Abstract
In this study, DEN-treated male mice were assigned to 4 groups: a 35% high fat ethanol liquid diet (EtOH), an EtOH liquid diet with soy protein isolate as the sole protein source (EtOH/SOY) an EtOH liquid diet supplemented with genistein (EtOH/GEN) and a chow group. EtOH feeding, final concentration 5% (v/v), continued for 16 wks. As expected, EtOH increased both the incidence and multiplicity of both basophilic lesions and adenomas compared to the chow fed group, (p<0.05). Soy protein supplementation in the EtOH/SOY group significantly reduced adenoma progression when compared to the EtOH and EtOH/GEN group, (p<0.05). Genistein supplementation alone in the EtOH diet had no protective effect. In saline-treated mice, soy feeding significantly reduced serum ALT concentrations (p<0.05), decreased hepatic TNFα and CD-14 expression and decreased nuclear accumulation of NFκB protein in the EtOH/SOY-treated mice compared to the EtOH group (p<0.05). With respect to ceramides, high resolution MALDI-FTICR Imaging mass spectrometry revealed changes in the accumulation of long acyl chain ceramide species, in particular C18, in the EtOH group when compared to the EtOH/SOY group. Additionally, expression of the enzymes acid ceramidase and sphingosine kinase 1 which degrade ceramide into sphingosine and convert sphingosine to sphingosine-1-phosphate respectively and expression of sphingosine-1-phosphate receptors S1PR2 and S1PR3 were all upregulated by EtOH and suppressed in the EtOH/SOY group, p<0.05. Chronic EtOH feeding also increased hepatocyte proliferation and mRNA expression of β-catenin targets, including cyclin D1, MMP7 and glutamine synthase, which were reduced in the EtOH/SOY group, p<0.05. These findings suggest that soy prevents tumorigenesis by reducing pro-inflammatory signaling resulting from EtOH-induced hepatic injury, and by reducing hepatocyte proliferation through inhibition of EtOH-mediated β-catenin signaling. These mechanisms may involve blockade of sphingolipid signaling.
Introduction
Alcohol-induced liver cancer occurs via several different interacting mechanisms at the level of both initiation and promotion [1, 2]. Formation of acetaldehyde and reactive oxygen species results in DNA damage as the result of ethanol (EtOH) metabolism by alcohol dehydrogenase and CYP2E1, and reduced DNA methylation as a result of disruption of one carbon metabolism may result in tumor initiation [2]. In addition, there is data demonstrating that EtOH can act as a tumor promoter [3, 4]. Our laboratory and others have shown that EtOH stimulates hepatocyte proliferation in rodent models coincident with development of liver injury and depletion of hepatic retinoid stores [4]. Treatment with retinoic acid can reverse the increase in hepatocyte proliferation after EtOH exposure [5]. Down regulation of retinoic acid receptor (RAR) signaling by use of a dominant negative has also been shown to increase hepatocyte proliferation and liver tumor promotion as a result of increased Wnt-β-catenin signaling [6]. Recently, we have developed a mouse model of EtOH-induced liver tumor promotion in which, in combination with tumor initiation during early development by the nitrosamine chemical carcinogen diethylnitrosamine (DEN). In this model, EtOH consumption during adulthood results in increased multiplicity of liver adenomas [4, 7]. We have shown that increased tumor promotion was associated with necroinflammatory injury, fibrosis, retinoid depletion and increased β-catenin signaling in both hepatocytes and the tumors in this model [4, 7]. Clinically, a significant percentage of liver tumors from alcoholics have also been shown to be β-catenin positive [8]. In many of these cases, mutations have been found in β-catenin, the phosphorylation site of GSK3β or in Axin resulting in β-catenin stabilization [9]. However, we did not observe β-catenin mutations in the mouse DEN-EtOH model [4, 7]. Transgenic mice expressing mutations in the β-catenin pathway do not have increased tumor initiation suggesting, consistent with our EtOH model, that increased Wnt-β-catenin signaling contributes to tumor promotion [8].
If this hypothesis is correct, then compounds which inhibit β-catenin signaling should be tumor protective in the mouse DEN-EtOH model. In this regard, there is ample epidemiological evidence and experimental data to suggest that dietary factors found in soy foods such as soy protein isolate (SOY) are cancer protective in multiple tissues. Meta-analyses have demonstrated reductions in risk of mammary, prostate and colon cancer in soy consumers [10]. In addition, animal studies of chemical carcinogenesis have shown protection against DMBA- and NMU-induced mammary tumors and against AOM-induced colon tumors after consumption of SOY [11–13]. Cancer protective effects of SOY have been ascribed to the presence of the isoflavone genistein in soy foods [14] working via several pathways including inhibition of cellular proliferation; induction of apoptosis; inhibition of angiogenesis and through anti-oxidant effects [14]. Genistein and SOY have also been suggested to interfere with Wnt-β-catenin signaling in a rat model of AOM-induced colon cancer coincident with significant decreases in formation of aberrant crypts [15]. In addition, in cell culture studies, genistein has been shown to inhibit various components of the Wnt-β-catenin signaling pathway [16–18] and has been shown to induce apoptosis in hepatocellular carcinoma cell lines [19]. The present study was designed to determine if feeding SOY or pure genistein at concentrations found in SOY diets are protective against EtOH-induced liver tumorigenesis in the mouse DEN-ETOH model and if protection was observed, it was associated with inhibition of β-catenin activation.
Materials and Methods
In Vivo Mouse Model of DEN-EtOH Liver Tumor Promotion
DEN-treated and saline-treated male C57Bl6 mice received EtOH-containing LieberDe Carli diets for 16 wks as previously described [29]. Briefly, DEN-injected mice (PND 13) were randomly assigned to four weight-matched diet groups: a chow diet (n=10, chow), an EtOH-containing liquid diet (n=21, EtOH) and an EtOH-containing liquid diet containing soy protein isolate (n=23, EtOH/SOY), and an EtOH-containing liquid diet containing genistein, 250 mg/kg diet, a level comparable to the concentration of genistein in SOY (n=24, EtOH/GEN). All groups had access to water ad libitum. Liquid diets were formulated according to the LiebeDeCarli diet of 35% of energy from fat, 18% from protein, and 47% from carbohydrates (Dyets, Inc., Bethlehem, PA). EtOH was added to the Lieber-DeCarli liquid diet slowly by substituting EtOH for carbohydrate calories in a stepwise manner until 28% total calories were reached as previously described [4]. This dose constitutes a final EtOH concentration of 5.0% (v/v). Additionally, saline-injected mice were randomized into three liquid diet groups, a chow diet (n=5), an EtOH (n=10), an EtOH/SOY (n=10) for 16 wks [29].
Tumor Pathology
For each DEN-treated mouse, formalin fixed lobes were embedded in paraffin, sectioned at 4 μm, stained with H&E, and examined under a light microscope and scored in a blinded manner by a veterinary pathologist (L.H). Within each lobe, lesions were counted at 40x magnification. Tumors were defined as follows – adenomas, a compressive lesion of any size without evidence of invasion or other criteria of malignancy; hepatocellular carcinoma, a compressive and invasive lesion with criteria of malignancy [4].
Biochemical Analysis of Liver Injury and Inflammation
Liver necrosis was assessed by measurement of serum alanine amino transferase (ALT) activity as described previously [4]. Kupffer cell activation (CD14 mRNA expression) and inflammation (expression of cytokine TNFα and IL-6 mRNA and the chemokine CXCL2 mRNA) were measured by real time RT-PCR analysis of expression of individual cDNA samples prepared from each group using SYBR green and an ABI 7500 sequence detection system (Applied Biosystems, Foster City, CA). Primer sequences are given in Table 1. Gene expression was normalized against 18S rRNA. In addition, nuclear fraction and cytosolic fractions were isolated from livers using NE-PER Nuclear and Cytoplsmic Extraction kit (Thermo Fisher Scientific) as per manufacturer’s instructions. Nuclear proteins were separated by SDS-PAGE and Western blotted with antibodies against the p65 subunit of NFκB (Cell Signaling, Danvers, MA). Protein loading was corrected for by staining for total protein with 0.1% amido black.
Table 1.
Real-Time RT-PCR primer sequences
| Gene | Forward Sequence (5'-3') | Reverse Sequence (5'-3') |
|---|---|---|
| TNFα | GACGTGGAACTGGCAGAAGAG | GCCACAAGCAGGAATGAGAAG |
| IL-6 | CTT CACAAGT CGGAGGCTTAAT | GCAAGTGCATCATCGTTGTTC |
| CXCL-2 | TAAGCACCGAGGAGAGTAGAA | GTCCAAGGGTTACTCACAACA |
| CD14 | CTAAGTATTGCCCAAGCACACTCA | CCCAACTCAGGGTTGTCAGACA |
| CyclinDl | TGCTGCAAATGCAACTGCTTCTTG | AAGGTCTGTGCATGTTTGCGGATG |
| GluS | TATTCCTCGTGCCCAGTTAATC | AAGAAAGGGTTGGTGTGTAGAG |
| MMP-7 | GACTTGCCTCGGTTCTTAGTAG3 | CCC TTGCGAAGCCAATTATG |
| SPTLC2 | CAGGAGCGTTCTGATCTTACAG | CCGGACACGATGTTGTAGTT |
| CerS1 | GCCTGACATTCCGTACTACTTC | GTCTTCCAGTTCACGCATCT |
| SPKH1 | GGTACGAGCAGGTGACTAATG | GGACAGACTGAGCACAGAATAG |
| ASAH1 | GTCCTCAACAAGCTGACTGTAT | CTATACAAGGGTCTGGGCAATC |
| S1PR1 | TTCACTCTGCTCCTGCTTTC | CTGGCCTTGGAGATGTTCTT |
| S1PR2 | CAACGGAGGCACTGACTAAT | TGGCAAATGTCTAGCCCTAAG |
| S1PR3 | GGGAGGCGTGATGTAGTTATTT | CAGAGGTGTCTTCTACGCATTT |
Analysis of Ceramide-Sphingosine Signaling
Ceramide species were detected in the livers of saline-treated mice receiving an EtOH or EtOH/SOY diet by MALDI-FTICR imaging mass spectrometry as previously described [21,29]. MALDI-IMS analysis was performed using a Bruker Solarix 7T FTICR mass spectrometer, equipped with a SmartBeam II laser operating at 1000 Hz, collecting spectra across the entire tissue in positive ion mode between (m/z 200–2000). A laser spot size of 25μm, and a raster width of 200 μm for general profiling or 75 μm for high resolution images was empolyed collecting 800 shots per pixel. Data was reduced to .98 ICR reduction and loaded into FlexImaging 4.0 software (Bruker Daltonics) for data analysis, and generation of lipid images of interest. Within FlexImaging, all data was normalized using root mean square and intensities were thresholded appropriately. Lipid species were assigned by mass accuracy, both to an internal ceramide database and to an external database Lipid Maps. mRNA expression of enzymes and receptors involved in ceramide-sphingosine signaling were quantitated by real time RT-PCR analysis of expression of individual cDNA samples prepared from each group using SYBR green and an ABI 7500 sequence detection system (Applied Biosystems, Foster City, CA). Primer sequences are given in Table 1. Gene expression was normalized against the housekeeping gene GAPDH mRNA. In addition, liver microsomes were prepared as previously described [20] and used for Western blot analysis with an antibody against sphingosine kinase H1 (Cell Signaling, Danvers, MA). Protein loading was corrected for by staining total protein with 0.1% amido black.
Hepatocyte Proliferation and β-Catenin Signaling
Hepatocyte proliferation was measured by histochemical analysis of PCNA staining as described previously [22]. Nuclear expression of β-Catenin protein was measured by Western blot [4]. In addition, mRNA expression of known downstream β-Catenin target genes cyclin D1, glutamine synthase (GluS) and matrix metalloproteinase 7 (MMP7) were measured by real time RT-PCR as described above and expressed relative to GAPDH mRNA.
Data and Statistical Analysis
Data are presented as mean ± SEM. For ALT, gene and protein expression data multiple group comparisons were made by One Way ANOVA or ANOVA of Ranks followed by Student-Neuman-Keuls post-hoc analysis. Adenoma incidence was determined using Fisher’s Exact Test. Multiplicity was determined One-Way ANOVA followed by Mann-Whitney U rank-sum test for post hoc comparisons. Statistical analysis was performed using Sigma Plot software package 11.0 (Systat Software, Inc. San Jose, CA) and Stata statistical software 13.1 (Stata Corporation, College Station, TX). Statistical significance was set at P<0.05.
Results and Discussion
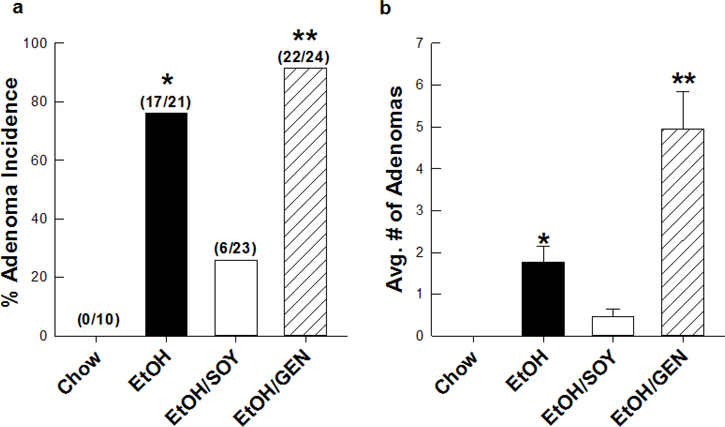
There is strong epidemiological data that alcohol can act as a tumor promoter. Chronic alcohol consumption increases the risk of HCC a further 2-fold when combined with factors such as HCV/HBV infection or diabetes [2]. We have developed a mouse model which replicates this phenomenon in which increased adenoma multiplicity occurs in mice where tumors are initiated with DEN on PND 13 and EtOH is administered chronically for 16 weeks in Lieber DeCarli liquid diets beginning in adulthood [4,7]. We have previously shown that at a final concentration of 28% total calories, the blood alcohol concentrations attained in this model average 75 mg/dL which is comparable to the human 80 mg/dL limit for DWI [7]. Tumor promotion was accompanied by appearance of steatohepatitis, fibrosis and stimulation of Wnt-β-cantenin-dependent hepatocyte proliferation coincident with loss of hepatic retinoids [4,7]. In the current study, we examined the possible protective effects of feeding soy protein isolate and of a major soy-associated phytochemical genistein in the DEN-EtOH mouse tumor promotion model based on literature showing ant-tumorigenic effects and inhibition of Wnt-β-catenin signaling in other cancer models. As expected, chronic EtOH feeding of adult mice as part of Lieber DeCarli liquid diets resulted in appearance of adenomas in 81% of DEN-treated mice with a multiplicity of 2 tumors/mouse (Figure 1). When the protein source in the EtOH-Lieber DeCarli diet was switched from casein to SOY, adenoma incidence was reduced to 26% and multiplicity was reduced by 75% (P<0.05) (Figure 1). Surprisingly, supplementation of the EtOH-Lieber DeCarli diet with 250 mg/kg diet genistein, a level comparable to that found in SOY actually increased adenoma incidence to 92% and multiplicity to 5 tumors/mouse (P<0.05). These data suggest that SOY contains factors that inhibit EtOH-induced tumor promotion but that the bioactive component may be either a phytochemical other than genistein or a protein/peptide. SOY contains over 100 phytochemicals and peptides and the major soy storage protein β-conglycenin may also give rise to bioactive peptides after digestion [23 24]. Identification of this cancer protective component of SOY remains the subject of future studies.
Figure 1.
Adenoma incidence (a) and tumor multiplicity (b) in DEN-treated male mice receiving a standard LieberDeCarli EtOH liquid diet using casein (EtOH) or soy protein isolate (EtOH/SOY) as the sole protein source as previously published [, 29]; and with a third group (EtOH/GEN) receiving the EtOH liquid diet supplemented with (250mg/kg diet) genistein for 16 wks. Data expressed as mean ± SEM. Adenoma incidence was determined using Fisher’s Exact Test. Multiplicity was determined One-Way ANOVA followed by Mann-Whitney U rank-sum test for post hoc comparisons, *p<0.05 EtOH vs. EtOH/SOY, **p<0.05 EtOH vs. EtOH/GEN.
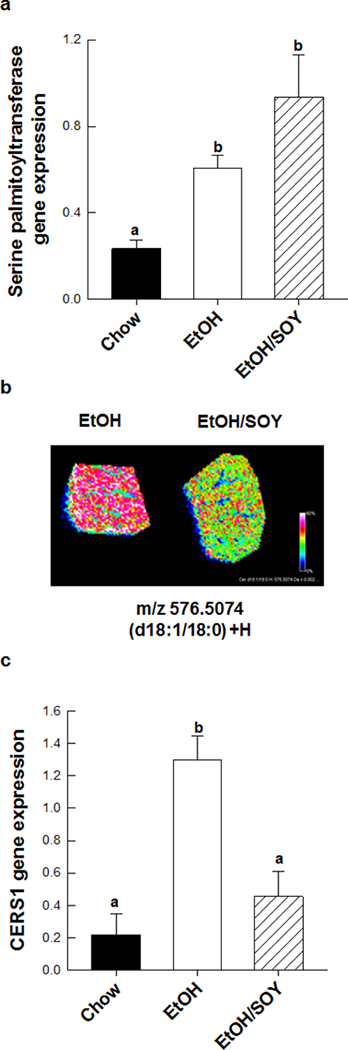
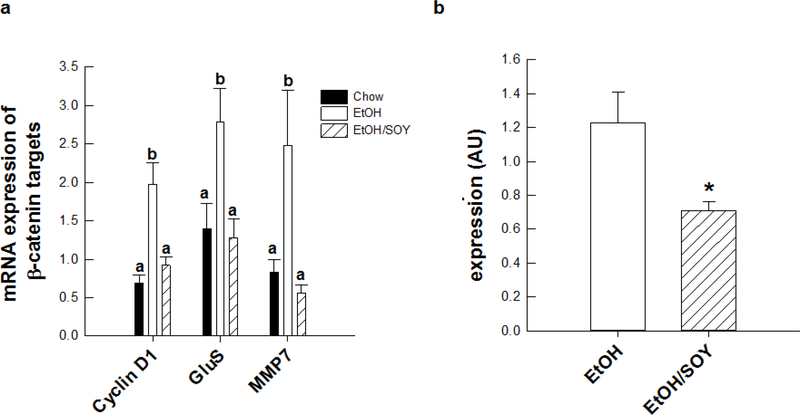
We conducted further analyses to examine the molecular mechanisms underlying the promotional protection afforded by feeding SOY with EtOH. In saline-treated mice, the EtOH/SOY group had significantly reduced necroinflammatory injury and Kupffer cell activation with lower levels of serum ALT, hepatic mRNA expression of CD14, TNFα, IL-6, CXCL-2 and nuclear expression of the NFκB p65 subunit when compared to the EtOH group (P<0.05) (Table 2). EtOH feeding increased hepatic concentrations of ceramide and hepatic mRNA expression of enzymes involved in synthesis of ceramide, sphingosine and sphingosine-1-phosphate (S1P); (ceramide synthase 1 – CERS1; acid ceramidase – ASAH1; serine palmitoyl transferase and sphingosine kinase-1 –SPKH1); compared to chow controls (P<0.05) and increased SPKH1 protein expression (Table 3, Figure 2). In addition mRNA encoding S1P receptors S1PR2 and S1PR3 were increased in the EtOH group relative to chow controls (Table 3). In contrast, feeding SOY with EtOH blocked the effects of EtOH on CERS1 mRNA and reduced hepatic ceramide concentrations compared to the EtOH group (P<0.05) (Figure 2). In addition, the EtOH/SOY group had lower SPKH1 mRNA and protein and reduced expression of S1PR2 and S1PR3 mRNA (Table 3). As previously reported, coincident with its promotional effects, EtOH increased hepatocyte proliferation in a β-catenin-dependent manner. EtOH increased expression of cyclin D1 mRNA and mRNA expression of other downstream β-catenin targets MMP7 and glutamine synthase (P< 0.05) (Figure 3). In saline-treated mice, feeding SOY with EtOH reversed these effects (P<0.05). Relative the EtOH group, the EtOH/SOY group had lower nuclear β-catenin protein (P<0.05) (Figure 3) and PCNA staining of proliferating hepatocytes was reduced from 2.5 ± 0.4 to 0.9 ± 0.1% (P<0.0.5). These data suggest that reduction in EtOH-dependent tumor promotional stimuli after SPI feeding are linked to lower necroinflammatory injury and normalization of ceramide/sphingosine signaling. Proliferative and regenerative repair responses are generally observed in the liver after injury [25]. It has been suggested that hepatocyte proliferation is a Wnt-regulated process linked to reduced retinoid signaling [5]. Sphingosine 1-phosphate signaling has been shown to negatively cross talk with retinoids and has been shown to activate hepatic stellate cells [26, 27]. Moreover, Wnt signals from other hepatic cell types including Kuffper cells have been shown to regulate hepatocyte proliferation under conditions of partial hepatectomy [28]. It remains to be seen if similar signals from activated Kupffer or stellate cells regulate hepatocyte and hepatic tumor cell proliferation after EtOH consumption and if the molecular mechanisms whereby SOY prevents these mechanisms are related to its effects on ceramide/sphingosine signaling pathways.
Table 2.
Biochemical analysis of liver injury and inflammatory response in saline-treated male mice receiving EtOH or EtOH/SOY diets for 16 wks [29].
| ALT | TNFα mRNA expression | IL6 mRNA expression | CD 14 mRNA expression | CXCL2 mRNA expression | NFkB nuclear expression | |
|---|---|---|---|---|---|---|
| Chow | 8.63±0.89a | 0.15±0.03a | 0.05±0.12a | 1.31±0.27a | 0.09±0.01a | -- |
| EtOH | 37.65±3.62b | 2.36±0.51b | 2.26±0.34b | 43.37±0.37b | 0.91±0.15c | 1.07±0.16 |
| EtOH/SOY | 8.67±1.58a | 1.23±0.21a | 0.72±0.13a | 2.71±0.29a | 0.46±0.11ab | 0.55±0.08* |
Data is expressed as mean ± St.Err; Groups: chow (n=5), EtOH (n=10), EtOH/SOY (n=10).
Liver injury was assessed by measuring serum alanine transferase (ALT), in S.F. units/ml (Ronis et at, 2011).
Gene expression was determined by real-time RT-PCR as previously described (Mercer et al., 2016), Significance, a<b<c, (p<0.05).
Table 3.
Changes in hepatic sphingosine signaling mediators in response to EtOH and EtOH/SOY diets in saline-treated male mice [29].
| mRNA expression (fold change) | ||||||
|---|---|---|---|---|---|---|
| SPKH1 membrane expression | ||||||
| SPKH1 | ASAH1 | S1PR1 | S1PR2 | S1PR3 | ||
| Chow | -- | 1.00±0.05a | 1.00±0.04a | 1.00±0.16a | 1.00±0.10a | 1.00±0.14a |
| EtOH | 1.60±0.15 | 23.45±5.67b | 1.70±0.29a’b | 0.96±0.22a | 3.59±0.93b | 2.64±0.54b |
| EtOH/SOY | 0.70±0.08* | 9.12±2.20a | 0.79±0.17a | 0.54±0.09a | 1.31±0.28a | 1.36±0.30a |
Data is expressed as mean ± St.Err; Groups: chow (n=5), EtOH (n=10), EtOH/SOY (n=10). Gene expression and protein determination were determined as previously described (Mercer et al., 2016), Significance, a<b<c, (p<0.05), Student’s T-Test
p<0.05.
Figure 2.
Alcohol feeding increased de novo ceramide synthesis as demonstrated by increased mRNA expression of (a) serine palmitoyltransferase; EtOH-specific increases in C18 ceramide (d18:1/18:0) as observed by (b) high resolution MALDI-FTICR imaging mass spectrometry, and (c) increased ceramide synthase 1 mRNA expression were prevented in EtOH/SOY-treated mice [29]. Significance a<b<c, (p<0.05).
Figure 3.
Changes in mRNA expression of (a) β-catenin targets, cyclin D1, GluS, and MMP7, and (b) nuclear expression of β-catenin in saline-treated mice receiving the EtOH or EtOH/SOY diet for 16 wks. Significance a<b<c, (p<0.05), Student T-test, *p<0.05 [29].
Acknowledgements
These studies were funded in part by NCI R21 CA169389 (M.J.R.) and the Lyon Foundation (K.E.M.).
References
- 1.Morgan TR, Mandayam S, Jamal MM (2004) Alcohol and hepatocellular carcinoma. Gatsroenterology 127:S87–S96. [DOI] [PubMed] [Google Scholar]
- 2.Seitz HK, Stickel F (2007) Molecular mechanisms of alcohol mediated carcinogenesis. Nat. Rev. Cancer 7:599–612. [DOI] [PubMed] [Google Scholar]
- 3.Brandon-Warner E, Walling TL, Schrum LW, McKillop IH (2012) Chronic ethanol feeding accelerates hepatocellular carcinoma progression in a sex-dependent manner in a mouse model of hepatocarcinogenesis. Alc. Clin. Exp. Res 36:641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercer KE, Hennings L, Sharma N, Lai K, Cleves MA, Wynne RA, Badger TM, Ronis MJJ (2014) Alcohol consumption promotes diethylnitrosamine-induced hepatocarcinogenesis in male mice through activation of the Wnt/β-catenin signaling pathway. Cancer Prev. Res 7:675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung J, Liu C, Smith DE, Seitz HK, Russell RM, Wang XD (2001). Restoration of retinoic acid concentrations suppress ethanol-enhanced c-Jun expression and hepatocyte proliferation in rat liver. Carcinogenesis 22:1213–1219. [DOI] [PubMed] [Google Scholar]
- 6.Yanagitani A, Yamada S, Yasui S, Simomura T, Marai R, Murawaki Y, Hashiguchi K, Kanbe T, Saeiki T, Ichiba M, Tanabe Y, Yoshida Y, Morino S, Kurimasa A, Usuda N, Yamazaki H, Kunisada T, Ito H, Murawaki Y, Shiota G (2004). Retinoic acid receptor alpha dominetn negative form causes stetohepatitis and liver tumors in transgenic mice. Hepatology 40:366–375. [DOI] [PubMed] [Google Scholar]
- 7.Mercer KE, Hennings L, Badger TM, Ronis MJJ (2015) Alcohol consumption, Wnt/β-catenin signaling and hepatocarcinogensis. Adv. Exp. Biol. Med 815:185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edamoto Y, Hara A, Biernat W, Terracciana L, Cathomas G, Riehle HM, Matsuda M, Fujii H, Scoazec JY, Ohgaki H (2003). Alterations in RB1, p53 and Wnt pathways in hepatocellular carcinomas associated with hepatitis C, hepatitis B and alcoholic liver cirrhosis. Int. J. Cancer 106:334–341. [DOI] [PubMed] [Google Scholar]
- 9.Nejak-Bowen KN, Monga SP, (2011). Beta catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin. Cancer Biol 21:44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badger TM, Ronis MJJ, Simmen R, Simmen F (2005) Soy protein isolate and protection against cancer. J. Am. Coll. Nutr 24:146S–149S. [DOI] [PubMed] [Google Scholar]
- 11.Hakkak R, Korourian S, Shelnutt SR, Lensing S, Ronis MJJ, Badger TM (2000). Diets Containing Whey Protein or Soy Protein Isolate Protect Against DMBA- Induced Mammary Tumors in Female Rats, Cancer Epidemiology Biomarkers and Prevention 9:113–117. [PubMed] [Google Scholar]
- 12.Simmen RC, Eason RR, Till SR, Chatman L Jr, Velarde MC, Geng Y, Korourian S, Badger TM (2005) Inhibition of NMU-induced mammary tumorigenesis by dietary soy. Cancer Lett. 224:45–52. [DOI] [PubMed] [Google Scholar]
- 13.Hakkak R, Korourian S, Ronis MJJ, Johnston S, Badger TM (2001) Lifetime soy protein isolate consumption protects against azoxymethane-induced colon tumors in male rats, Cancer Letts. 166:27–32. [DOI] [PubMed] [Google Scholar]
- 14.Adjakly M, Ngollo M, Boiteux J-P, Bignon Y-J, Guy L, Bernard-Gallon D (2013) Geniostein and daidzein: Different molecular effects on prostate cancer. Anticancer Res. 33:39–44. [PubMed] [Google Scholar]
- 15.Zhang Y, Li Q, Zhou D, Chen H (2013) Genistein, a soya isoflavone, prevents azoxymethane-induced up-regulation of Wnt/β-catenin signaling and reduces colon pre-neoplasia in rats. Br. J. Nutr 109:33–42, [DOI] [PubMed] [Google Scholar]
- 16.Su Y, Simmen RC (2009) Soy isoflavone genistein upregulates epithelial adhesion moleculae E-cadherin expression and attenuates beta catenin signaling in mammary epithelial cells. Canrcinogenesis 30:331–339. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Chen H (2011) Genistein attenuates WNT signaling by upregulating sFRP2 in a human colon cancer cell line. Exp. Biol. Med 236:714–722. [DOI] [PubMed] [Google Scholar]
- 18.Park S, Choi J (2010) Inhibition of beta-catenin/Tcf signaling by flavonoids. J. Cell Biochem 110:1376–1385. [DOI] [PubMed] [Google Scholar]
- 19.Dastjerdi MN, Kavoosi F, Valiani A, Esfandiari E, Sanaei M, Sobhanian S, Hakemi MG, Mobarakian M. (2015) Inhibitory effect of genostein on PLC/PRF5 hepatocellular carcinoma cell line. Int. J. Prev. Med 6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronis MJJ, Badger TM, Chen Y, Badeaux J (2009) Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAR, LXR and SREBP-1c signaling, J. Nutr 139: 1431–1438. [DOI] [PubMed] [Google Scholar]
- 21.Jones EE, Dworski S, Canals D, Casas J, Fabrias G, Schoenling D, Levade T, Denlinger C, Hannun YA, Medin JA, Drake RR (2014) On-tissue localization of ceramides and other sphingolipids by MALDI mass spectrometry imaging Anal. Chem 86:8303–8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumgardner JN, Shankar K, Badger TM, Ronis MJJ (2007) Undernutrition enhances alcohol-induced hepatocyte proliferation in the liver of rats fed via total enteral nutrition, Am. J. Physiol 293:G355–364. [DOI] [PubMed] [Google Scholar]
- 23.Fang N, Yu S, Badger TM (2004) Comprehensive phytochemical profile of soy protein isolate. J. Agric. Food Chem 52:4012–4020. [DOI] [PubMed] [Google Scholar]
- 24.Burris RL, Ng H-P, Nagarajan S (2014) Soy protein inhibits inflammation-induced VCAM-1 and inflammatory cytokine induction by inhibiting NFkB and Akt signaling pathway in apolipoprotein E-deficient mice. Eur. J. Nutr 53:135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehendale H (2005) Tissue repair: an important determinant of final outcome of toxicant-induced injury. Toxicol. Pathol 33:41–51. [DOI] [PubMed] [Google Scholar]
- 26.Osawa Y, Nagaki M, Banno Y, Nozawa Y, Moriwaki H, Nakashima S (2001) Sphingosine kinase regulates hepatoma cell differentiation: roles of hepatocyte nuclear factor and retinoid receptor. Biochem. Biophys. Res. Cummun 286:673–677. [DOI] [PubMed] [Google Scholar]
- 27.Bi Y, Li J, Ji B, Kang N, Yang L, Simonetto DA, Kwon JH, Kamath M, Cao S, Shah V (2014) Sphinogosine-1-phosphate mediates a reciprocal signaling pathway between stellate cells and cancer cells that promotes pancreatic cancer growth. Am. J. Pathol 184:2791–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Mowry LE, Nejak-Bowen KN, Okabe H, Diegel CR, Lang RA, Williams BO, Monga SP (2014) β-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation! Hepatology 60: 964–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercer KE, Pulliam CF, Hennings L, Lai K, Cleves MA, Jones EE, Drake RR, and Ronis MJJ (2016) Soy protein isolate protects against ethanol-mediated adenoma progression in DEN-treated male mice. Cancer Prev. Res (Submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]