Abstract
Objective:
The objective of this study was to evaluate the antibacterial efficacy of two doses of vetiver and chamomile essential oils compared with chlorhexidine and calcium hydroxide against Enterococcus faecalis.
Materials and Methods:
The growth inhibition and minimum inhibitory concentration of all tested materials were determined ex vivo following agar diffusion and broth dilution assay procedure. Human maxillary anterior teeth were prepared with protaper rotary files, followed by incubation with standard broth of E. faecalis. A total of 140 teeth were included in the study. These teeth are randomized and equally divided into seven groups and were treated with low (1.25 μl) and high doses (2.5 μl) of essential oils of vetiver and chamomile and calcium hydroxide (0.1/1.0 mL), 2% chlorhexidine (2.5 μl). Microbial sampling of six teeth from each group was done with paper points and Gates–Glidden burs at 1, 7, and 14 days and colony-forming unit (CFU)/mL was determined.
Results:
There was a significant reduction in mean ± standard deviation of CFU (log10) in vetiver oil high dose (3.32 ± 0.036) and chlorhexidine (3.34 ± 0.030), followed by calcium hydroxide (3.46 ± 0.015) and chamomile oil high dose (3.48 ± 0.20) on day 1. On 7th day, a significant reduction was seen in chlorhexidine (2.74 ± 0.212), chamomile oil (2.81 ± 0.035, low dose and 2.97 ± 0.119, high dose), followed by calcium hydroxide (3.25 ± 0.028). However, on 14th day, it was 2.32 ± 0.088 for chlorhexidine, 2.91 ± 0.029 for chamomile oil high dose, 3.15 ± 0.010 for vetiver oil high dose, and 3.09 ± 0.068 for calcium hydroxide.
Conclusion:
The study showed a good effectiveness of chamomile oils in root canal infection of E. faecalis at different time intervals compared to chlorhexidine and calcium hydroxide vetiver oil did not sustain their activity for a longer duration.
Key words: Antibacterial, endodontics, essential oils, intracanal medicaments, root canal treatment
INTRODUCTION
Despite so many advancements in equipment and materials, effective disinfection during the endodontic treatment is still a cumbersome and challenging issue. The complex morphology of dentine and the presence of bacteria, especially in the apical part of root canal, even after the disinfection protocols, make this task very tedious, especially in Enterococcus faecalis infection, which is the most common pathogen responsible for the failure of root canal therapy.[1]
Calcium hydroxide has a long history of its usage as an intracanal medicament as well as antimicrobial agent; however, recent findings questioned about its potential, especially against E. faecalis.[2] Several antimicrobial agents including linezolid, ampicillin, as well as laser or ultrasonic techniques also have been used as disinfecting agents during root canal procedure; however, these medicaments and procedures have some clinical drawbacks and some of them are not cost-effective.[3,4,5]
Hence, the field of dentistry is constantly evolving and adopting the best suitable and available antimicrobial agent for the treatment. Since long time, many agents have been used to eradicate or reduce endodontic microflora; however, most of them are found ineffective to combat complete infection and led to the failure of root canal treatment. This has prompted the scientists worldwide to explore novel materials which can serve with the better output, less shortcomings, and low investment. The material with properties, such as good biocompatibility, good biological properties, favorable physical properties, cost-effectiveness to the patient, and dentist too, are more in demand than other substance. Therefore, the search for the novel materials and new techniques which can take dentistry to new heights and make the patient more comfortable is incessant.
Given these facts, we planned our study to evaluate the antimicrobial potential of two plant-derived essential oils, namely, chamomile and vetiver, along with traditional intracanal medicaments chlorhexidine and calcium hydroxide against E. faecalis.
MATERIALS AND METHODS
Preparation of plant extracts
The plant materials (roots) of Chrysopogon zizanioides (L.) Roberty (Poaceae) and flowers of Matricaria chamomilla L.(Asteraceae) were subjected to steam distillation for 4–6 h using a Clevenger-type apparatus and the essential oils were collected after decantation. The antimicrobial activity of the essential oil was tested by making a stock/working concentration in dimethyl sulfoxide/phosphate-buffered saline (PBS).[6,7]
Media preparation
To prepare the solid agar media, 37 g of brain heart infusion broth (BHIB) and 15 g of agar powder were weighed and dissolved in 1000 mL by stirring in distilled water and sterilized by autoclaving at 15 lbs pressure (121°C) for 15 min.
Maintenance of bacterial strain
The antibacterial activity was tested against clinical isolate and wild-type strain of E. faecalis. For the experiments, each of the microbial strain was revived in liquid media (BHIB). The turbidity was adjusted to 0.5 McFarland equivalents (approximately 1–2 × 108 colony-forming units [CFU]/mL). The culture of E. faecalis was incubated for 24 h at 37°C in BHIB. After the incubation period, the growth of microbe was confirmed by identifying specific morphology in the culture plates.[7]
Disc diffusion assay
The antibacterial activity of the vetiver and chamomile oil along with standard antimicrobials was determined by disc diffusion assay described by Bauer et al.[8] E. faecalis strain was inoculated in BHIB from preserved culture and then grown for overnight at 37°C then streaked on BHI agar plate to get single-isolated colony. The antibacterial susceptibility testing was performed by taking single-isolated colony of E. faecalis from culture plate and suspended in the sterile saline solution and was mixed thoroughly to get 0.5 McFarland bacterial suspensions equivalents (approximately 1–2 × 108 CFU/mL). The plating was done, and the plates were incubated at 37°C overnight in an incubator, the magnitude of the zone of growth inhibition (ZOGI) was measured which indicated the strength of antibacterial activity. The ZOGI of the tested antimicrobials was compared with that of standard agents.
Minimum inhibitory concentration determination
The antibacterial activity of vetiver and chamomile oil against E. faecalis was quantified by the determination of minimum inhibitory concentration (MIC) using broth dilution assay which is based on serial dilution of the media.[9,10] In brief, a sterile 96-well tissue culture microplates were used for the MIC determination. The first 10 wells of the row were used for serial dilution of the chosen compounds, 11th column was kept as positive control whereas 12th was labeled as media (negative) control. To each well, 150 μl of liquid media (BHIB) was added in the well of the first column followed by the addition of 3 μl of antimicrobials to be tested (to achieve 1000 and 200 μg/mL of concentration for oil and standard drug, respectively). It was mixed properly, and the volume was made 300 μl with the addition of 147 μl of media. Subsequently, 150 μl of media containing antimicrobials was transferred to subsequent wells, and the same was repeated till 10th well column. The 10 μl of working bacterial culture suspension was added to each well (1–11th column) except media control (12th column). The plates were incubated at 37°C overnight and the growth was observed by considering the turbidity in the media. The MIC was determined accordingly and expressed as μg/mL.
Extracted teeth collection and preparation for the experiments
A prior ethical clearance was taken from the Institutional Ethical Committee (Ref. No. 77th ECM II-B IMR-faculty/P16) and signed written consent was obtained by each and every individual who volunteered to donate their teeth for this study.
A total of 140 extracted single-rooted human teeth were stored in the saline solution after extraction. The specimens were decoronated to a standard 14-mm root segment length with a rotating diamond saw (HORICO DENTAL Hopf, Ringleb and Co., GmbH and Cie, Germany) under water coolant. The cleaning and shaping of the specimens were done using Protaper universal file system (Dentsply Maillefer, Ballaigues, Switzerland) from Sx–F3 according to the manufacturer's instructions, using Glyde Prep Canal (Dentsply Maillefer, Ballaigues, Switzerland) as a lubricant. In between the instrumentation, the canals were irrigated with 3% NaOCl solution (Parcan, Septodent Healthcare India Pvt. Ltd, India). After instrumentation, the canals were irrigated with 17% ethylenediaminetetraacetic acid solution (Dentochlor, Ammdent India) for 3 min and NaOCl (3 min) to remove the smear layer and to permit microorganisms and medicaments to penetrate the dentinal tubules. The final canal irrigation was done with 10 mL of physiological saline solution. The roots were dried with sterile gauze, and two layers of nail polish were applied over the entire external surface of all roots to avoid external microbial contamination. Care was taken not to occlude the root canal entrance. Each specimen was placed in a microcentrifuge tube containing 500 mL of PBS (0.01 M, pH 7.4) and they were subsequently autoclaved at 121°C for 30 min. To confirm the efficacy of the sterilization process, PBS from the microcentrifuge tubes was aspirated under sterile conditions and inoculated into BHI broth then plated onto blood agar plates and incubated at 37°C for 24 h and checked for any microbial growth. All the remaining specimen manipulations were performed under a laminar flow hood to avoid contamination from outside organisms.[11]
Infection of root canals
All the root specimens were transferred and mounted into sterile flat bottom 24-well cell culture well plates under sterile conditions with 2% sterile agar which was allowed to solidify so that root specimens can be stabilized.[12] Once the root specimens were mounted and stabilized on cell culture well plates, 10 μl of E. faecalis culture was injected into the respected root specimens using pipettes. After inoculation of E. faecalis, the hole of each specimen was entirely filled with BHIB and incubated at 37°C for 24 h. Every alternate day, about 10 μl of fresh BHIB was added to ensure the viability of E. faecalis. With the help of sterile 3 mL syringe and 30-gauge Navitip needle (Ultradent Products, Inc., USA), medicaments were injected under aseptic conditions into the canals of all experimental group samples until the canals were totally fill. The samples were withdrawn and cultivated on blood agar plates. The bacterial growth was observed by counting colony-forming units and compared with the standard drugs. These samples were randomly divided into seven groups (n = 20) according to the medicament used.
Group 1: Control group treated with sterile water only
Group II: Treated with 2.5 μl of chlorhexidine (2%) solution
Group III: Treated with 1.25 μl of chamomile oil
Group IV: Treated with 2.50 μl of chamomile oil
Group V: Treated with 1.25 μl of vetiver oil
Group VI: Treated with 2.50 μl of vetiver oil
Group VII: Treated with 10% (w/v) of calcium hydroxide.
Incubation and microbiological sampling
The specimens in all groups were randomly divided equally into three subgroups. The samples were incubated at 37°C for 1 day (subgroup 1) or 7 days (subgroup 2) and 14 days (subgroup 3). The first bacteriological sampling was taken from six samples of each group using sterile paper points (size 30). A paper point was placed inside each root canal for 1 min. After sampling, each paper point was placed in a microcentrifuge tube containing 1 mL of BHI broth and shaken for 30 s on a vortex. 0.1 mL aliquot of the microbial suspension was incubated on a BHI agar plate for 24 h at 37°C. For the second bacteriological sampling, Gates–Glidden burs #5 ((Dentsply Maillefer, Ballaigues, Switzerland) and an Endo IT electromotor (VDW, Munich, Germany) at 2000 rpm were used to obtain dentine samples from the lumen. The same procedure described for paper points was applied to the burs. Each bur was used three times up to 10 mm of the canal length in 10 s. After confirming the purity of the positive cultures using colony morphology, the number of CFUs of each specimen was determined. The identical procedure was applied for microbial sampling at day 7 and 14.[13]
RESULTS
At day 1, the mean CFU (log10) was maximum in Group I (3.67 ± 0.015) and minimum in Group VI (3.32 ± 0.036) [Table 1]. The maximum and minimum percentage reduction compared to control was seen in Group VI (9.39%) and Group III (2.28%), respectively, which was statistically significant [Table 1]. At day 7, the mean CFU (log10) was maximum in Group I (3.63 ± 0.009) and minimum in Group II (2.74 ± 0.212) [Table 2]. The maximum and minimum percentage reduction compared to control was seen in Group II (24.75%) and Group V (6.32%), respectively [Table 2]. At day 14, the mean CFU was maximum in Group I (3.64 ± 0.009) and minimum in Group II (2.32 ± 0.087) [Table 3]. The maximum and minimum percentage reduction compared to control was seen in Group II (36.16%) and Group III (7.05%), respectively, which was statistically significant [Table 3].
Table 1.
Analysis of colony-forming unit (log10) of control and different medicament at day 1
| Group | Mean±SD | Minimum | Maximum | Mean ranks | Percentage reduction in CFU (log10) compared to control | Kruskal–Wallis test (χ2, df, P) |
|---|---|---|---|---|---|---|
| I | 3.67±0.015 | 3.65 | 3.7 | 39.50 | - | 38.68, 6, 0.00 |
| II | 3.34±0.030 | 3.31 | 3.39 | 7.0 | 8.99 | |
| III | 3.59±0.020 | 3.56 | 3.62 | 33.17 | 2.28 | |
| IV | 3.48±0.020 | 3.46 | 3.51 | 20.08 | 5.26 | |
| V | 3.54±0.026 | 3.50 | 3.57 | 27.65 | 3.56 | |
| VI | 3.32±0.036 | 3.27 | 3.37 | 6.0 | 9.39 | |
| VII | 3.46±0.015 | 3.44 | 3.48 | 17.08 | 5.69 |
CFU: Colony-forming unit, SD: Standard deviation
Table 2.
Analysis of colony-forming unit (log10) of control and different medicament at day 7
| Groups | Mean±SD | Minimum | Maximum | Mean rank | Percentage reduction in CFU (log10) compared to control | Kruskal–Wallis test (χ2, df, P) |
|---|---|---|---|---|---|---|
| I | 3.63±0.009 | 3.61 | 3.64 | 39.50 | - | 38.485, 6, 0.00 |
| II | 2.74±0.212 | 2.48 | 3.09 | 6.83 | 24.75 | |
| III | 2.81±0.345 | 2.77 | 2.86 | 8.42 | 22.60 | |
| IV | 2.97±0.119 | 2.79 | 3.09 | 13.75 | 18.17 | |
| V | 3.40±0.239 | 3.37 | 3.44 | 27.50 | 6.32 | |
| VI | 3.33±0.018 | 3.30 | 3.35 | 21.50 | 8.31 | |
| VII | 3.25±0.028 | 3.21 | 3.29 | 38.48 | 10.44 |
CFU: Colony-forming unit, SD: Standard deviation
Table 3.
Analysis of colony-forming unit (log10) of control and different medicament at day 14
| Groups | Mean ± SD | Minimum | Maximum | Mean ranks | Percentage reduction in CFU (log10) compared to control | Kruskal–Wallis test (χ2, df, P) |
|---|---|---|---|---|---|---|
| I | 3.64 ± 0.009 | 3.63 | 3.65 | 39.50 | - | 38.775, 6, 0.00 |
| II | 2.32 ± 0.088 | 2.2 | 2.45 | 3.50 | 36.16 | |
| III | 3.38 ± 0.022 | 3.36 | 3.41 | 33.50 | 7.05 | |
| IV | 2.91 ± 0.029 | 2.87 | 2.94 | 9.50 | 20.10 | |
| V | 3.10 ± 0.021 | 3.07 | 3.13 | 19.33 | 14.62 | |
| VI | 3.15 ± 0.010 | 3.13 | 3.16 | 25.50 | 13.51 | |
| VII | 3.09 ± 0.068 | 3.01 | 3.18 | 19.67 | 14.99 |
CFU: Colony-forming unit, SD: Standard deviation
DISCUSSION
This in vitro study investigated the antibacterial potential of two essential oils, that is, vetiver and chamomile, and it was observed that activity was comparable with that of standards, namely, chlorhexidine and calcium hydroxide against E. faecalis in infected root canal models. This study was planned to provide an alternative for quick, preliminary assessment of antibacterial potential of essential oils of vetiver and chamomile, and its role as an intracanal medicament in an infected root canal models. Previously published reports advocate the antimicrobial action of vetiver and chamomile oils against E. faecalis. The effectiveness and clinical efficacy of chamomile have also been reported selectively in removing smear layer, oral mucositis, plaque, scurvy, gingivitis, and patient undergoing orthodontic treatment.[14,15,16] The result of the present investigation exhibited at day 1, the highest antibacterial potential of chlorhexidine and it maintains its activity up to 14th day. Earlier reports depict the antibacterial efficacy of chlorhexidine and calcium hydroxide combination for the treatment of teeth with primary endodontic lesions.[17,18] However, a number of factors are also associated with the failure of root canal therapy due to E. faecalis infection such as its collagen adherence,[19] long starvation survival potential,[20] proton pump,[21] bidirectional horizontal transfer of resistance factors from one species to another[22] which make this bacteria a versatile and commonly associated with failed root canal cases (77%), and approximately 50% in chronic apical periodontitis.[1,23] These are some of the known reasons which prompt us to choose this microbe for our study. The MICs of all medicaments included in the study was measured before the study on root canal models. The calcium hydroxide has a long history of its usage as an intracanal medicament; however, few conflicting results have also been reported, such as its high pH (12.5–12.8), low solubility in aqueous medium, and its ability to absorb CO2which make it lethal for most of the pathogenic bacteria in endodontic infections.[2,24] It also inactivates glycolipids of bacterial virulence factors.[25] In our experimental analysis, at day 1, calcium hydroxide significantly reduced the CFU values compare to control (5.69%); however, it was less effective than chlorhexidine (8.99%) and vetiver oil high dose (9.39%) [Table 1]. Chlorhexidine is positively charged particle reacts with negatively charged molecule present on the bacterial cell wall and destroyed cell hemostasis. It has been known for its activity against E. faecalis.[13,19,22,26] At day 1, the maximum reduction in CFU was shown by vetiver oil at higher concentration (9.39%) followed by chlorhexidine (8.99%). The effectiveness of chlorhexidine was increased at 7th day (24.75%), and it even showed an increasing trend on 14th day (36.16%) [Tables 1–3 and Graph 1]. Thus, it remains effective up to 14th day and proved to be the best medicament in our experimental analysis. Supportively, other studies also reported similar results.[27,28] Chamomile oil at low dose was least effective at day 1 but showed a better activity at 7th day, and finally, its effectiveness reduced at 14th day which explains its short-term effect [Graph 1]. The chamomile oil at higher dose shows significant reduction in bacterial count at day 1 (5.26%) and its effectiveness increased up to day 7 (18.17%) and even remains stationary at day 14 (20.1%), these findings show that higher dose regimen of this oil was more effective and long-lasting for endodontic purposes. The chamomile plant is known to have antibacterial, anti-inflammatory, antiviral, and antioxidant.[29] Its antibacterial properties are mainly due to the presence of α-bisabolol, luteolin, quercetin, and apigenin against many bacteria including Bacillus subtilis, Staphylococcus aureus, Streptococcus mutans, Streptococcus salivarius, and fungi like Candida albicans.[30] Its antifungal action has been speculated due to a specific inhibition of ergosterol biosynthesis by α-bisobolol.[31] The chamomile oil has a high level of safety, acute oral LD50, and dermal LD50was found >5 g/kg body weight, and Food and Drug Administration classified it as generally regarded as safe.[32] The vetiver oil at higher dose regimen works excellent at day 1 (9.39%) but effectiveness decreases afterward. The low-dose regimen did not show any significant antibacterial property at any stage of experimental analysis [Tables 1-3 and Graph 1]. Other natural products, that is, propolis produced by honey bee tasted against E. faecalis and found to be highly effective for 2 days when compared with calcium hydroxide and triple antibiotic mixture.[33] Another study compared Morinda citrifolia, papain, and aloe vera. They concluded that M. citrifolia having good antibacterial activity against E. faecalis; however, it was not as effective as chlorhexidine.[34]
Graph 1.
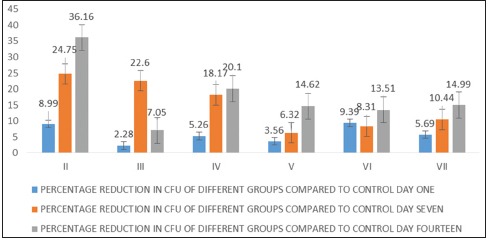
Graph showing percentage reduction in colony-forming unit (log10) of different groups compared to control
The present study proved the antibacterial role of chamomile essential oil for a long time up to 14 days. It can be a herbal alternative intracanal medicament to disinfect the root canal.
Since this was an in vitro study, further ex vivo studies are required regarding the same to corroborate with the results of this study before this can be used for the clinical purpose.
CONCLUSION
The present study provides an evidence for the effectiveness of chamomile essential oils in root canal infection against E. faecalis at different time intervals compared to chlorhexidine and calcium hydroxide, while vetiver oil did not sustain their activity for a longer duration.
Financial support and sponsorship
This study was financially supported by Research Cell, King George's Medical University, Lucknow, India. Under Intramural seed grant program for faculty.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are grateful to the Director, CSIR-CIMAP, Lucknow, for supporting us to carry out this collaborative work.
REFERENCES
- 1.Siqueira JF, Jr, Rôças IN. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:85–94. doi: 10.1016/s1079-2104(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 2.Kim D, Kim E. Antimicrobial effect of calcium hydroxide as an intracanal medicament in root canal treatment: A literature review – Part I. In vitro studies. Restor Dent Endod. 2014;39:241–52. doi: 10.5395/rde.2014.39.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavaskar R, de Ataide Ide N, Chalakkal P, Pinto MJ, Fernandes KS, Keny RV, et al. An in vitro study comparing the intracanal effectiveness of calcium hydroxide- and linezolid-based medicaments against Enterococcus faecalis. J Endod. 2012;38:95–100. doi: 10.1016/j.joen.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 4.Cheng X, Chen B, Qiu J, He W, Lv H, Qu T, et al. Bactericidal effect of er:YAG laser combined with sodium hypochlorite irrigation against Enterococcus faecalis deep inside dentinal tubules in experimentally infected root canals. J Med Microbiol. 2016;65:176–87. doi: 10.1099/jmm.0.000205. [DOI] [PubMed] [Google Scholar]
- 5.Krithikadatta J, Indira R, Dorothykalyani AL. Disinfection of dentinal tubules with 2% chlorhexidine, 2% metronidazole, bioactive glass when compared with calcium hydroxide as intracanal medicaments. J Endod. 2007;33:1473–6. doi: 10.1016/j.joen.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Clevenger JF. Apparatus for the determination of volatile oil. J Pharm Sci. 1928;17:345–9. [Google Scholar]
- 7.Luqman S, Dwivedi GR, Darokar MP, Kalra A, Khanuja SP. Antimicrobial activity of Eucalyptus citriodora essential oil. Int J Essent Oil Ther. 2008;2:69–75. [Google Scholar]
- 8.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 9.Jorgensson JH, Turnigde DJ, Washington JA. Antibacterial 40. Susceptibility tests: Dilution and disc diffusion methods. In: Murray PR, editor. Manual of Clinical Microbiology. Washington, DC: American Society for Microbiology; 1999. pp. 1526–43. [Google Scholar]
- 10.Luqman S, Dwivedi GR, Darokar MP, Kalra A, Khanuja SP. Potential of rosemary oil to be used in drug-resistant infections. Altern Ther Health Med. 2007;13:54–9. [PubMed] [Google Scholar]
- 11.Vidana R, Sullivan A, Billström H, Ahlquist M, Lund B. Enterococcus faecalis infection in root canals – Host-derived or exogenous source? Lett Appl Microbiol. 2011;52:109–15. doi: 10.1111/j.1472-765X.2010.02972.x. [DOI] [PubMed] [Google Scholar]
- 12.Hecker S, Hiller KA, Galler KM, Erb S, Mader T, Schmalz G, et al. Establishment of an optimized ex vivo system for artificial root canal infection evaluated by use of sodium hypochlorite and the photodynamic therapy. Int Endod J. 2013;46:449–57. doi: 10.1111/iej.12010. [DOI] [PubMed] [Google Scholar]
- 13.Afkhami F, Akbari S, Chiniforush N. Entrococcus faecalis elimination in root canals using silver nanoparticles, photodynamic therapy, diode laser, or laser-activated nanoparticles: An in vitro study. J Endod. 2017;43:279–82. doi: 10.1016/j.joen.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 14.Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol. 1999;86:985–90. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 15.Venkataram V, Gokhale ST, Kenchappa M, Nagarajappa R. Effectiveness of chamomile (Matricaria recutita L.), MTAD and sodium hypochlorite irrigants on smear layer. Eur Arch Paediatr Dent. 2013;14:247–52. doi: 10.1007/s40368-013-0062-3. [DOI] [PubMed] [Google Scholar]
- 16.Goes P, Dutra CS, Lisboa MR, Gondim DV, Leitão R, Brito GA, et al. Clinical efficacy of a 1% Matricaria chamomile L. mouthwash and 0.12% chlorhexidine for gingivitis control in patients undergoing orthodontic treatment with fixed appliances. J Oral Sci. 2016;58:569–74. doi: 10.2334/josnusd.16-0280. [DOI] [PubMed] [Google Scholar]
- 17.Donyavi Z, Ghahari P, Esmaeilzadeh M, Kharazifard M, Yousefi-Mashouf R. Antibacterial efficacy of calcium hydroxide and chlorhexidine mixture for treatment of teeth with primary endodontic lesions: A randomized clinical trial. Iran Endod J. 2016;11:255–60. doi: 10.22037/iej.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narayanan LL, Vaishnavi C. Endodontic microbiology. J Conserv Dent. 2010;13:233–9. doi: 10.4103/0972-0707.73386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kayaoglu G, Erten H, Ørstavik D. Possible role of the adhesin ace and collagen adherence in conveying resistance to disinfectants on Enterococcus faecalis. Oral Microbiol Immunol. 2008;23:449–54. doi: 10.1111/j.1399-302X.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- 20.Figdor D, Davies JK, Sundqvist G. Starvation survival, growth and recovery of Enterococcus faecalis in human serum. Oral Microbiol Immunol. 2003;18:234–9. doi: 10.1034/j.1399-302x.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 21.Evans M, Davies JK, Sundqvist G, Figdor D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J. 2002;35:221–8. doi: 10.1046/j.1365-2591.2002.00504.x. [DOI] [PubMed] [Google Scholar]
- 22.Sedgley CM, Lee EH, Martin MJ, Flannagan SE. Antibiotic resistance gene transfer between Streptococcus gordonii and Enterococcus faecalis in root canals of teeth ex vivo. J Endod. 2008;34:570–4. doi: 10.1016/j.joen.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Peciuliene V, Reynaud AH, Balciuniene I, Haapasalo M. Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int Endod J. 2001;34:429–34. doi: 10.1046/j.1365-2591.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 24.Kontakiotis E, Nakou M, Georgopoulou M. In vitro study of the indirect action of calcium hydroxide on the anaerobic flora of the root canal. Int Endod J. 1995;28:285–9. doi: 10.1111/j.1365-2591.1995.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 25.Baik JE, Kum KY, Yun CH, Lee JK, Lee K, Kim KK, et al. Calcium hydroxide inactivates lipoteichoic acid from Enterococcus faecalis. J Endod. 2008;34:1355–9. doi: 10.1016/j.joen.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Manzur A, González AM, Pozos A, Silva-Herzog D, Friedman S. Bacterial quantification in teeth with apical periodontitis related to instrumentation and different intracanal medications: A randomized clinical trial. J Endod. 2007;33:114–8. doi: 10.1016/j.joen.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Kanisavaran ZM. Chlorhexidine gluconate in endodontics: An update review. Int Dent J. 2008;58:247–57. doi: 10.1111/j.1875-595x.2008.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 28.Gomes BP, Vianna ME, Zaia AA, Almeida JF, Souza-Filho FJ, Ferraz CC, et al. Chlorhexidine in endodontics. Braz Dent J. 2013;24:89–102. doi: 10.1590/0103-6440201302188. [DOI] [PubMed] [Google Scholar]
- 29.Gosztola B, Sárosi S, Németh E. Variability of the essential oil content and composition of chamomile (Matricaria recutita L.) affected by weather conditions. Nat Prod Commun. 2010;5:465–70. [PubMed] [Google Scholar]
- 30.Cinco M, Banfi E, Tubaro A, Della LR. A microbiological survey on the activity of a hydroalcoholic extract of camomile. Int J Crude Drug Res. 1983;21:145–51. [Google Scholar]
- 31.Pauli A. α-Bisabolol from chamomile – A specific ergosterol biosynthesis inhibitor? Int J Aromather. 2006;16:21–5. [Google Scholar]
- 32.Opdyke D. Fragrance raw materials monographs. Chamomile oil German. Food Cosmet Toxicol. 1974;12(Suppl):851–2. [PubMed] [Google Scholar]
- 33.Madhubala MM, Srinivasan N, Ahamed S. Comparative evaluation of propolis and triantibiotic mixture as an intracanal medicament against Enterococcus faecalis. J Endod. 2011;37:1287–9. doi: 10.1016/j.joen.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 34.Bhardwaj A, Ballal S, Velmurugan N. Comparative evaluation of the antimicrobial activity of natural extracts of Morinda citrifolia, papain and Aloe vera (all in gel formulation), 2% chlorhexidine gel and calcium hydroxide, against Enterococcus faecalis: An in vitro study. J Conserv Dent. 2012;15:293–7. doi: 10.4103/0972-0707.97964. [DOI] [PMC free article] [PubMed] [Google Scholar]


