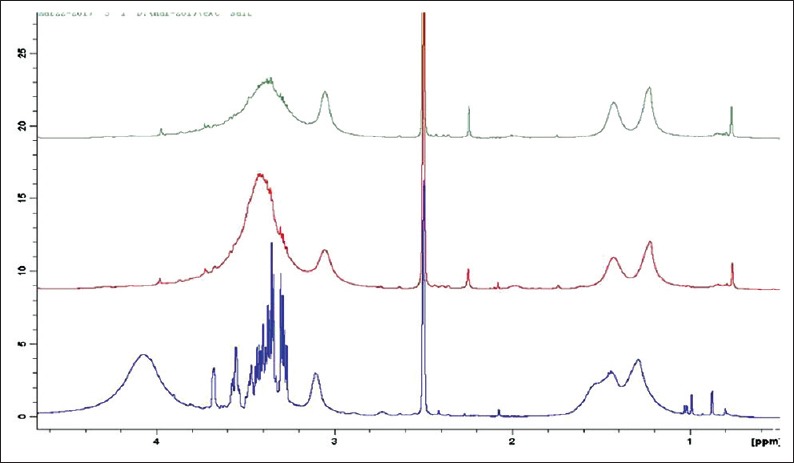
Consolidated nuclear magnetic resonance spectra
DISCUSSION
PCA is a compound used in the manufacture of dyes, pigments, agricultural chemicals, and pharmaceuticals according to the IARC and is also known to induce DNA damage in bacteria and gene mutation.
Although there is sufficient evidence to prove carcinogenicity of PCA in animals, however the same has not been validated in humans till date. A cytotoxicity study done on human fibroblasts using the MTT assay reported that PCA exerted a toxicity of 14.8 mm at an effective concentration of 50%.[20]
Furthermore, it was shown in a study done by Cintra et al. 2014 that the precipitate is more cytotoxic than NaOCl and CHX alone when implanted under the skin of an animal model. Nocca et al. 2017 demonstrated that PCA and its by-products formed on a combination of CHX with NaOCl can delay wound healing and cause potential damage to periapical tissues.
In the present study, the combination of NaOCl and CHX formed a reddish-brown precipitate. The brownish flocculates formed due to the presence of calcium, iron, and magnesium are easily oxidized, which was confirmed in another study by analyzing the metals in different concentrations by atomic absorption spectrophotometer.[21] CHX is a dicationic acid (pH 5.5–6.0) that has the ability to donate protons whereas NaOCl is alkaline being able to accept protons from the dicationic CHX. This exchange between protons leads to the formation of a precipitate which is an insoluble and neutral substance.
Neem (Azadirachta indica) shows both antioxidant and antimicrobial properties and can be used as an alternative to NaOCl.[22] It is also known to possess antifungal, antiviral and anti-carcinogenic activity. The main active component of neem is nimbidin, and other isolated components such as nimbin, nimbinin, nimbidinin, nimbolide, and nimbidic acid are also responsible for its biological effect. The antimicrobial activity against Streptococcus mutans, Enterococcus faecalis, and Candida albicans is well documented. Phytochemical screening of neem revealed that it contains alkaloids, steroids, flavonoids, tannins, saponins, and amino acid. The greenish precipitate formed on a combination of neem and CHX may be due to the interaction of steroids with the latter.[23]
Tulsi (Ocimum sanctum) has been used in ayurvedic medicine since time immemorial. It possesses antibacterial, antioxidant, analgesic, antipyretic, anti-ulcer, antidiabetic and anticancer activities. Tulsi is composed of 71% eugenol and 20% methyl eugenol where eugenol is the key phytochemical constituent responsible for antimicrobial activity. A rise in antibacterial activity against E. faecalis was elicited with increase in the concentration of tulsi.[24] Phytochemical screening of tulsi revealed that it contains alkaloids, steroids, and tannins. The combination of tulsi and CHX yielded a dark greenish precipitate possibly due to the interaction of steroids with components of CHX.[25]
In a pilot study (unpublished), done before the commencement of the present study, it was observed that no precipitate was formed following the interaction of CHX with garlic or Aloe vera. On the other hand, neem and tulsi yielded a green and dark greenish precipitate. Furthermore, according to the literature, steroids were absent in both garlic and Aloe vera, a possible reason for no precipitate being formed when combined with CHX.
TLC is an easy, inexpensive and visual method used for determination of purity and identification of components in a given mixture. This method is based on a stationary phase usually silica gel and a mobile phase. In this study, TLC was used to detect the presence of PCA where RF values have confirmed the same in the precipitate.
HPLC coupled with UV is an analytical technique used to separate, identify and quantify each component in a mixture. The advantage of this study lies in the fact that non-destructive methods such as TLC and HPLC were used instead of MS. In MS, the sample being studied is ionized by light or radiation or electron beams, hence it is destructive to the sample. The amount of PCA in percentage of dry weight were found to be 14, 10.57, and 9 respectively which corresponds to CHX + NaOCl, CHX + neem and CHX + tulsi. In addition, the retention time for CHX + NaOCl, CHX + neem and CHX + tulsi was found to be 4.537, 4.527 and 4.548 min, close to the standard PCA values. Thus, HPLC results points to the presence of PCA in precipitate. UV is also known as electronic spectroscopy which is an analytical tool used for quantitative determination of different analytics such as transition metal ions, highly conjugated organic compounds and biological macromolecules. In this study, peak values of reddish brown, green, dark green precipitate were found to be coinciding with the standard values of PCA.
NMR is a nondestructive technique used to analyze molecules in a mixture. NMR is highly predictable, unique, well-resolved, and analytically tractable for small molecules. Proton and carbon-13 NMR are two types of NMR spectroscopy, but is only applicable to those kind of samples that contains nuclei possessing spin. Since it is an insensitive method, better methods exist for detecting impurities although large amounts of impurities do show on the NMR spectrum. In this study, both proton and carbon 13-NMR were used. The chemical shift of standard PCA were found to be 7.00 and 6.55 ppm whereas for other three precipitates the chemical shift was observed to be between 7.33 and 7.55 ppm. In the present study, the NMR values were in accordance with the values of a previous study conducted by Ekim et al. whereas HPLC, TLC values were not found to be coinciding with the aforesaid study. Also in the present study, NMR detected para-chlorophenyl urea and para-chlorophenylguanidyl-1,6-diguanidyl-hexane but failed to detect the presence of PCA. Since PCA being aniline, is volatile, hence there is a possibility that lack of detection was due to this characteristic property. PCA may not be found in an environment that allows sample to evaporate.
ESI-MS is sensitive and robust, a reliable tool for studying femtomole quantities in microliter sample volumes, nonvolatile and thermally labile bio-molecules that are not amenable to analysis by other conventional techniques. It is used with MS to simplify complicated sample purification that is commonly used in gas chromatography. It was observed in all three precipitate samples that peak values were found to be similar with standard PCA values, i.e., 125,149,167 m/z.
The null hypothesis stands rejected simply for the fact that precipitate was formed in all groups incorporating CHX and also the presence of PCA was detected in all the sensitive methods used in this study. Since we have used more sensitive and coupled spectroscopy, we can affirm the presence of PCA in the tested samples.
Neem and tulsi were particularly included in this study to rule out the possibility that CHX does not form precipitate with irrigants other than NaOCl. Interestingly, the green and dark green precipitate formed on combination with neem and tulsi contained PCA which was confirmed by TLC, CC, HPLC, ESI-MS, and UV.
Krishnamurthy et al. 2010, Ahmed et al. 2012 and Vouzara et al. 2016 disclosed that thorough intermediate flushes with distilled water, absolute alcohol or saline followed by drying of the canals can limit or possibly prevent the formation of PCA and its by-products. Even though Magro et al. 2015 had objected to the above-stated, a set of guidelines should be followed to prevent or minimize precipitate formation.
CONCLUSION
CHX on combination with sodium hypochlorite, neem and tulsi produced reddish brown, green and dark green precipitate respectively
The highest amount of precipitate was seen in group 3 followed by group 2 and group 1
The presence of PCA in precipitate was confirmed by TLC, CC, HPLC, ESI-MS, and UV.
Based on the results of the present study, we assume that components in CHX are responsible for precipitate formation which contains PCA as well.
At a clinical level, researchers and dental practitioners alike must follow a set of guidelines which can help in preventing or minimizing precipitate formation:
Firstly, since the results of the study demonstrate that the amount of precipitate formed with neem and tulsi is almost 4–7.5 times greater than that formed with NaOCl, caution should be exercised when combining any natural product irrigant with CHX.
Secondly, in light of the above-said statement, increase in precipitate content will lead to occlusion of dentinal tubules to a greater extent hence the right combination of irrigants to be used in certain circumstances is of paramount importance.
Thirdly, extrusion of precipitate beyond the apex may also cause periapical tissue damage and delayed wound healing at the same time therefore careful rinsing of the canals between each irrigant is advocated.
All things considered, the combination of CHX with NaOCl and other natural products can be used provided that thorough intermediate flushing with alcohol, saline or distilled water is strictly followed along with proper drying of the canals to achieve a favorable outcome.
Future scope
The active component in any natural product which is responsible for forming a precipitate with CHX has to be evaluated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to acknowledge the technical assistance provided to us by the Sophisticated Analytical Instrument Facility-IIT, Madras and Monarch Biotech Private Limited, Chennai. The authors would also like to acknowledge the skillful support of Mr. Narendran K.
REFERENCES
- 1.Kuruvilla JR, Kamath MP. Antimicrobial activity of 2.5% sodium hypochlorite and 0.2% chlorhexidine gluconate separately and combined, as endodontic irrigants. J Endod. 1998;24:472–6. doi: 10.1016/S0099-2399(98)80049-6. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner JC, Mader CL. A scanning electron microscopic evaluation of four root canal irrigation regimens. J Endod. 1987;13:147–57. doi: 10.1016/s0099-2399(87)80132-2. [DOI] [PubMed] [Google Scholar]
- 3.Haapasalo M, Shen Y, Qian W, Gao Y. Irrigation in endodontics. Dent Clin North Am. 2010;54:291–312. doi: 10.1016/j.cden.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–9. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 5.Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89:321–8. doi: 10.1111/j.1600-0722.1981.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 6.Leonardo MR, Tanomaru Filho M, Silva LA, Nelson Filho P, Bonifácio KC, Ito IY, et al. In vivo antimicrobial activity of 2% chlorhexidine used as a root canal irrigating solution. J Endod. 1999;25:167–71. doi: 10.1016/s0099-2399(99)80135-6. [DOI] [PubMed] [Google Scholar]
- 7.Okino LA, Siqueira EL, Santos M, Bombana AC, Figueiredo JA. Dissolution of pulp tissue by aqueous solution of chlorhexidine digluconate and chlorhexidine digluconate gel. Int Endod J. 2004;37:38–41. doi: 10.1111/j.1365-2591.2004.00749.x. [DOI] [PubMed] [Google Scholar]
- 8.Bui TB, Baumgartner JC, Mitchell JC. Evaluation of the interaction between sodium hypochlorite and chlorhexidine gluconate and its effect on root dentin. J Endod. 2008;34:181–5. doi: 10.1016/j.joen.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Basrani BR, Manek S, Fillery E. Using diazotization to characterize the effect of heat or sodium hypochlorite on 2.0% chlorhexidine. J Endod. 2009;35:1296–9. doi: 10.1016/j.joen.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 10.Yeung SY, Huang CS, Chan CP, Lin CP, Lin HN, Lee PH, et al. Antioxidant and pro-oxidant properties of chlorhexidine and its interaction with calcium hydroxide solutions. Int Endod J. 2007;40:837–44. doi: 10.1111/j.1365-2591.2007.01271.x. [DOI] [PubMed] [Google Scholar]
- 11.Basrani BR, Manek S, Sodhi RN, Fillery E, Manzur A. Interaction between sodium hypochlorite and chlorhexidine gluconate. J Endod. 2007;33:966–9. doi: 10.1016/j.joen.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Orhan EO, Irmak Ö, Hür D, Yaman BC, Karabucak B. Does para-chloroaniline really form after mixing sodium hypochlorite and chlorhexidine? J Endod. 2016;42:455–9. doi: 10.1016/j.joen.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Basrani BR, Manek S, Mathers D, Fillery E, Sodhi RN. Determination of 4-chloroaniline and its derivatives formed in the interaction of sodium hypochlorite and chlorhexidine by using gas chromatography. J Endod. 2010;36:312–4. doi: 10.1016/j.joen.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Mortenson D, Sadilek M, Flake NM, Paranjpe A, Heling I, Johnson JD, et al. The effect of using an alternative irrigant between sodium hypochlorite and chlorhexidine to prevent the formation of para-chloroaniline within the root canal system. Int Endod J. 2012;45:878–82. doi: 10.1111/j.1365-2591.2012.02048.x. [DOI] [PubMed] [Google Scholar]
- 15.Arslan H, Uygun AD, Keskin A, Karatas E, Seçkin F, Yıldırım A, et al. Evaluation of orange-brown precipitate formed in root canals after irrigation with chlorhexidine and QMix and spectroscopic analysis of precipitates produced by a mixture of chlorhexidine/NaOCl and QMix/NaOCl. Int Endod J. 2015;48:1199–203. doi: 10.1111/iej.12427. [DOI] [PubMed] [Google Scholar]
- 16.Ballal NV, Moorkoth S, Mala K, Bhat KS, Hussen SS, Pathak S, et al. Evaluation of chemical interactions of maleic acid with sodium hypochlorite and chlorhexidine gluconate. J Endod. 2011;37:1402–5. doi: 10.1016/j.joen.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 17.Chhabra RS, Huff JE, Haseman JK, Elwell MR, Peters AC. Carcinogenicity of p-chloroaniline in rats and mice. Food Chem Toxicol. 1991;29:119–24. doi: 10.1016/0278-6915(91)90166-5. [DOI] [PubMed] [Google Scholar]
- 18.Messmer AS, Nickel CH, Bareiss D. P-chloroaniline poisoning causing methemoglobinemia: A case report and review of the literature. Case Rep Emerg Med. 2015;2015:208732. doi: 10.1155/2015/208732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waris G, Ahsan H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lueken A, Juhl-Strauss U, Krieger G, Witte I. Synergistic DNA damage by oxidative stress (induced by H2O2) and nongenotoxic environmental chemicals in human fibroblasts. Toxicol Lett. 2004;147:35–43. doi: 10.1016/j.toxlet.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Marchesan MA, Pasternak Júnior B, Afonso MM, Sousa-Neto MD, Paschoalato C. Chemical analysis of the flocculate formed by the association of sodium hypochlorite and chlorhexidine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e103–5. doi: 10.1016/j.tripleo.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Bohora A, Hegde V, Kokate S. Comparison of the antibacterial efficiency of neem leaf extract and 2% sodium hypochlorite against E. faecalis, C. albicans and mixed culture-An in vitro study. Endodontology. 2010;22:8–12. [Google Scholar]
- 23.Al-Hashemi ZS, Hossain MA. Biological activities of different neem leaf crude extracts used locally in Ayurvedic medicine. Pac Sci Rev Nat Sci Eng. 2016;18:128–31. [Google Scholar]
- 24.Chandrappa PM, Dupper A, Tripathi P, Arroju R, Sharma P, Sulochana K, et al. Antimicrobial activity of herbal medicines (tulsi extract, neem extract) and chlorhexidine against Enterococcus faecalis in endodontics: An in vitro study. J Int Soc Prev Community Dent. 2015;5:S89–92. doi: 10.4103/2231-0762.172952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi B, Sah GP, Basnet BB, Bhatt MR, Sharma D, Subedi K, et al. Phytochemical extraction and antimicrobial properties of different medicinal plants: Ocimum sanctum (Tulsi), Eugenia caryophyllata (Clove), Achyranthes bidentata (Datiwan) and Azadirachta indica (Neem) J Microbiol Antimicrob. 2011;3:1–7. [Google Scholar]