Abstract
A water soluble metallosupramolecular hexagon containing pendant methyl viologen (MV) and trimethylammonium units at the vertices has been synthesized via an organoplatinum(II)←pyridyl coordination driven self-assembly reaction. The MV units of the metallacycle were further utilized in the formation of a hetero-ternary complex with cucurbit[8]uril and a galactose functionalized naphthalene derivative, yielding a metallacycle-cored carbohydrate cluster that was subsequently ordered into nano-spheres and tapes, depending upon the concentration.
Graphical Abstract:
Carbohydrates play crucial roles in various biological processes: bacterial or viral infection, fertilization, immune response, tumor-cell metastasis, etc., via enabling specific recognition interactions between a glyco-cluster and a protein.1 Given these intricate functions, various synthetic carbohydrate containing functional scaffolds including glycoproteins, polymers, dendrimers, carbon nano-materials, calixarenes, pillararenes, etc. have been prepared via covalent synthesis.2 In contrast to the tedious covalent approach, supramolecular chemistry, in particular coordination driven self-assembly via metal-ligand bonding is a viable strategy, whereby a fast and efficient one pot synthesis of diverse supramolecular coordination complexes (SCCs) can be accomplished.3 Due to their well-defined shapes and sizes, SCCs are excellent platforms to install functional units either on the periphery or at the vertices, resulting in functional systems, such as sensors and catalysts, etc.4 Functionalized SCCs are currently synthesized from appropriate prefunctionalized precursors whose preparations involve some additional covalent synthesis of organic ligands or metal acceptors.5 Recently, hierarchical self-assembly techniques have emerged as a new strategy, wherein multiple orthogonal interactions between molecular precursors can produce functional structures while keeping the synthetic steps at a minimum. For example, a precise unification of coordination-driven self-assembly with crown ether based host-guest interactions leads to the formation of robust metallacycle-cored supramolecular polymer gels in organic solvents.6 The hierarchical approach is, however, much less explored in aqueous medium due to the intrinsic hydrophobicity of SCCs, restricting the implementation of biologically relevant functionalities, such as sugars, into the SCC platforms.7
Cucurbit[n]urils (CB[n], n = 5–8, 10, 14) are a family of pumpkin-shaped macrocyclic cavitands, having repetitive glycoluril units interconnected by methylene groups.8 Among the CB[n] homologues, CB[8] is capable of accommodating two identical or different small guest molecules in its cavity in water.9 For example, amino acids and peptides form non-covalent homo-dimers (1:2) with CB[8], while hetero-dimerizations (1:1:1) were observed with dicationic species such as methyl viologen (MV), 2,7-dimethyldiazaphenanthrenium and electron-rich aromatic molecules such as naphthalene, indole etc. (Ka ≥ 1011 M−2).10 This unique property of CB[8] has been utilized for the preparation of microcapsules for on-demand release, tunable emission, supramolecular polymerization, catalysis, protein dimerization, enzymatic activity, etc.11
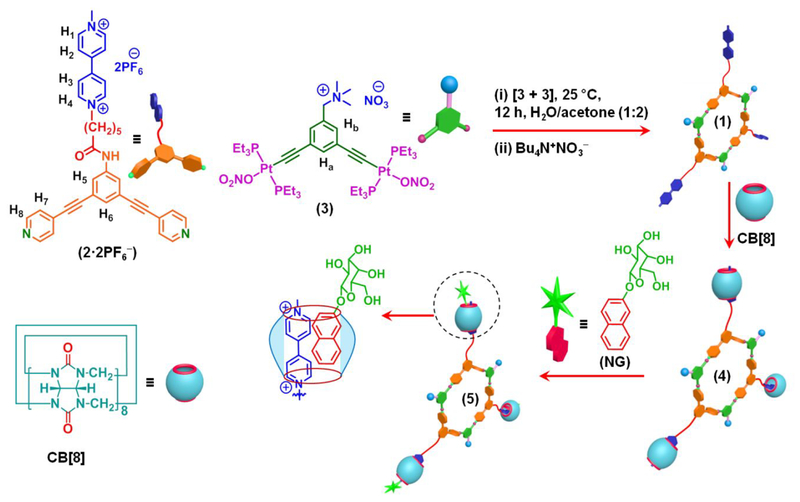
Herein, we combine the organoplatinum(II)←pyridyl coordination driven self-assembly with CB[8] mediated host-guest interactions to report the synthesis of a metallacycle-cored carbohydrate cluster (Scheme 1) that subsequently formed various nanostructures, such as nano-spheres and tapes, depending upon the concentration. In these hierarchical nanostructures, the metal-ligand coordination provides the first level of organization, while host-guest interaction and hydrogen bonding derived from CB[8] and sugar units, enable the higher order organizations. The approach described herein provides a facile strategy to prepare carbohydrate functional materials that can be useful for biomedical research. As shown in Scheme 1, the synthesis of hexagon 1 requires a 120° dipyridyl donor 2·2PF6− and a 120° organoplatinum acceptor 3. The former was prepared by a 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide hydrochloride (EDC·HCl) mediated amidation reaction between 612 and 713 in 70% yield (Scheme 2A). While the multi-step synthesis of the new metal acceptor 3 commenced with a nucleophilic substitution reaction that transformed 814 into 9 (Scheme 2B), a palladium-catalyzed Sonogashira reaction of 9 with (trimethylsilyl)acetylene delivered a trimethylsilyl-protected acetylene derivative that provided precursor 10 after hydrolysis (65% isolated yield). Compound 10 was then reacted with 4 equiv of trans-PtI2(PEt3)2 to prepare 11, which upon reaction with methyl iodide, followed by anion metathesis with AgNO3 resulted in 3 in good yield. Subsequently, the self-assembled [3 + 3] hexagon 1 was obtained by stirring a mixture of MV-functionalized 120° dipyridyl donor, 2·2PF6−, and 120° organoplatinum(II) acceptor 3 in a 1:1 stoichiometry in H2O/acetone (1:2 v/v) at room temperature for 12 h, followed by anion exchange using tetrabutylammonium nitrate (Scheme 1). The resulting hexagon 1 is water soluble, although the corresponding precursors 2·2PF6− and 3 are water insoluble.
Scheme 1.
Preparation of discrete metallacyclic hexagon 1, and cartoon representation of the formation of hetero-ternary host-guest complex 5 from 1, CB[8] and NG.
Scheme 2.
Synthesis of (A) MV-functionalized dipyridyl donor 2·2PF6−, (B) TMA-pendent organoplatinum(II) acceptor 3.a aConditions: (i) EDC·HCl, DMAP, DMF, 48 h, room temperature, 70%; (ii) aq. Me2NH, K2CO3, THF, 6 h, 60 °C (90%); (iii) and (iv) (trimethylsilyl)acetylene, Pd(PPh3)4, CuI, Et3N, THF, 24 h, 60 °C; KOH, methanol, 24 h, room temperature, (95%); (v) trans-PtI2(Et3)2, CuI, Et2NH, toluene, room temperature, 16 h (57%); (vi) and (vii) MeI, CH2Cl2, reflux, 24 h; AgNO3, CH2Cl2, room temperature, 12 h (90%).
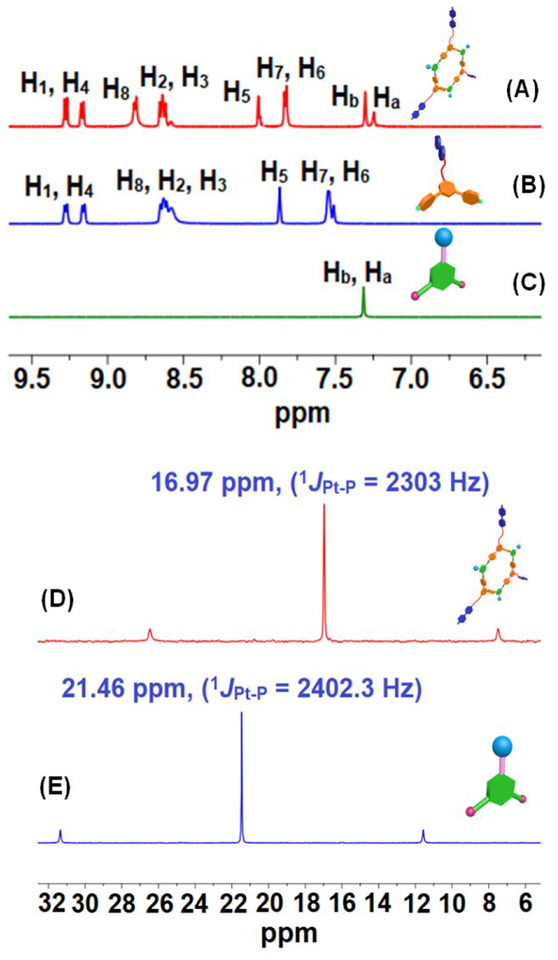
In the 1H NMR spectrum of 1 (Figure 1A), the pyridyl protons H8 and H7 of 2 showed downfield shifts (Δδ[H8] = 0.24 ppm; Δδ[H7] = 0.28 ppm) with reference to those of the free 2·2NO3− (Figure 1B) due to the coordination of the pyridyl N-atoms to the Pt centers. Likewise, the 31P{1H} NMR spectrum of 1 revealed a single sharp singlet at ~16.97 ppm with concomitant 195Pt satellites (JPt-P = 2303 Hz), consistent with a single phosphorus environment (Figure 1D). This signal shifted upfield by 4.49 ppm relative to that of acceptor 3 (Figure 1E). The decrease in the coupling of 195Pt satellites (ca. ΔJ = 99 Hz) in 1 relative to that of 3 is consistent with the electron backdonation from the platinum centers.
Figure 1.
(A-C) Partial 1H- and (D, E) 31P-NMR spectra (CD3OD, 25 °C) of (A,D) hexagonal metallacycle 1, (B) donor 2·2NO3−, and (C,E) acceptor 3.
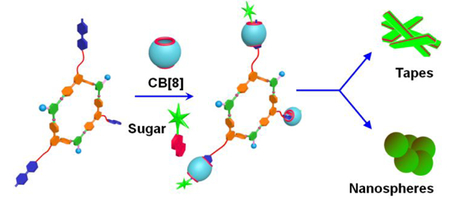
The formation of 1 was further supported by electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS). In the mass spectrum of 1, two peaks were identified that support the formation of a [3 + 3] assembly: m/z = 1387.51 Da for [M – 4ONO2]4+ and m/z = 1097.61 Da for [M – 5ONO2]5+. Both peaks were isotopically resolved and matched well with their calculated theoretical distributions (Figure S26, Supporting Information (SI)). All attempts to grow X-ray single crystals of 1 have thus far proven unsuccessful, likely due to the flexibility of the alkyl chains. PM6 semiempirical computations of 1 (Figure S27, SI) featured a planar hexagonal ring at the core with an internal diameter of 2.80 nm. The ring is surrounded by three MV units and three trimethylammonium (TMA) groups alternatively at the vertices. The host-guest complexation between 1 and CB[8] was studied by 1H-NMR experiments in D2O (Figure S29, SI). The signal at 9.05 and 9.15 ppm of 1 was assigned to the H1 and H4 protons of the MV units (Figure S28 and S29A, SI). They shifted upfield in 4 = [1·(CB[8])3], merged with the peak of pyridyl H8 protons and appeared as a broad peak at 8.8 ppm due to host-guest complexation (Figure S29B, SI). Upon subsequent hetero-ternary host-guest complexation with three equiv of NG, this peak of 4 split into two sets and appeared at 8.4 and 8.8 ppm, where the former was assigned to the H1 and H4 protons of the MV units and the latter was assigned to the pyridyl H8 protons. (Figure S29C, SI). At the same time, the NG protons appeared in the range of 7.4–8.0 ppm and experienced a significant upfield shift in 5 = [4·(NG)3], (Figures S29C and D, SI). These upfield shifts in the 1H-NMR spectra are diagnostic for the hetero-ternary complexation.15 The presence of galactose units does not interfere with such hetero-ternary complexation (Figure S30, SI).
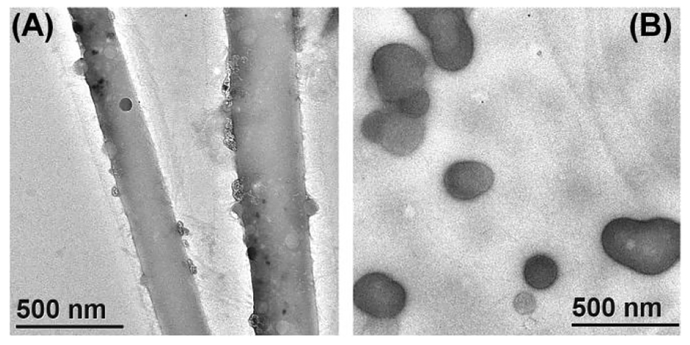
The morphologies of the self-assembled aggregates of 1, 4 and 5 in water were examined using transmission electron microscopy (TEM). Spherical nano-particles with a diameter of ca. 14 nm were formed by 1 at a concentration of 17 μM (Figure S31A, SI). A dynamic light scattering (DLS) experiment performed with a 17 μM aqueous solution of 1 showed a narrow size distribution (Figure S33A, SI). The average hydrodynamic diameter (Dh) of 1 was observed to be 15 nm in good accordance with the TEM results. The host-guest complex 4 formed globular aggregates with an average size of 100 nm (Figure S31B and S33B, SI). The hetero-ternary complex 5 showed tape-like morphology with widths of about 30–200 nm at a concentration of 17 μM (Figure S31C, SI). However, it is evident from the TEM image that the tape-like structure is not well developed (Figure S31C, SI). Nicely formed tapes with widths of about 200–300 nm were obtained after keeping the aqueous solution of 5 (17 μM) at room temperature for one week (Figure 2A).
Figure 2.
TEM images of the aggregates obtained from the aqueous solutions of 5 after standing for one week at concentrations of (A) 17 and (B) 3.4 μM respectively.
At a concentration of 3.4 μM, 5 exhibited lump-like aggregates (Figure S31D, SI) which transformed into nano-spheres with diameters of 80–200 nm upon standing of the aqueous solution at room temperature for one week (Figure 2B). DLS data revealed the average size of the nano-spheres to be ca. 140 nm (Figure S33C, SI), which is consistent with the TEM result (Figure 2B). Likewise, Yan et al. observed morphological transition from sphere-to-fiber/ribbon upon increasing the concentration of organoplatinum rhomboids having hydrophilic PEG groups at the vertices.16 Additionally, STEM-EDS (STEM, scanning transmission electron microscopy; EDS, energy-dispersive spectroscopy) spectra of the above samples further confirmed the presence of carbon, nitrogen, oxygen, phosphorus and platinum in the aggregates (Figure S32, SI). We further investigated the effect of adding an excess 2-naphthol to 5. Lumps-like aggregates of 5 were still observed after addition of three equiv (with respect to 1) of 2-naphthol (Figure S31G, SI), whereas upon addition of 9 equiv of 2-naphthol the lumps were disassembled (Figure S31I, SI).
In summary, a multi-cationic organoplatinum(II) hexagon 1 was prepared by means of coordination driven self-assembly. The cationic groups at the periphery of 1 overcome its hydrophobicity attributed to the hexagonal aromatic core and triethylphosphine groups and render it water soluble. The MV units of 1 form a non-covalent host-guest complex with CB[8] that can further undergo hetero-ternary complexation with NG forming 5. The host-guest complexation processes were accompanied by morphological transformations that were studied by 1H-NMR spectroscopy, TEM and DLS experiments. The hetero-ternary complex 5 resulted in a tape-like morphology after its aqueous solution was kept at room temperature for 1 week. The galactose units at the surface of the tape-like structures may endow them with interesting biofunction. Exploration of biological applications of these nano-structures is underway in our laboratory.
Supplementary Material
ACKNOWLEDGMENT
P.J.S. and S.D., respectively, thank the NIH (R01-CA215157) and SERB Indo-U.S. Postdoctoral fellowship for financial support.
Footnotes
Supporting Information
Experimental details, NMR spectra, and other materials. These material are available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.(a) Illescas BM; Rojo J; Delgado R; Martín NJ Am.Chem. Soc 2017, 139, 6018–6025. [DOI] [PubMed] [Google Scholar]; (b) Bhatia S; Camacho LC; Haag RJ Am. Chem. Soc 2016, 138, 8654–8666. [DOI] [PubMed] [Google Scholar]; (c) Sato S; Yoshimasa Y; Fujita D; Yagi-Utsumi M; Yamaguchi T; Kato K; Fujita M Angew. Chem., Int. Ed 2015, 54, 8435–8439. [DOI] [PubMed] [Google Scholar]
- 2.(a) Liu Y; Zhang Y; Wang Z; Wang J; Wei K; Chen G; Jiang MJ Am.Chem. Soc 2016, 138, 12387–12394. [DOI] [PubMed] [Google Scholar]; (b) Park S; Kim G-H; Park S-H; Pai Rathwell D; J.; Park J-Y; Kang Y-S; Shin I. J. Am. Chem. Soc 2015, 137, 5961–5968. [DOI] [PubMed] [Google Scholar]; (c) Zhang S; Moussodia R-O; Sun H-J; Leowanawat P; Muncan A; Nusbaum C-D; Chelling KM; Heiney PA; Klein ML; André S; Roy R; Gabius H-J; Percec V Angew. Chem., Int. Ed 2014, 53, 10899–10903. [DOI] [PubMed] [Google Scholar]; (d) Yu G; Ma Y; Han C; Yao Y; Tang G; Mao Z; Gao C; Huang FJ Am. Chem. Soc 2013, 135, 10310–10313. [DOI] [PubMed] [Google Scholar]; (e) Pasparakis G; Cockayne A; Alexander CJ Am. Chem. Soc 2007, 129, 11014–11015. [DOI] [PubMed] [Google Scholar]; (f) El-Boubbou K; Gruden C; Huang XJ Am. Chem. Soc 2007, 129, 13392–13393. [DOI] [PubMed] [Google Scholar]; (g) Wang H; Gu L; Lin Y; Lu F; Meziani MJ; Luo PG; Wang W; Cao L; Sun Y-PJ Am. Chem. Soc 2006, 128, 13364–13365. [DOI] [PubMed] [Google Scholar]
- 3.(a) Li Z; Yan X; Huang F; Sepehrpour H; Stang PJ Org. Lett 2017, 19, 5728–5731. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang S; McGuirk CM; Ross MB; Wang S; Chen P; Xing H; Liu Y; Mirkin CA J. Am. Chem. Soc 2017, 139, 9827–9830. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Xie T-Z; Wu X; Endres KJ; Guo Z; Lu X; Li J; Manandhar E; Ludlow JM; III.; Moorefield, C. N.; Saunders, J. M.; Wesdemiotis, C.; Newkome, G. R. J. Am. Chem. Soc 2017, 139, 15652–15655. [DOI] [PubMed] [Google Scholar]; (d) Mosquera J; Szyszko B; Ho SKY; Nitschke JR Nat. Commun 2017, 8, 14882. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Cook TR; Stang PJ Chem. Rev 2015, 115, 7001–7045. [DOI] [PubMed] [Google Scholar]
- 4.(a) Sinha I; Mukherjee PS Inorg. Chem 2018, 57, 4205–4221. [DOI] [PubMed] [Google Scholar]; (b) Chan AK-W; Ng M; Wong Y-C; Chan M-Y; Wong W-T; Yam VW-W J. Am. Chem. Soc 2017, 139, 10750–10761. [DOI] [PubMed] [Google Scholar]; (c) Bloch WM; Holstein JJ; Hiller W; Clever GH Angew. Chem., Int. Ed 2017, 56, 8285–8289. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Saha ML.; Yan X; Stang PJ Acc. Chem. Res 2016, 49, 2527–2539. [DOI] [PubMed] [Google Scholar]; (e) Sun B; Wang M; Lou Z; Huang M; Xu C; Li X; Chen L-J; Yu Y; Davis GL; Xu B; Yang H-B; Li X-PJ Am. Chem. Soc 2015, 137, 1556–1564. [DOI] [PubMed] [Google Scholar]; (f) Kishi N; Akita M; Kamiya M; Hayashi S; Hsu H-F; Yoshizawa MJ Am. Chem. Soc 2013, 135, 12976–12979. [DOI] [PubMed] [Google Scholar]
- 5.Zhou F; Li S; Cook TR; He Z; Stang PJ Organometallics 2014, 33, 7019–7022 [Google Scholar]
- 6.Yan X; Cook TR; Pollock JB; Wei P; Zhang Y; Yu Y; Huang F; Stang PJ J. Am. Chem. Soc 2014, 136, 4460–4463. [DOI] [PubMed] [Google Scholar]
- 7.(a) Datta S; Saha ML; Stang PJ Acc. Chem. Res 2018, 51, 2047–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wei P; Yan X; Huang F Chem. Soc. Rev 2015, 44, 815–832. [DOI] [PubMed] [Google Scholar]
- 8.Murray J; Kim K; Ogoshi T; Yao W; Gibb BC Chem. Soc.Rev 2017, 46, 2479–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assaf KI; Nau WM Chem. Soc. Rev 2015, 44, 394–418. [DOI] [PubMed] [Google Scholar]
- 10.(a) Datta S; Misra SK; Saha ML; Lahiri N; Louie J; Pan D; Stang PJ Proc. Natl. Acad. Sci. U.S.A 2018, 115, 8093–8098. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Barrow SJ; Kasera S; Rowland MJ; del Barrio J; Scherman OA Chem. Rev 2015, 115, 2320–12406. [DOI] [PubMed] [Google Scholar]
- 11.(a) Liu J; Lan Y; Yu Z; Tan CSY; Parker RM; Abell C; Scherman OA Acc. Chem. Res 2017, 50, 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ni X-L; Chen S; Yang Y; Tao ZJ Am. Chem. Soc 2016, 138, 6177–6183. [DOI] [PubMed] [Google Scholar]; (c) Zheng L; Sonzini S; Ambarwati M; Rosta E; Scherman OA; Herrmann A Angew. Chem., Int. Ed 2015, 54, 13007–13011. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Huang Z; Yang L; Liu Y; Wang Z; Scherman OA; Zhang X Angew. Chem., Int. Ed 2014, 53, 5351–5355. [DOI] [PubMed] [Google Scholar]; (e) Zhang K-D; Tian J; Hanifi D; Zhang Y; Sue AC-H; Zhou T-Y; Zhang L; Zhao X; Liu Y; Li Z-TJ Am. Chem. Soc 2013, 135, 17913–17918. [DOI] [PubMed] [Google Scholar]; (f) Dang DT; Nguyen HD; Merkx M; Brunsveld L Angew. Chem., Int.Ed 2013, 52, 2915–2919. [DOI] [PubMed] [Google Scholar]; (g) Reczek JJ; Kennedy AA; Halbert BT; Urbach AR J. Am. Chem. Soc 2009, 131, 2408–2415. [DOI] [PubMed] [Google Scholar]
- 12.Pollock JB; Cook TR; Stang. P. J. J. Am. Chem. Soc 2012, 134, 10607–10620. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi H; Harada A Biomacromolecules 2002, 3, 1163–1169. [DOI] [PubMed] [Google Scholar]
- 14.Gök Y; Noël T; Van der Eycken J Tetrahedron: Asymmetry 2010, 21, 2768–2774. [Google Scholar]
- 15.(a) Samanta SK; Moncelet D; Briken V; Isaacs LJ Am. Chem.Soc 2016, 138, 14488–14496. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kim H-J; Heo J; Jeon WS; Lee E; Kim J; Sakamoto S; Yamaguchi K; Kim K Angew. Chem., Int. Ed 2001, 40, 1526–1529. [PubMed] [Google Scholar]
- 16.Yan X; Li S; Cook TR; Ji X; Yao Y; Pollock JB; Shi Y; Yu G; Li J; Huang F; Stang PJ J. Am. Chem. Soc 2013, 135, 14036–14039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.