Abstract
Crosstalk between the gut microbiota and the host has attracted considerable attention owing to its involvement in diverse diseases. Chronic kidney disease (CKD) is commonly associated with hypertension and is characterized by immune dysregulation, metabolic disorder and sympathetic activation, which are all linked to gut dysbiosis and altered host-microbiota crosstalk. In this Review, we discuss the complex interplay between the brain, the gut, the microbiota and the kidney in CKD and hypertension and explain our brain-gut-kidney axis hypothesis for the pathogenesis of these diseases. Consideration of the role of the brain-gut-kidney axis in the maintenance of normal homeostasis and of dysregulation of this axis in CKD and hypertension could lead to the identification of novel therapeutic targets. In addition, the discovery of unique microbial communities and their associated metabolites and the elucidation of brain-gut-kidney signalling are likely to fill fundamental knowledge gaps leading to innovative research, clinical trials and treatments for CKD and hypertension.
Chronic kidney disease (CKD) affects approximately 10% of the global population and has a financial impact of ~$48 billion per year in the United States alone1. Hypertension is an important risk factor for CKD, and approximately 85–90% of patients with stage 3–5 CKD have hypertension2. Long-term hypertension leads to high intraglomerular pressure, which subsequently impairs glomerular filtration3. Thus, blood-pressure lowering is an important and widely used approach to slow CKD progression. Current management of early-stage CKD focuses on blood pressure control, reduction of protein and salt intake, prevention of acute kidney injury and glycaemic control4. No cure or strategy for prevention of CKD exists, and timely treatment is extremely challenging owing to a lack of symptoms in the early stages of the disease5. Moreover, with the exception of dialysis and kidney transplantation, effective treatments for end-stage renal disease (ESRD) are lacking. Thus, paradigm-shifting concepts and innovative approaches are needed to detect, manage, control and ultimately cure these diseases.
Increasing evidence indicates an important role of the gut microbiota in the development of hypertension and CKD. The gut microbiota constantly communicates with vital organ systems of the host, such as the brain6, bone marrow7, vasculature8, kidney9, immune system10 and autonomic nervous system (ANS)11,12. This communication contributes to the homeostasis and health of the host. Bone-marrow-derived immune cells are activated by the gut microbiota, leading to low-grade inflammation that affects the brain, ANS and the kidney via the circulation13–15. Peripheral stimuli influence the ANS to subsequently modify neural inputs to the kidney, intestine and lymphoid organs16. In addition, immune and gut microbiota-derived products affect renal function and have important effects on CKD17. Gut dysbiosis has an important role in many chronic diseases, and amelioration of this dysbiosis could be a potential strategy for the prevention and management of these diseases18.
In this Review, we provide evidence for a gut-kidney axis and its potential regulation by the brain. We describe the gut microbiota and its interactions with major components in the brain-gut-kidney axis, such as the neural, hormonal, bone marrow and immune systems, and discuss this communication in the context of CKD and hypertension.
The gut microbiota
The gut harbours trillions of microorganisms, including commensal bacteria (FIG. 1). Initial microbial colonization is generally accepted to occur transvertically during birth and to continuously evolve to a fairly stable, adult-like composition within the first 3–5 years of life19. However, evidence that the maternal microbiota affects the fetal microbiota has challenged this concept20.
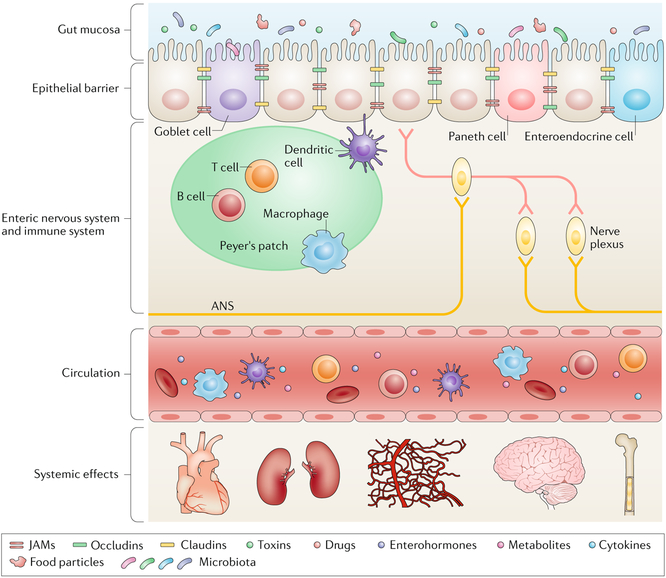
Fig. 1 |. The anatomy of the gut and its interactions with multiple systems.
The epithelial barrier, which is mainly composed of epithelial cells, goblet cells, Paneth cells and enteroendocrine cells, physically separates the gut mucosa from the submucosa. The gut mucosa is the most dynamic reservoir of the gut microbiota, which is constantly influenced and modified by factors including diet, toxins, pathogens and drugs. Tight junction proteins seal the epithelial layer and prevent translocation of pathogenic gut microorganisms across the epithelial barrier. Immune cells residing inside lymph nodes monitor the intestinal environment and maintain gut homeostasis. The enteric nervous system, which is composed of numerous nerve plexuses, perceives mechanical and chemical changes within the gut and communicates with the autonomic nervous system (ANS). Enterohormones, metabolites, immune cells and cytokines derived from this complex mucosal and submucosal network have systemic impacts on other organs such as the kidney, cardiovascular system, bone marrow and brain via the circulation. JAMs, junctional adhesion molecules.
The gut microbiota of adults can be divided into two major enterotypes according to the dominant bacterial phylotype; both of these enterotypes are strongly associated with long-term diet21. The predominant bacterial population of enterotype 1 is Bacteroides, which predominantly metabolize protein, whereas enterotype 2 contains predominantly saccharolytic Prevotella. Not surprisingly, microbial metabolite profiles (for example, short-chain fatty acids (SCFAs) and bile acids) are strongly associated with enterotypes22.
Dynamic evolution of the gut microbiota during early life begins with colonization by facultative anaerobic bacteria (predominantly Proteobacteria), followed by growth of anaerobic bacteria (generally Lactobacillus and Bifidobacterium) and, finally, diversification of bacteria — mainly different genera within the Bacteroidetes phylum — according to energy supply19. Neonates have an immature immune system that does not adequately respond to or defend against pathogenic invasions, which can potentially lead to severe infections. Breastfeeding and maternal interactions supply the conditions that are required for optimal development of the immune system23. Lactose, the primary carbohydrate of human milk, greatly promotes the growth of Lactobacillus and shapes the gut microbiota in infants24. The subsequent introduction of solid food substantially reshapes the gut microbiota, indicating that environmental factors have critical roles in determining its composition19.
Establishment of an intact gut-blood barrier, characterized by complete physiological and immunological protection, preserves the digestive and absorptive functions of the intestine and restricts the invasion of pathogens and toxic metabolites into the circulation. This essential process depends to a great extent on the presence of a balanced gut microbiota25. In adults, microbial metabolic pathways in the gut are fairly stable, although, as mentioned above, environmental factors, especially diet, profoundly modify the gut microbiota26. Age-related changes in the gut microbiota have also been identified in the elderly population, characterized by a decrease in diversity, contraction in saccharolytic bacteria, expansion in proteolytic bacteria, increases in certain Proteobacteria and a decline in Bifidobacterium counts27. Plasma markers of increased intestinal permeability are elevated in the healthy elderly population, indicating disruption of intestinal barrier function with ageing28. Moreover, probiotic supplementation has been shown to have healthy lifespan-promoting effects, including suppressing chronic low-grade inflammation and increasing longevity in mice29, indicating the importance of the gut microbiota in the maintenance of overall health.
The gut virome shows more interindividual variation and is less affected by environmental changes than is the gut microbiome30. However, the human gut virome carries a collection of hypervariable sequences that are considered to be a reservoir of viral evolution for adaption to a new environment31,32. In patients with type 1 diabetes mellitus, changes in the gut virome seem to precede the development of autoimmunity33, indicating a potential role of the virome in disease development. Fungal communities in the gut do not seem to cause illness directly but may exhibit dysbiosis that could potentially contribute to systemic inflammation34.
Gut physiology
The small intestine (duodenum, jejunum and ileum) and large intestine (colon) differ substantially in their structure and composition. For example, goblet cells are enriched in the proximal colon, whereas Peyer’s patches are primarily found in the small intestine35,36. In addition, the mucin layers are thinner and the microvilli are more numerous in the small intestine than in the colon. This diversity is associated with multidimensional functions that are important in host-microbiota interactions. Approximately 70% of the immune cells in the body reside in the gut; these cells maintain a balance of immune activation and tolerance to the gut microbiota37. The gut is also the second-most innervated organ in the body, facilitating communication with the brain38. The complex vascular bed of the gut enables efficient absorption of nutrients and water and maintains a gradient of oxygenation along the gastrointestinal tract39. The gut is one of the first major organs to encounter environmental factors such as diet, toxins and pathogens, and its interactions with endocrine, circulatory, neural and immune systems have a substantial impact on host physiological responses (FIG. 1).
Endocrines and metabolites.
Enteroendocrine cells are specialized endocrine cells of the gastrointestinal tract. Upon stimulus, these cells secrete hormones that are transported via the circulation to target receptors on recipient cells and regulate intestinal and/or systemic physiological functions40. Gut microbial metabolites, including SCFAs that are generated by the fermentation of dietary fibre, influence the host endocrine system. For example, the SCFA propionate stimulated release of glucagon-like peptide 1 (GLP1) and the gut hormone peptide YY (PYY) from murine primary intestinal cultures via a free fatty acid receptor 2 (FFAR2)-dependent mechanism41,42. Another study in mice reported that PYY was induced by gut microbiota in an FFAR3-dependent manner43. These results suggest a role of gut-microbiota-derived SCFAs in the production of endocrine hormones.
SCFAs also have multiple roles in the maintenance of intestinal homeostasis, including control of the balance between proliferation and apoptosis of intestinal epithelial cells44, induction of the secretion of endogenous antimicrobial peptides from intestinal epithelial cells45,46 and of the differentiation of regulatory T (Treg) cells47, modulation of cytokine production37 and maintenance of gut barrier function48. Therefore, enteroendocrine cell-derived hormones and gutmicrobiota-derived metabolites exert profound effects on gut homeostasis.
Neural control of the gut.
Intricate neural control of gastrointestinal function is achieved through the autonomic (extrinsic) and enteric (intrinsic) nervous systems38. The ANS conveys physiological conditions in the gut, such as acidity, levels of nutrients, osmolarity and pain, to the brain49. The enteric nervous system (ENS), which consists of the myenteric plexus and submucosal plexus, contributes to in situ neural communication within the intestine and connection to the ANS38.
The ENS and its neural pathways are responsible for intestinal motor and sensory functions independent of central nervous system (CNS) control50. In germ-free mice, colonization of the gut microbiota is critical for the development and maturation of the ENS51. The gut microbiota and its metabolites are potent stimulators of the production of serotonin by enterochromaffin cells52. This key neurotransmitter mediates gut secretion, motility and local nerve reflexes. In addition, treatment with medium fermented by the probiotic bacteria Bifidobacterium longum reduced anxiety and decreased the excitability of the ileal myenteric plexus neurons in mice with infectious colitis11, indicating communication of probiotics with the CNS via the ENS and the vagal nerve. Further investigations are required to identify the neurons that are affected by probiotics and the signals that are involved in this communication and to identify other alterations in gut microbiota that may also affect the ENS.
The ENS communicates bidirectionally with the brain through the vagus nerve, which sends sensory signals from the gut to the nucleus of the solitary tract (NTS) in the CNS. In a rat model of obesity, changes in the gut microbiota induced by an energy-dense diet were associated with alterations in brain-gut vagal (NTS) communication53, which may alter vagal satiety signalling and stimulate energy intake and adiposity54. A series of beneficial effects of treatment with probiotics (Lactobacillus rhamnosus and B. longum) on stress and anxiety have been demonstrated to be vagus-nerve-dependent11,12. Vagal afferents express receptors that sense SCFAs55, and activation of this pathway has been implicated in glucose homeostasis56.
Role of the gut in the immune system.
The gut is the largest immune organ in the body, with a complex mucosal immune system located at its inner surface and exposed to the lumen. Lymphocytes and innate immune cells, such as macrophages and dendritic cells, are found throughout the epithelial layers37. Mucosal immunity is characterized by individually compartmentalized gut-associated lymphoid tissues (GALTs) that form an interface between the blood and the intestinal lymph. This structural feature enables the GALT to constantly supply mature immune cells to the intestinal epithelium and lamina propria, where mucosal immunity interacts with the gut microbiota to produce immune responses and tolerance37. Harmonious immune responses within the physiological range ensure intestinal and systemic homeostasis. The gut microbiota therefore has a critical role not only in determining local immune outcomes but also in maintaining systemic physiology57.
A lack of gut microbiota leads to deficient development of the GALT58 and abnormal systemic59 and central immunity60. Germ-free animals have a substantial reduction in the levels of T helper 17 (TH17) cells61, B cells, immunoglobulin A (IgA) and plasma cells62–63, an imbalance of TH1 and TH2 responses64 and impaired Treg cell function65. Intestinal infiltration of pro-inflammatory TH17 cells is induced by segmented filamentous bacteria66, and gut microbial diversity, particularly colonization by Bacteroidetes, is critical for balancing TH1 and TH2 responses64. Treg cells are induced by a variety of bacterial groups67,68 and by the SCFA butyrate47, which is produced by bacterial fermentation. Innate immunity is also regulated by the gut microbiota as evidenced by reduced numbers and compromised functions of antigen-presenting cells and microglia in germ-free animals60,69. These findings indicate that the gut microbiota has a global impact on host immunity.
Given the aforementioned immune abnormalities, the observed alterations in gut and systemic physiological functions in germ-free mice are not surprising. These mice exhibit considerable alterations in the size and number of goblet cells in the caecum70 (but not in the colon71), in mucus properties72 and in intestinal tight junction proteins73. Other alterations in physiological parameters in germ-free animals include impaired blood-brain barrier integrity74, an exaggerated hypothalamic-pituitary-adrenal response to stress75, increased anxiety-like behaviour76, altered neurotransmitter levels52,77 and a reduced metabolic rate in the liver78. In the kd/kd mouse model of collapsing glomerulopathy, germ-free conditions postponed the onset of renal mitochondrial ultrastructural defects79, indicating a contribution of the gut microbiota to the pathogenesis of this kidney disease. Therefore, alteration and disruption of homeostasis in the gut have negative effects on intestinal and systemic physiological functions.
The gut microbiota in hypertension
Dysregulation of multiple contributing factors has been demonstrated in hypertension80, including the reninangiotensin system81,82, the ANS82,83 and the immune system84. Environmental factors in association with epi-genetic85 and genetic86 components have critical roles in the initiation, maintenance and progression of hyper-tension. In addition, emerging evidence indicates that the gut microbiota has an essential role in hypertension development.
Gut dysbiosis has been reported in animal models87–89 and in patients with hypertension87,90 (Table 1). Moreover, spontaneously hypertensive rats (SHRs) showed patho-physiological changes in the gut, including decreased numbers of goblet cells and villi length and increased fibrosis compared with age-matched normotensive Wistar Kyoto (WKY) controls91. Although these changes were more profound in adult SHRs than in juvenile SHRs that had not yet developed hypertension, the prehypertensive juvenile SHRs had reduced levels of multiple tight junction proteins but similar gut permeability compared with juvenile WKY rats91. These findings indicate that gut pathology occurs before the onset of blood pressure elevation in the SHRs. Further evidence for a causative role of gut dysbiosis in the genesis of hypertension came from faecal microbiota transplantation (FMT) experiments in which transferring dysbiotic faecal samples from patients with hypertension to germ-free mice90 or faeces from hypertensive stroke-prone SHRs to normotensive WKY rats92 increased blood pressure in the recipients. As gut pathophysiological changes, immune responses and autonomic responses to FMT were not evaluated, further investigation is required to identify the potential mechanisms that underlie this FMT-induced increase in blood pressure.
Table 1 |.
Changes in the gut microbial composition in hypertension and CKD
| Bacteria | Hypertension | CKD | ||
|---|---|---|---|---|
| Change (organism) | Refs | Change (organism) | Refs | |
| Actinobacteria | ||||
| Bifidobacterium | ↓ (rat) | 87,89 | ↓ (human and rat) | 103,217 |
| Bacteroidetes | ||||
| Bactemides | ↓ (human and rat) | 87,89,90,95 | ↓ (human and rat) | 101,104 |
| Prevotella | ↑ (human) | 90 | ↓ (human) | 107,110 |
| Parabactewides | ↑ (human and rat) | 87,90 | ↑ (human) | 110 |
| Firmicutes | ||||
| Lactobacillus | ↓ (human and mouse) | 92 | ↓ (human and rat) | 101,104 |
| Ruminococcaceae | NA | NA | ↓ (human) | 107 |
| Roseburia | ↓ (human) | 90 | ↓ (human) | 107 |
| Allobaculum | ↓ (rat) | 87 | NA | NA |
| Enterococcus | NA | NA | ↑ (human) | 107 |
| Faecalibacterium | ↓ (human) | 90 | ↓ (human) | 107 |
| Proteobacteria | ||||
| Enterobacteriaceae | NA | NA | ↑ (human) | 101,105 |
| Klebsiella | ↓ (human) | 203 | ↑ (human) | 107 |
| Verrucomicrobia | ||||
| Akkermansia | ↓ (human and rat) | 87,90 | NA | NA |
Changes in microbial composition in comparison with the microbiota of healthy controls. ↑, proportion increased; ↓, proportion decreased; CKD, chronic kidney disease; NA, not available.
Finally, studies in animal models and in patients with hypertension have reported that interventions that target the gut microbiota, such as a high-fibre diet, probiotics and antibiotics, have blood-pressure lowering effects87,92–99. For example, salt-sensitive mice treated with Lactobacillus murinus had lower systolic blood pressure (SBP; ~5 mmHg) and diastolic blood pressure (DBP; ~5 mmHg) than untreated controls92. The findings of these studies, which are discussed further below, provide further support for a role of the microbiota in hypertension.
The gut microbiota in CKD
The gut microbiota also seems to be a key factor that mediates the onset of kidney disease. In 1984, a study using a mouse strain that developed a spontaneous renal cystic disease (CFWwd mice) reported that mice that were raised in a germ-free environment rarely displayed this disease, whereas all those that were conventionally housed died of the disease100. Similar results were observed in the kd/kd mouse model of collapsing glomerulopathy, which spontaneously develops inter-stitial nephritis79. Transfer of these mice from specific pathogen-free (SPF) conditions to a germ-free environment resulted in a marked decrease in the incidence of this disease.
As gut dysbiosis and altered gut pathology are associated with hypertension, and hypertension is an important factor that contributes to the development of CKD, the finding that changes in the composition of the gut microbiota (TABLE 1) are associated with CKD and ESRD is not surprising101,102. Compared with healthy individuals, a decrease in culturable anaerobic bacteria was observed in the faeces of patients with stage 3–4 CKD103. By contrast, an increase in culturable aerobic bacteria was reported in the faeces of patients with CKD who were not yet on dialysis compared with healthy adults104. As culture of most gut bacteria is not currently possible, the fact that these findings were confirmed using non-culture-dependent methods, such as PCR or pyrosequencing, is reassuring. In addition, patients with ESRD and healthy individuals had distinct faecal microbial compositions, characterized by differences in the abundance of 190 microbial operational taxonomic units, akin to bacterial species101. Rats with CKD induced by 5/6 nephrectomy also differed substantially from sham controls in their abundance of bacterial taxa and showed increases in blood pressure, serum urea and creatinine levels and urinary protein levels101.
In a study that included 30 patients with ESRD not on dialysis, bacterial DNA was detected in the blood of six (20%) patients and the bacterial genera found in the blood were overgrown in the guts of these patients105. Moreover, the levels of C-reactive protein and IL-6 (biomarkers of low-grade inflammation) were significantly higher in patients with circulating bacterial DNA than in those in whom bacterial DNA was not detected. These findings suggest that overgrown bacteria trans-locate from the gut to the blood, where they contribute to increased levels of low-grade inflammation and thus exacerbate CKD pathology.
Although different sequencing methods and bacterial taxonomic levels have been used, studies have consistently reported that animals and patients with CKD had decreases in the genus Lactobacillus in their gut micro-biota101,106, whereas the levels of the Enterobacteriaceae family were increased101,105. In patients with ESRD in China, a switch from enterotype 2 (Prevotella dominant) to enterotype 1 (Bacteroides dominant) was associated with a decrease in butyrate-producing bacteria107. This shift in enterotype is characterized by a change in predominant microbial metabolism from saccharolytic to proteolytic fermentation.
Interestingly, in the 5/6 nephrectomy CKD model, the levels of uraemic toxins in serum correlated with the abundance of Clostridia-affiliated and Bacteroidia-affiliated species in the indigenous gut microbiota106. These species have a gene that encodes a tryptophanase-tyrosine phenol-lyase, suggesting that they have an important role in the production of uraemic toxins. To date, more than 80 uraemic toxins have been reported to accumulate in patients with CKD108. Most of these toxins are widely considered to contribute to uraemic syndromes. For example, the plasma levels of trimethylamine N-oxide (TMAO), an amine oxide metabolite of trimethylamine (TMA) that is associated with an increased risk of adverse cardiovascular events109, are elevated in patients with CKD compared with levels in healthy individuals110. Consistent with this finding, a phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) analysis showed that the expression of three genes involved in the production of TMA was also significantly increased in the gut microbiota of patients with CKD110. Antibiotic-treated mice that received FMT from patients with CKD showed notable elevations of TMAO in their plasma compared with mice that received FMT from healthy individuals, indicating a critical role of gut dysbiosis in the overproduction of TMAO in CKD110. Unfortunately, glomerular filtration rate (GFR) was not measured in these mice; therefore, the role of gut dysbiosis in the initiation and progression of CKD remains to be determined.
The gut-kidney axis
The gut-kidney axis can be subdivided into metabolism-dependent and immune pathways9. The metabolism-dependent pathway is primarily mediated by metabolites produced by the gut microbiota that have the capability to regulate host physiological functions. In the immune pathway, components of the immune system (for example, lymphocytes, monocytes and cytokines) have a critical role in communication between the gut and the kidney (FIG. 2). Crosstalk between the metabolism-dependent and immune pathway also has an important role in maintaining the balance of the gut-kidney axis.
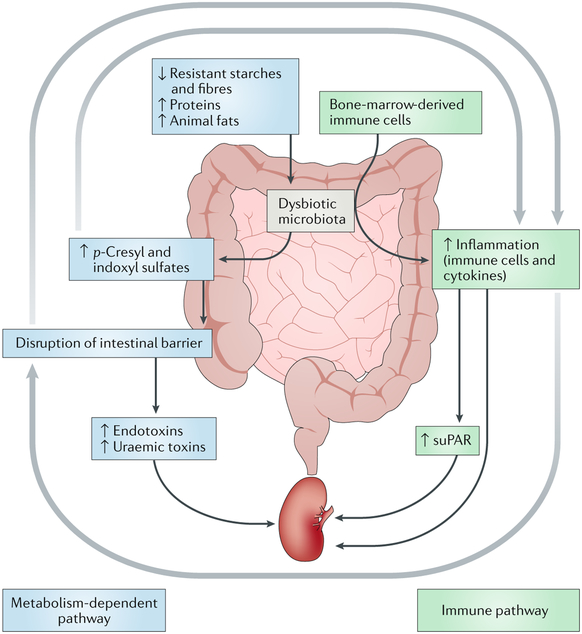
Fig. 2 |. The metabolism-dependent and immune pathways of the gut-kidney axis.
In the metabolism-dependent pathway, dysbiosis induced by an imbalanced diet (for example, a diet that is low in dietary fibres and high in protein and animal fats) leads to overproduction and accumulation of p-cresyί and indoxyl sulfates in the intestine. This accumulation disrupts the gut barrier and thus increases gut permeability. Consequently, influx of endotoxins and uraemic toxins into the kidney via the circulation contributes to renal inflammation. In the immune pathway, immune cells originating from the bone marrow encounter dysbiotic microbiota and become overactivated within the intestine. Inflammatory cells, cytokines and soluble urokinase plasminogen activator surface receptor (suPAR) generated in the gut contribute to renal inflammation via the circulation. Crosstalk between metabolism-dependent and immune pathways is achieved through the contributory effects of dysbiotic metabolites on intestinal and renal immunity, inflammation-induced gut barrier disruption and the resultant influx of dysbiotic metabolites into the kidney through the circulation.
Metabolism-dependent pathway.
Diet is increasingly recognized to be a fundamental regulator of gut microbiomes. Dietary fermentable fibres, rather than protein, are the main source of energy for gut epithelial cells111. With sufficient supply of dietary fibres, the protein-derived α-amino nitrogen is almost totally incorporated into the faecal biomass. Lack of dietary fibres or excessive protein or animal fats leads to overaccumulation of α-amino nitrogen, which can be converted into uraemic toxins by the gut microbiota112. Patients on haemodialysis who had intact colons had significantly higher levels of p-cresyl sulfate and indoxyl sulfate than those who did not have colons, indicating an important contribution of colonic microorganisms to the production of uraemic toxins113. Colonic transit time is a modifiable determinant of uraemic toxin production114. A prolonged transit time decreases the availability of carbohydrates in the colon, facilitating increased protein fermentation and expanding the proteolytic bacterial population9. Therefore, the colonic microbiota makes a considerable contribution to the production of uraemic toxins.
In CKD, a reduction in renal filtering capacity results in the deposition and accumulation of waste products in the blood. Accumulation of products of protein fermentation (for example, α-amino nitrogen) in the intestine and blood increases the gut intraluminal pH, deranges gut homeostasis and triggers intestinal disorders102. In addition, as renal function declines, the colon replaces the kidney as the primary site of excretion of urea and uric acid115. Constant exposure of colonic epithelial cells to urea reduces their viability and decreases epithelial barrier function in vitro116 and disrupts colonic tight junction proteins (for example, claudin 1, occludin and zonula occludens 1) both in vitro and in vivo116,117. Consequently, the levels of endotoxins and bacterial products in the circulation are elevated in patients with CKD compared with healthy individuals118,119. Deficits in renal function associated with a leaky gut exacerbate the accumulation of metabolic wastes in the blood and may eventually cause uraemia.
Immune pathway.
Another pathway that links the gut microbiota and the kidney is mediated by the immune system. Colonization of commensal microbiota in germ-free mice induced changes in the inflammatory cytokine profile in the bone marrow120, which is the primary site of origin of immune cells. Cytokines have important effects in haematopoiesis, and antibiotic-mediated depletion of the intestinal microbiota in mice led to the suppression of multipotent progenitors in the bone marrow7. Therefore, the gut microbiota modulates not only the activation of intestinal immune cells but also the profile of immune progenitor cells in the bone marrow.
The relationship between the bone marrow, cardiovascular system, hypertension and CKD has long been recognized15,121. Following bone marrow ablation, reconstitution of WKY rats with bone marrow from SHRs led to an elevation in blood pressure and inflammation, whereas reconstitution of SHRs with WKY bone marrow had the opposite effect15. In a clinical setting, renal dysfunction has been found in recipients of bone marrow transplants122, suggesting a contributory role of the bone marrow in the initiation of kidney inflammation. As the levels of pro-inflammatory cytokines positively correlate with the development of albuminuria and proteinuria, early intrarenal inflammation has been suggested as an important pathogenic mechanism in the onset of kidney disease123. In addition, immature myeloid cells derived from the bone marrow have been reported to be responsible for elevation in the circulating levels of soluble urokinase plasminogen activator surface receptor (suPAR)124, which has been implicated in the onset and progression of CKD125. Evidence also indicates that multipotent cells in bone marrow repair damaged tissues, including the vasculature and kidney, by undergoing proliferation, mobilization, differentiation and eventually incorporation into these tissues126–128.
After exiting from the bone marrow, mature immune cells in the gut are activated by the gut microbiota in peripheral lymphoid organs, such as GALT129. Gut per meability leads to accumulation of bacteria and bacterial products in the circulation and substantially contributes to chronic and systemic low-grade inflammation.
Low-grade inflammation has a critical role in the maintenance of many chronic diseases, including hypertension and CKD130,131. A number of studies have demonstrated contributory roles for macrophages132, T cells133 and B cells134 in the genesis of hypertension. For example, the blood pressure of germ-free mice is comparable to that of conventionally raised mice8, but angiotensin-II-induced increases in blood pressure are blunted in germ-free mice, likely owing to inefficient induction of oxidative stress and inflammation by angiotensin II8. In the prehepatic portal hypertension model, mice with absent intestinal bacteria exhibited lower portal pressure than controls with intestinal microbiota; this lower portal pressure was associated with reduced densities of intestinal lymphatic and blood vessels135. These data suggest the involvement of gut microbiota in the immune-cell-mediated genesis of hypertension.
The gut microbiota also has a crucial role in systemic metabolic syndrome and CKD136–138. Mice with adenine-induced renal failure housed in germ-free conditions had significantly lower levels of uraemic toxins than those housed in SPF conditions138. However, more severe renal damage was observed in the germ-free mice, presumably owing to reduced production of renoprotective SCFAs and inefficient utilization of amino acids compared with the SPF mice. This finding highlights the importance of maintaining an exquisitely balanced gut microbiota in CKD.
Communication between the pathways.
The gut microbial metabolites p-cresyl sulfate and indoxyl sulfate bind albumin in the circulation139 and are rapidly released from albumin immediately before being eliminated by tubular secretion140. The levels of p-cresyl and indoxyl sulfates increase concomitantly with CKD progression141, and this increase has been attributed to decreased renal clearance142 and increased production due to gut dysbiosis138. Gut-microbiota-derived uraemic toxins induce inflammation in the gastrointestinal tract, as evidenced by increased intestinal permeability in patients and animals with uraemia143,144, increased penetration of bacteria across the intestinal wall in uraemic rats145, the detection of endotoxaemia in patients with ESRD105,119 and histological evidence of chronic enterocolitis in patients on dialysis146. Pathological accumulation of p-cresyl and indoxyl sulfates in the circulation results in systemic inflammation in blood vessels, endothelial dysfunction147, insulin resistance148 and activation of the renin-angiotensin-aldosterone system149, which are all common features of hypertension and CKD. Furthermore, high concentrations of uraemic toxins in plasma due to CKD lead to increased concentrations of these toxins in the gastrointestinal tract, where they affect the composition of the gut microbiome106. The resulting dysbiosis and deregulation of local gut immune responses perpetuate loss of renal function, accumulation of metabolic wastes and changes in metabolic state in a positive feedback loop.
In addition to expansion of indole-forming and p-cresol-forming bacteria, contraction of families of SCFA-producing bacteria has been reported in patients with ESRD compared with healthy individuals150,151. These changes included reductions in the Lactobacillaceae and Prevotellaceae families, which express genes that encode butyrate-forming enzymes (phosphotransbutyrylase and butyrate kinase)150, and in the butyrate-producing bacteria Roseburia spp. and Faecalibacterium prausnitzii151. Beneficial effects of butyrate on colonic inflammation have been reported152. Moreover, in uninephrectomized rats, infusion of sodium butyrate into the intramedullary area of the kidney resulted in improvement in angiotensin-II-induced glomerulosclerosis, renal fibrosis and urinary albumin levels and led to decreases in the levels of (pro)renin receptor, angiotensinogen, renin, angiotensin-I-converting enzyme and renal inflammatory markers153. In the deoxycortico sterone acetate (DOCA) hypertension model, a high-fibre diet that promoted the growth of acetate-producing bacteria and acetate supplementation attenuated renal fibrosis95. These findings indicate that SCFAs regulate immune responses and attenuate kidney pathology.
The brain-gut-kidney axis
Our research group was the first to demonstrate a contribution of the brain-gut-bone-marrow axis to blood pressure elevation15,87,91,154–159. Emerging evidence has led to expansion of this concept to that of the brain-gut-kidney axis9,118,160,161 (TABLE 2). The brain has considerable involvement in the gut-kidney axis through communication with metabolism-dependent and immune pathways via the sympathetic nervous system (SNS).
Table 2 |.
Evidence for a gut-kidney axis and a brain-gut-kidney axis
| Evidence | Species | Refs |
|---|---|---|
| Gut-kidney axis | ||
| •Gut dysbiosis in hypertension and CKD | Human and rat | 87,89,90,101,103–106 |
| •Altered gut metabolite profile in hypertension | Human and mouse | 106,118,203,233 |
| •Increased levels of gut-microbiota-derived uraemic toxins in CKD | ||
| •Intestinal pathology and inflammation in hypertension | Human and rat | 234,235 |
| •Intestinal and renal inflammation in CKD | ||
| •Proteinuria, renal failure and uraemia in intestinal inflammatory bowel disease | Human | 236 |
| •Angiotensin-ll-induced hypertension is attenuated in germ-free mice | Mouse and rat | 8,79,100,106 |
| •Rodent models of spontaneous renal diseases do not develop severe disease in germ-free conditions | ||
| Brain-gut-kidney axis | ||
| •Altered autonomic nervous system in hypertension and CKD | Human and rat | 156,162,164,170,237 |
| •Increased microglialactivation and neuroinflammation in hypertension | Human, rat and mouse | 175,177,178,205 |
| •Increased neuroinflammation and cognitive impairment in CKD |
CKD, chronic kidney disease.
Sympathetic nervous system and brain.
The occur-rence of increased SNS activity in hypertension and CKD is well established83,162. Efferent fibres of the SNS innervate the renal vasculature and juxtaglomerular cells, and afferent fibres convey mechanical and chemical information from the kidney163. Rapid turnover of noradrenaline in autonomic brain centres has been shown in rats with 5/6-nephrectomy-induced CKD164. In addition, the sympathetic dampening agent moxonidine lowers urinary albumin excretion and reduces glomerulosclerosis in subtotally nephrectomized rats165. These data indicate altered bidirectional autonomic communication between the brain and the kidney in CKD. Uraemic toxins do not have a direct effect on renal afferents, as evidenced by a study that showed similar levels of muscle sympathetic nerve activity in patients with uraemia on haemodialysis and in nonuraemic kidney transplant recipients with diseased native kidneys166.
The SNS directly innervates both primary (bone marrow) and secondary (spleen) immune organs80. Expression of adrenergic receptors on immune cells residing in immune organs indicates regulatory effects of sympathetic catecholamines on the immune system154,167. Both anti-inflammatory and pro-inflammatory effects of adrenergic signalling have been demonstrated, depending upon the subtype of adrenergic receptors expressed167, the level of activation of specific cell types and the stage of disease progression168. However, persistent activation of the SNS results in changes in signalling within immune organs and cells towards pro-inflammatory pathways168, as observed in hypertension and CKD169,170.
In addition to peripheral blood vessel control, the SNS regulates water and sodium balance through direct innervation of the nephron, the renal vasculature and juxtaglomerular cells. The renorenal reflex is an inhibitory feedback loop that constitutes renal afferent nerves that convey signals to the CNS, governing sympathetic outflow163. An impaired renorenal reflex in hypertension and CKD leads to augmented sympathetic excitation to the blood vessels, heart and kidney161.
Multiple central neural sites have been implicated in the regulation of sympathetic outflow, including the paraventricular nucleus of hypothalamus (PVN), NTS and rostral ventrolateral medulla (RVLM). These regions communicate with each other and integrate diverse inputs to determine the tonicity of sympathetic outflow171. Neuroinflammation in central sympathetic regions is observed in hypertension172,173, and the central renin-angiotensin system has an important role in mediating neuroinflammation174. In CKD, indoxyl sulfate increases neuroinflammation, which may facilitate the neurodegeneration that has been observed in some patients175. In the 5/6 nephrectomized mouse, renal denervation lowers blood pressure, and reduced sympathetic nerve activity is associated with increased GABA input into the PVN176, indicating crosstalk between the kidney and the brain in the context of hypertension and CKD.
In cross-sectional and longitudinal studies, each 10 ml/min/1.73 m2 reduction in estimated GFR (eGFR) below 60 ml/min/1.73 m2 was associated with an approximately 11% increase in the prevalence of cognitive impairment177,178. Other studies failed to identify statistically significant correlations between eGFR and cognitive impairment but reported that albuminuria and the rate of eGFR decline were associated with cognitive decline179–181. Uraemic guanidino compounds with neuroexcitatory effects have been found in brain regions responsible for cognition (thalamus and mammillary bodies) in patients with CKD182, suggesting a direct role of these compounds in cognitive impairment.
Another potential mechanism that links cognitive impairment to the gut-brain-kidney axis is dysregulation of the tryptophan kynurenine pathway. Such dys-regulation was associated with eGFR decline and CKD incidence in a population-based study183. Germ-free mice had reduced kynurenine pathway activity that was normalized by colonization of a conventional gut microbiota184. This finding indirectly suggests a role of immune activation in the regulation of the kynure-nine pathway. Activation of Toll-like receptor 3 (TLR3) in peripheral monocytes facilitates production of the metabolite quinolinic acid185 (an end product of the kynurenine pathway), which is an excitotoxin with high affinity for glutamate N-methyl-d-aspartate (NMDA) receptors186. As TLR3 is abundantly expressed in the brain187, abnormal activation of NMDA receptor signalling by quinolinic acid might be at least partially responsible for numerous neurological diseases186,188.
Abnormal sympathetic drive to the bone marrow in hypertension and CKD dramatically shifts the immune properties of haematopoietic cells to a pro-inflammatory state, and the release of these inflammatory immune cells from the bone marrow contributes to the pathogenesis of hypertension and CKD15,156,189. Therefore, the immune pathway of the brain-kidney axis involves input from the CNS and/or SNS to the bone marrow and the effects of inflammatory cells released from the bone marrow on the kidney.
Epigenetic factors.
Epigenetic factors might also have a role in the brain-gut-kidney axis. Microbial metabolites including folate, butyrate and acetate are cofactors and allosteric regulators of epigenetic processes such as DNA methylation, histone acetylation and RNA interference190–192. The gut microbiome has been shown to induce host epigenetic changes that might contribute to the development of cancer193,194, and notable changes in epigenetic modifications have been reported in hyper-tension and CKD85,195. For example, podocyte-specific inactivation of Dicer, one of the enzymes responsible for production of microRNAs, results in proteinuria and glomerulosclerosis196. In a genome-wide DNA methylation study of human kidney tubules, several genes that are associated with kidney fibrosis were characterized by methylation changes and alterations of downstream transcript levels in CKD samples compared with controls197. In a rat model of salt-sensitive hypertension, stimulation of sympathetic signalling led to reduced expression of a regulator of sodium reabsorption, protein kinase lysine-deficient 4 (WNK4), owing to hyper-acetylation of the promoter198. Moreover, upregulation of angiotensin-converting enzyme 1 in SHR compared with WKY controls was associated with multiple epi-genetic modifications in several tissues, such as the adrenal gland, aorta, heart and kidney199.
In germ-free mice, colonization by gut microbiota normalized the deregulation of microRNA in the amygdala and prefrontal cortex of the brain200, indicating an epigenetic connection between the gut and the brain. The chromatin accessibility of intestinal intraepithelial lymphocytes was quantitatively changed in germ-free mice colonized by conventional microbiota, resulting in determination of functional features ofhost immune cell lineages201. Epigenetic inheritance may explain much of the heritability of hypertension202. For example, in mice, perinatal exposure to a high-fat, high-sucrose diet epi-genetically primed the central renin-angiotensin system leading to hypertension, potentially owing to limited plasticity of the autonomic system202. Unfortunately, the effects of this diet on the gut microbiome were not investigated in this study.
Together, the available data suggest that epigenetic changes mediated by the gut microbiota are involved in the pathogenesis of dysbiosis-associated hypertension and CKD. However, further studies are needed to provide direct evidence of a role of the microbiota in the induction of host epigenetic changes in these diseases.
Pathogenesis of hypertension and CKD.
On the basis of the available evidence, we propose a triangular brain-gut-kidney hypothesis for the pathogenesis of hypertension and CKD (Fig. 3). Environmental, dietary and other pro-hypertensive and/or CKD-relevant stimuli are perceived at the autonomic brain regions, where they are integrated into signals that lead to increased sympathetic nervous drive to the gut and bone marrow. Sympathetic drive to the bone marrow shifts the balance of physiological inflammation towards overactivation, which perpetuates low-grade systemic inflammation and results in a reduction in the production of pluripotent stem cells from the bone marrow for vascular, intestinal and renal repair. Activation of the SNS initiates a sequence of events in the gut that leads to increased gut wall permeability, dysbiosis, migration of pro-inflammatory cells from the bone marrow and the release of microbial products and metabolites into the blood. The resulting imbalance in the plasma metabolome adversely affects various cardio-renal tissues; for example, accumulation of uraemic toxins or a lack of SCFAs leads to activation of systemic and tissue inflammation147,203. In addition, activation of renal sympathetic nerve activity might directly influence renal physiology, altering body fluid balance and plasma metabolite secretion and retention. These events culminate in the development of CKD and hypertension. Consistent with our hypothesis, transplantation of bone marrow15,122, gut microbiota90 or kidney204 has been demonstrated to induce disorders not only in the organs of the proposed brain-gut-kidney axis but also in the interconnected pathways of this axis. Therefore, the brain-gut-kidney hypothesis represents a novel conceptual shift that can potentially be applied in clinical settings.
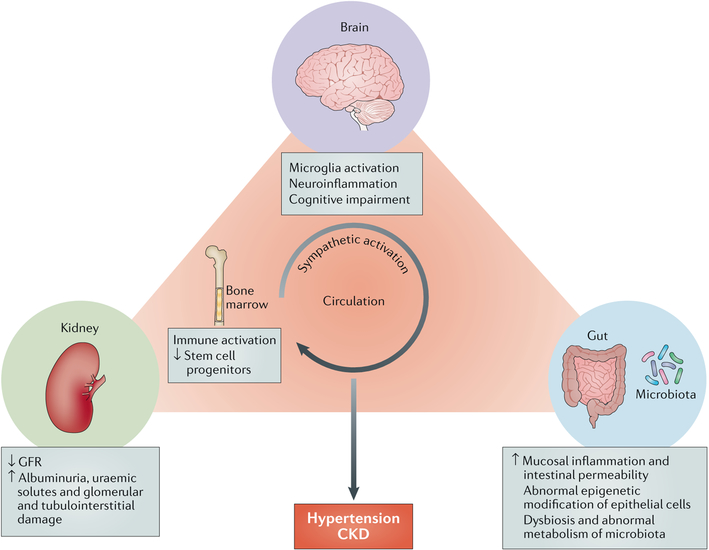
Fig. 3 |. The brain-gut-kidney axis hypothesis for the pathogenesis of hypertension and CKD.
Sympathetic activation is a common feature in disorders of the brain, gut and kidney. Persistent microglial activation and neuroinflammation in presympathetic regions of the brain responsible for sympathetic outflow contribute to an increase in blood pressure and to pathogenesis in the gut and kidney. Immune cells that develop in the bone marrow are activated by microbiota in the gut and enter the circulation; these cells contribute to gut and kidney inflammation. Local mucosal immunity is also regulated by the intestinal environment owing to close communication between the gut and the gut microbiota. Dysbiosis and disorders in intestinal metabolism result in an imbalance of intestinal homeostasis, which is characterized by increased mucosal inflammation, intestinal permeability and abnormal epigenetic modification of epithelial cells. A decline in renal function leads to reduced glomerular filtration rate (GFR), increased albuminuria and uraemic toxins and glomerular and tubuίointerstitiaί damage. These pathological events in the brain, gut and kidney substantially contribute to the development of hypertension and chronic kidney disease (CKD).
Potential therapeutic strategies
According to the brain-gut-kidney axis hypothesis, treatments that target the brain, the SNS or the gut could potentially be beneficial for patients with hypertension and/or CKD. Consistent with this postulate, intracerebroventricular administration of minocycline suppresses microglial and sympathetic activation and lowers blood pressure in animal models of hypertension205. Moreover, beneficial effects of renal denervation, which targets sympathetic drive, have been reported in patients with resistant hypertension and CKD206,207. Modulation of the gut microbiota reduces systemic inflammation and SNS activity, both of which contribute to hypertension and CKD80,162,208. Therefore, various approaches to modulate the gut microbiota are being explored for the treatment of these diseases.
Dietary interventions.
Most patients with CKD are advised to limit their intake of sodium, protein, potassium, cholesterol and phosphorus, whereas patients with hypertension are advised to avoid sodium and cholesterol. By contrast, foods rich in fibre, vitamins and minerals are highly recommended. The Dietary Approach to Stop Hypertension (DASH) diet of the National Kidney Foundation was developed for the treatment of hypertension and kidney disease according to these principles209.
In DOCA-salt hypertensive mice, a high-fibre diet led to substantial reductions in both SBP and DBP (~20 mmHg)95. Clinical studies have shown a moderate blood-pressure lowering effect of dietary fibre210,211. Moreover, a meta-analysis of 25 randomized placebo-controlled trials reported that high versus low dietary fibre intake was associated with a modest but statistically significant reduction in DBP (1.65 mmHg) but not in SBP97. Importantly, more pronounced blood pressure reductions were found when the analysis was restricted to trials conducted in patients with hyper-tension (SBP 5.95 mmHg, DBP 4.2 mmHg) or to trials with long interventions (≥8 weeks; SBP 3.12 mmHg, DBP 2.57 mmHg).
A meta-analysis of 14 controlled feeding trials involving 143 participants with CKD showed that supplementation of dietary fibre intake significantly reduced serum levels of uraemic toxins, urea and creatinine212. Similarly, a study that investigated the association of dietary fibre intake with CKD-related parameters in 157 patients in China reported that a fibre intake of ≥25 g per day compared with <25 g per day was associated with a smaller reduction in eGFR and lower levels of serum C-reactive protein, indoxyl sulfate, cholesterol and IL-6 during 18 months offollow-up213. Thus, high fibre intake seems to retard loss of GFR and is negatively associated with cardiovascular risk. However, caution must be used when selecting high-fibre foods with high potassium contents. Therefore, a need exists to identify or formulate low-potassium, high-fibre foods for patients with CKD.
Probiotics, prebiotics and synbiotics.
The therapeutic use of probiotics, prebiotics and synbiotics is an area of increasing interest among renal health-care professionals. A meta-analysis of nine clinical trials with a total of 543 participants demonstrated that probiotic consumption modestly but significantly reduced both SBP (3.65 mmHg) and DBP (2.38 mmHg) in populations with baseline BP ≥135/85 mmHg98. Probiotics that contained multiple rather than single species of bacteria, a longer duration of the intervention (≥8 weeks) and higher daily doses (≥1011 colony-forming units) were associated with more effective blood-pressure lowering in this study. Notably, the conclusions of the individual trials included in this meta-analysis were inconsistent, likely owing to substantial differences in participant numbers, baseline blood pressures, treatment durations and type and dose of probiotics.
Numerous clinical trials and experimental studies in CKD have shown that the administration of prebiotics, probiotics and synbiotics can reduce the levels of uraemic p-cresyl and indoxyl sulfates and inflammatory mediators160,214,215 and attenuate colonic epithelial tight junction disruption215, resulting in substantial improvements in endotoxaemia, blood urea nitrogen levels and quality of life160,216. A diet with high levels of resistant starch (a prebiotic) favourably altered the gut microbiota and caecal, serum and urine metabolite profiles in rats with adenine-induced CKD217.
As the abundance of Lactobacillus is decreased in CKD101,106, this bacterium is one of the most common probiotics used in CKD studies. In patients with uraemia undergoing haemodialysis, oral administration of a preparation of antibiotic-resistant lactic acid bacteria (known as Lebenin) restored the composition of the gut microbiota to normal and inhibited the accumulation of uraemic toxins in the blood218. Such beneficial effects of Lactobacillus could be the result of effects of this bacteria on the permeability and immune status of the gut epithelium.
Antibiotics.
Manipulation of the gut microbiota through the use of antibiotics influences blood pressure and may be a useful intervention for hypertension control. For example, in rats with angiotensin-II-induced hypertension, minocycline administration changed the gut micro-biota and lowered blood pressure87. Administration of propionate has also been reported to modulate blood pressure in mice, likely via binding to the G protein-coupled receptor 41 (GPR41) and olfactory receptor 78 (OLFR78; also known as OR51E2)93,94. In a case of patient with treatment-resistant hypertension, antibiotic therapy using a combination of vancomycin, rifampin and ciprofloxacin decreased SBP by ~70 mmHg in the absence of antihypertensive drugs99.
As the kidney has an important role in the elimination of metabolites, drug use in patients with CKD requires caution to minimize the risk of adverse effects owing to the accumulation of active metabolites219,220. For example, antibiotic treatment of Escherichia coli O157:H7 infection increases the risk of haemolytic uraemic syndrome221. In contrast to Lactobacillus, the population of Enterobacteriaceae of the Proteobacteria phylum is expanded in CKD101,105. Bacteriophage therapy against Enterobacteriaceae has been proposed as an alternative strategy to antibiotics for controlling this bacterial population222,223.
Faecal transplantation.
FMT and transplantation of sterile faecal filtrate (FFT), which is enriched in all components of the gut contents except bacteria and particulate matter, are alternative approaches to modify the gut microbiota. To our knowledge, no studies of FMT or FFT for the treatment of patients with hyper-tension or CKD have been published to date. However, FMT and FFT have been utilized for the treatment of Clostridium difficile infection, which is a common complication in patients with CKD, particularly in those undergoing haemodialysis224–226. Following successful treatment of C. difficile infection by FFT transplantation, the gut microbiota of a patient with loss of renal function resembled that of the faecal donor with remarkable elimination of Proteobacteria, which had dominated before FFT226. The risk of secondary infection may be lower with FFT than with FMT, as FFT does not involve the transfer of live bacteria226. Further investigation is needed to understand how the various components of sterile faecal filtrate, including viruses (that is, bacteriophages sensu stricto), bacterial DNA and metabolites, lead to long-term changes in the gut microbiota.
Metabolite modulation.
Haemodialysis removes most uraemic toxins with the exception of protein-bound uraemic toxins such as indoxyl sulfate and p-cresyl sulfate, which bind serum albumin227. The spherical carbon adsorbent AST-120 absorbs indole produced in the intestine and thereby reduces serum and urinary levels of indoxyl sulfate228. AST-120 has been approved as a treatment to delay the initiation of haemodialysis in patients with CKD in Japan but not in Europe or the United States, owing to a lack of proof of an unequivocal therapeutic benefit in large randomized clinical trials with hard renal and cardiovascular end points228.
To date, metabolomics studies of hypertension are limited, but changes in lipid and fatty acid profiles have been reported229. In addition, numerous plasma metabolites have been associated with longitudinal eGFR183 or cross-sectional eGFR decline230 in the general population. In particular, metabolites of the spermidine and kynurenine pathways are associated with the gut microbiota as well as the physiology of the host231,232. Such metabolites are potential therapeutic targets for the prevention of disease progression in CKD.
Conclusions and future perspectives
The gut microbiota has a critical role in a variety of diseases, including hypertension and CKD. Thus, we propose expansion of our brain-gut axis hypothesis for the pathogenesis of hypertension to explain the role of communication between the gut, the brain and the kidney in CKD. Numerous studies have demonstrated pathways that connect the brain and the gut in hyper-tension, but relatively little is known about the role of brain-gut-kidney connections in CKD pathogenesis. In particular, whether sympathetic activation to the bone marrow and gut is increased in CKD and whether changes in kidney function are associated with increased gut permeability remain to be addressed. Further studies in the settings of hypertension and CKD are needed to elucidate the mechanisms and provide proof of concept for the brain-gut-kidney axis.
Potential therapeutic strategies for CKD and hyper-tension that target the gut microbiota are already being investigated. However, whether hypertension and CKD are associated with specific gut microbial profiles and microbial metabolomes remains unclear. Such profiles could provide useful biomarkers for establishing disease diagnosis and severity as well as potential therapeutic targets. The gut epithelium is dynamic, has high regenerative capacity and is epigenetically modified by the gut microbiota and their metabolites194. Therefore, targeting epigenetic modification of epithelia might be a promising direct approach to control the progression and perhaps also the initiation of gut-dysbiosis-associated diseases. Large-scale, well-controlled translational and preclinical studies are required to evaluate the therapeutic implications of the brain-gut-kidney hypothesis.
Probiotics
A group of microorganisms with beneficial effects on human health.
Nucleus of the solitary tract
(NTS). A brainstem region that receives and integrates peripheral afferent inputs from the baroreceptors, chemoreceptors and subdiaphragmatic organs of the gastrointestinal tract. The NTS projects selectively to the paraventricular nucleus of hypothalamus or caudal ventrolateral medulla to modulate sympathetic outflow.
TH1 and TH2 responses
CD4+ T cells can be divided into two subsets on the basis of their pattern of cytokine production. The TH1 response is characterized by the production of iFNγ and is generally more effective against intracellular pathogens, whereas the TH2 response is characterized by the production of i L-4 and is generally more effective against extracellular bacteria and parasites.
Uraemic toxins
Various compounds, mainly derived from the gut microbiota, that accumulate in the blood and tissue with progression of renal failure. Some compounds exhibit high affinity for albumin and are difficult to remove by haemodialysis.
Paraventricular nucleus of hypothalamus
(PVN). An important region in the central nervous system that contributes to sympathetic nervous system efferent transmission. Stimulation of the PVN with inflammatory cytokines or angiotensin ii increases sympathetic outflow.
Rostral ventrolateral medulla
(RVLM). The RVLM receives projections from the paraventricular nucleus of hypothalamus and caudal ventrolateral medulla to control sympathetic activity associated with cardiovascular functions.
Kynurenine pathway
The kynurenine pathway catabolizes approximately 95–99% of ingested tryptophan that is not utilized for protein synthesis in mammalian cells. Dysregulation of the kynurenine pathway results in overproduction of quinolinic acid, which has been implicated in inflammatory neurological diseases, such as Alzheimer and Huntington diseases.
Excitotoxin
A collection of chemical compounds that overactivate and exhaust neurons by binding to their receptors.
Prebiotics
Food ingredients that promote growth of beneficial microorganisms.
Synbiotics
Combinations of prebiotics and probiotics.
Key points.
The gut microbiota has crucial roles in a variety of diseases, including hypertension and chronic kidney disease (CKD).
The gut microbiota communicates with the endocrine, nervous and immune systems to regulate host homeostasis, including blood pressure and kidney functions.
The gut-kidney axis is mediated through metabolism-dependent and immune pathways.
The brain-gut-kidney axis involves connections between these organs that are mediated by descending autonomic regulation from the brain and signals from the gut and the kidney, such as immune products and microbial metabolites.
Potential therapeutic strategies for CKD and hypertension that target the gut microbiota include dietary interventions, probiotics, prebiotics, synbiotics, faecal microbiota transplant and metabolome modulation.
Glossary
- Low-grade inflammation
A chronic systemic immune response that occurs without acute clinical symptoms.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jha V et al. Chronic kidney disease: global dimension and perspectives. Lancet 382, 260–272 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Rao MV, Qiu Y, Wang C & Bakris G Hypertension and CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES), 1999–2004. Am. J. Kidney Dis 51, S30–S37 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Taler SJ et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am. J. Kidney Dis. 62, 201–213 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inker LA et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 63, 713–735 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Andrassy KM Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int. 84, 622–623 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Dinan TG & Cryan JF Gut-brain axis in 2016: Brain-gut-microbiota axis — mood, metabolism and behaviour. Nat. Rev. Gastroenterol. Hepatol 14, 69–70 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Josefsdottir KS, Baldridge MT, Kadmon CS & King KY Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood 129, 729–739 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karbach SH et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J. Am. Heart Assoc 5, e003698 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evenepoel P, Poesen R & Meijers B The gut-kidney axis. Pediatr. Nephrol 32, 2005–2014 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Rooks MG & Garrett WS Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol 16, 341–352 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bercik P et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil 23, 1132–1139 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bravo JA et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA 108, 16050–16055 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akchurin OM & Kaskel F Update on inflammation in chronic kidney disease. Blood Purif. 39, 84–92 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Shankland SJ & Jefferson JA A bone marrow factor contributes to kidney disease. Nat. Med 23, 13–14 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Santisteban MM et al. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ. Res 117, 178–191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carabotti M, Scirocco A, Maselli MA & Severi C The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol 28, 203–209 (2015). [PMC free article] [PubMed] [Google Scholar]
- 17.Cigarran Guldris S, Gonzalez Parra E & Cases Amenós A Gut microbiota in chronic kidney disease. Nefrologia 37, 9–19 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Konturek PC et al. Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J. Physiol. Pharmacol 66, 483–491 (2015). [PubMed] [Google Scholar]
- 19.Rodriguez JM et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis 26, 26050 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller NT, Bakacs E, Combellick J, Grigoryan Z & Dominguez-Bello MG The infant microbiome development: mom matters. Trends Mol. Med 21, 109–117 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu GD et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ou J et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am. J. Clin. Nutr 98, 111–120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker WA The importance of appropriate initial bacterial colonization of the intestine in newborn, child, and adult health. Pediatr. Res 82, 387–395 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francavilla R et al. Effect of lactose on gut microbiota and metabolome of infants with cow’s milk allergy. Pediatr. Allergy Immunol 23, 420–427 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Ulluwishewa D et al. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr 141, 769–776 (2011). [DOI] [PubMed] [Google Scholar]
- 26.The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claesson MJ et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4586–4591 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi Y et al. Intestinal permeability biomarker zonulin is elevated in healthy aging. J. Am. Med. Dir. Assoc 18, 810.e1–810.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto M, Kurihara S, Kibe R, Ashida H & Benno Y Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS ONE 6, e23652 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minot S et al. The human gut virome: inter-individual variation and dynamic response to diet. Genome Res. 21, 1616–1625 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minot S, Grunberg S, Wu GD, Lewis JD & Bushman FD Hypervariable loci in the human gut virome. Proc. Natl Acad. Sci. USA 109, 3962–3966 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minot S et al. Rapid evolution of the human gut virome. Proc. Natl Acad. Sci. USA 110, 12450–12455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao G et al. Intestinal virome changes precede autoimmunity in type I diabetes-susceptible children. Proc. Natl Acad. Sci. USA 114, E6166–E6175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iliev ID & Leonardi I Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat. Rev.Immunol 17, 635–646 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen TL, Vieira-Silva S, Liston A & Raes J How informative is the mouse for human gut microbiota research? Dis. Model. Mech 8, 1–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atuma C, Strugala V, Allen A & Holm L The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol 280, G922–G929 (2001). [DOI] [PubMed] [Google Scholar]
- 37.McDermott AJ & Huffnagle GB The microbiome and regulation of mucosal immunity. Immunology 142, 24–31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furness JB, Callaghan BP, Rivera LR & Cho HJ The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol 817, 39–71 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Zheng L, Kelly CJ & Colgan SP Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol. 309, C350–C360 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Worthington JJ, Reimann F & Gribble FM Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 11, 3–20 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Psichas A et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes 39, 424–429 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tolhurst G et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samuel BS et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl Acad. Sci. USA 105, 16767–16772 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donohoe DR et al. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 48, 612–626 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raqib R et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc. Natl Acad. Sci. USA 103, 9178–9183 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng X et al. Induction of porcine host defense peptide gene expression by short-chain fatty acids and their analogs. PLoS ONE 8, e72922 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furusawa Y et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Kelly CJ et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF Augments tissue barrier function. Cell Host Microbe 17, 662–671 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berthoud HR, Blackshaw LA, Brookes SJ & Grundy D Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol. Motil 16 (Suppl. 1), 28–33 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Costa M, Brookes SJ & Hennig GW Anatomy and physiology of the enteric nervous system. Gut 47 (Suppl. 4), iv15–iv19 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McVey Neufeld KA, Perez-Burgos A, Mao YK, Bienenstock J & Kunze WA The gut microbiome restores intrinsic and extrinsic nerve function in germ-free mice accompanied by changes in calbindin. Neurogastroenterol. Motil 27, 627–636 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Yano JM et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaughn AC et al. Energy-dense diet triggers changes in gut microbiota, reorganization of gut-brain vagal communication and increases body fat accumulation. Acta Neurobiol. Exp 77, 18–30 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Lartigue G, de La Serre CB & Raybould HE Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol. Behav 105, 100–105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lal S, Kirkup AJ, Brunsden AM, Thompson DG & Grundy D Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol 281, G907–G915 (2001). [DOI] [PubMed] [Google Scholar]
- 56.Zadeh-Tahmasebi M et al. Activation of short and long chain fatty acid sensing machinery in the ileum lowers glucose production in vivo. J. Biol. Chem 291, 8816–8824 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chow J, Lee SM, Shen Y, Khosravi A & Mazmanian SK Host-bacterial symbiosis in health and disease. Adv. Immunol 107, 243–274 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eberl G & Lochner M The development of intestinal lymphoid tissues at the interface of self and microbiota. Mucosal Immunol. 2, 478–485 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Macpherson AJ & Harris NL Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol 4, 478–485 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Erny D et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci 18, 965–977 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ivanov II et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4, 337–349 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bunker JJ et al. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science 358, eaan6619 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crabbé PA, Bazin H, Eyssen H & Heremans JF The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract. Int. Arch. Allergy Appi. Immunol 34, 362–375 (1968). [DOI] [PubMed] [Google Scholar]
- 64.Mazmanian SK, Liu CH, Tzianabos AO & Kasper DL An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Ostman S, Rask C, Wold AE, Hultkrantz S & Telemo E Impaired regulatory T cell function in germ-free mice. Eur. J. Immunol 36, 2336–2346 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Ivanov II et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Atarashi K et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Round JL & Mazmanian SK Inducible Foxp3+ regulatory T cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107, 12204–12209 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thaiss CA, Zmora N, Levy M & Elinav E The microbiome and innate immunity. Nature 535, 65–74 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Kandori H, Hirayama K, Takeda M & Doi K Histochemical, lectin-histochemical and morphometrical characteristics of intestinal goblet cells of germfree and conventional mice. Exp. Anim 45, 155–160 (1996). [DOI] [PubMed] [Google Scholar]
- 71.Nowacki MR Cell proliferation in colonic crypts of germ-free and conventional mice — preliminary report. Folia Histochem. Cytobiol 31, 77–81 (1993). [PubMed] [Google Scholar]
- 72.Johansson ME et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe 18, 582–592 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kozakova H et al. Colonization of germ-free mice with a mixture of three lactobacillus strains enhances the integrity of gut mucosa and ameliorates allergic sensitization. Cell. Mol. Immunol 13, 251–262 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Braniste V et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl Med 6, 263ra158 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sudo N et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol 558, 263–275 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crumeyrolle-Arias M et al. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 42, 207–217 (2014). [DOI] [PubMed] [Google Scholar]
- 77.Mayer EA, Knight R, Mazmanian SK, Cryan JF & Tillisch K Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci 34, 15490–15496 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sewell DL, Wostmann BS, Gairola C & Aleem MI Oxidative energy metabolism in germ-free and conventional rat liver mitochondria. Am. J. Physiol 228, 526–529 (1975). [DOI] [PubMed] [Google Scholar]
- 79.Hallman TM et al. The mitochondrial and kidney disease phenotypes of kd/kd mice under germfree conditions. J. Autoimmun 26, 1–6 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang T & Zubcevic J Gut-brain axis in regulation of blood pressure. Front. Physiol 8, 845 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aroor AR et al. The role of tissue Renin-Angiotensinaldosterone system in the development of endothelial dysfunction and arterial stiffness. Front. Endocrinol 4, 161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Young CN & Davisson RL Angiotensin-II, the brain, and hypertension: an update. Hypertension 66, 920–926 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mancia G & Grassi G The autonomic nervous system and hypertension. Circ. Res 114, 1804–1814 (2014). [DOI] [PubMed] [Google Scholar]
- 84.Harrison DG The immune system in hypertension. Trans. Am. Clin. ClimatolAssoc 125, 130–140 (2014). [PMC free article] [PubMed] [Google Scholar]
- 85.Wise IA & Charchar FJ Epigenetic modifications in essential hypertension. Int. J. Mol. Sci 17, 451 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahn SY & Gupta C Genetic programming of hypertension. Front. Pediatr 5, 285 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang T et al. Gut dysbiosis is linked to hypertension. Hypertension 65, 1331–1340 (2015).This study demonstrates a clear association between gut dysbiosis and hypertension in rats and a small cohort of human patients with hypertension
- 88.Mell B et al. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol. Genom 47, 187–197 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Durgan DJ et al. Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertension 67, 469–474 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li J et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5, 14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Santisteban MM et al. Hypertension-linked pathophysiological alterations in the gut. Circ. Res 120, 312–323 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilck N et al. Salt-responsive gut commensal modulates T. Nature 551, 585–589 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pluznick JL et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl Acad. Sci. USA 110, 4410–4415 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Natarajan N et al. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom 48, 826–834 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marques FZ et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135, 964–977 (2017). [DOI] [PubMed] [Google Scholar]
- 96.Aleixandre A & Miguel M Dietary fiber and blood pressure control. Food Fund. 7, 1864–1871 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Whelton SP et al. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J. Hypertens 23, 475–481 (2005). [DOI] [PubMed] [Google Scholar]
- 98.Khalesi S, Sun J, Buys N & Jayasinghe R Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension 64, 897–903 (2014). [DOI] [PubMed] [Google Scholar]
- 99.Qi Y, Aranda JM, Rodriguez V, Raizada MK & Pepine CJ Impact of antibiotics on arterial blood pressure in a patient with resistant hypertension — a case report. Int. J. Cardiol 201, 157–158 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Werder AA, Amos MA, Nielsen AH & Wolfe GH Comparative effects of germfree and ambient environments on the development of cystic kidney disease in CFWwd mice. J. Lab Clin. Med 103, 399–407 (1984). [PubMed] [Google Scholar]
- 101.Vaziri ND et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 83, 308–315 (2013).This is a comprehensive study demonstrating that the gut microbiota is linked to CKD in rats and humans. Uraemia significantly altered gut microbial composition.
- 102.Felizardo RJ, Castoldi A, Andrade-Oliveira V & Câmara NO The microbiota and chronic kidney diseases: a double-edged sword. Clin. Transl Immunol 5, e86 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ranganathan N et al. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: a 6-month pilot scale trial in Canada. Curr. Med. Res. Opin 25, 1919–1930 (2009). [DOI] [PubMed] [Google Scholar]
- 104.Fukuuchi F et al. Intestinal bacteria-derived putrefactants in chronic renal failure. Clin. Exp. Nephr 6, 99–104 (2002). [Google Scholar]
- 105.Wang F et al. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology 17, 733–738 (2012). [DOI] [PubMed] [Google Scholar]
- 106.Kikuchi M, Ueno M, Itoh Y, Suda W & Hattori M Uremic toxin-producing gut microbiota in rats with chronic kidney disease. Nephron 135, 51–60 (2017). [DOI] [PubMed] [Google Scholar]
- 107.Jiang S et al. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci. Rep 7, 2870 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duranton F et al. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol 23, 1258–1270 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang WH et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl.J. Med 368, 1575–1584 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu KY et al. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci. Rep 7, 1445 (2017).This functional analysis of gut microbial communities in CKD identifies several altered genes responsible for TMAO production. Transplantation of faecal samples from patients with CKD induced an increased TMAO levels in the mouse recipients.
- 111.Scheppach W Effects of short chain fatty acids on gut morphology and function. Gut 35, S35–38 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sirich TL, Plummer NS, Gardner CD, Hostetter TH & Meyer TW Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol 9, 1603–1610 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aronov PA et al. Colonic contribution to uremic solutes. J. Am. Soc. Nephrol 22, 1769–1776 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stephen AM, Wiggins HS & Cummings JH Effect of changing transit time on colonic microbial metabolism in man. Gut 28, 601–609 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hatch M & Vaziri ND Enhanced enteric excretion of urate in rats with chronic renal failure. Clin. Sci 86, 511–516 (1994). [DOI] [PubMed] [Google Scholar]
- 116.Vaziri ND, Yuan J & Norris K Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am. J. Nephrol 37, 1–6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vaziri ND et al. Disintegration of colonic epithelial tight junction in uremia: a likely cause of CKD-associated inflammation. Nephrol. Dial. Transplant 27, 2686–2693 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Al Khodor S & Shatat IF Gut microbiome and kidney disease: a bidirectional relationship. Pediatr. Nephrol 32, 921–931 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shi K et al. Gut bacterial translocation may aggravate microinflammation in hemodialysis patients. Dig. Dis. Sci 59, 2109–2117 (2014). [DOI] [PubMed] [Google Scholar]
- 120.Yan J et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl Acad. Sci. USA 113, E7554–E7563 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Callen IR & Limarzi LR Blood and bone marrow studies in renal disease. Am. J. Clin. Pathol 20, 3–23 (1950). [DOI] [PubMed] [Google Scholar]
- 122.Hingorani S, Guthrie KA, Schoch G, Weiss NS & McDonald GB Chronic kidney disease in long-term survivors of hematopoietic cell transplant. Bone Marrow Transplant. 39, 223–229 (2007). [DOI] [PubMed] [Google Scholar]
- 123.Hingorani S, Gooley T, Pao E, Sandmaier B & McDonald G Urinary cytokines after HCT: evidence for renal inflammation in the pathogenesis of proteinuria and kidney disease. Bone Marrow Transplant. 49, 403–409 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hahm E et al. Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat. Med 23, 100–106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hayek SS, Quyyumi AA & Reiser J Soluble urokinase receptor and chronic kidney disease.N. Engl. J. Med 374, 891 (2016). [DOI] [PubMed] [Google Scholar]
- 126.Napoli C, Maione C, Schiano C, Fiorito C & Ignarro LJ Bone marrow cell-mediated cardiovascular repair: potential of combined therapies. Trends Mol. Med 13, 278–286 (2007). [DOI] [PubMed] [Google Scholar]
- 127.Sugimoto H et al. Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc. Natl Acad. Sci. USA 103, 7321–7326 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huls M, Russel FG & Masereeuw R Insights into the role of bone marrow-derived stem cells in renal repair. Kidney Blood Press Res. 31, 104–110 (2008). [DOI] [PubMed] [Google Scholar]
- 129.Jung C, Hugot JP & Barreau F Peyer’s patches: the immune sensors of the intestine. Int. J. Inflam 2010, 823710 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pedrinelli R et al. Low-grade inflammation and microalbuminuria in hypertension. Arterioscler Thromb. Vasc. Biol 24, 2414–2419 (2004). [DOI] [PubMed] [Google Scholar]
- 131.Costello-White R, Ryff CD & Coe CL Aging and low-grade inflammation reduce renal function in middle-aged and older adults in Japan and the USA. Age 37, 9808 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wenzel P et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124, 1370–1381 (2011). [DOI] [PubMed] [Google Scholar]
- 133.Guzik TJ et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med 204, 2449–2460 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chan CT et al. Obligatory role for B cells in the development of angiotensin II-dependent hypertension. Hypertension 66, 1023–1033 (2015). [DOI] [PubMed] [Google Scholar]
- 135.Moghadamrad S et al. Attenuated portal hypertension in germ-free mice: function of bacterial flora on the development of mesenteric lymphatic and blood vessels. Hepatology 61, 1685–1695 (2015). [DOI] [PubMed] [Google Scholar]
- 136.Chassaing B & Gewirtz AT Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol. Pathol 42, 49–53 (2014). [DOI] [PubMed] [Google Scholar]
- 137.Cani PD, Osto M, Geurts L & Everard A Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 3, 279–288 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mishima E et al. Evaluation of the impact of gut microbiota on uremic solute accumulation by a CE-TOFMS-based metabolomics approach. Kidney Int. 92, 634–645 (2017).This paper examines metabolite profiles of plasma, faeces and urine in germ-free animals compared with SPF controls and outlines the contributions of gut microbiota to the production of uraemic solutes.
- 139.Meijers BK, Bammens B, Verbeke K & Evenepoel P A review of albumin binding in CKD. Am. J. Kidney Dis 51, 839–850 (2008). [DOI] [PubMed] [Google Scholar]
- 140.Sirich TL, Aronov PA, Plummer NS, Hostetter TH & Meyer TW Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int. 84, 585–590 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu IW et al. p- Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transplant 26, 938–947 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lin CJ et al. p- Cresylsulfate and indoxyl sulfate level at different stages of chronic kidney disease.J. Clin. Lab. Anal 25, 191–197 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Magnusson M, Magnusson KE, Sundqvist T & Denneberg T Increased intestinal permeability to differently sized polyethylene glycols in uremic rats: effects of low- and high-protein diets. Nephron 56, 306–311 (1990). [DOI] [PubMed] [Google Scholar]
- 144.Magnusson M, Magnusson KE, Sundqvist T & Denneberg T Impaired intestinal barrier function measured by differently sized polyethylene glycols in patients with chronic renal failure. Gut 32, 754–759 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.de Almeida Duarte JB, de Aguilar-Nascimento JE, Nascimento M & Nochi RJ Bacterial translocation in experimental uremia. Urol. Res 32, 266–270 (2004). [DOI] [PubMed] [Google Scholar]
- 146.Vaziri ND, Dure-Smith B, Miller R & Mirahmadi MK Pathology of gastrointestinal tract in chronic hemodialysis patients: an autopsy study of 78 cases. Am. J. Gastroenterol 80, 608–611 (1985). [PubMed] [Google Scholar]
- 147.Ito S & Yoshida M Protein-bound uremic toxins: new culprits of cardiovascular events in chronic kidney disease patients. Toxins 6, 665–678 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Koppe L et al. p-Cresyl sulfate promotes insulin resistance associated with CKD. J. Am. Soc. Nephrol 24, 88–99 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sun CY, Chang SC & Wu MS Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS ONE 7, e34026 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wong J et al. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol 39, 230–237 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression. Antonie Van Leeuwenhoek 109, 1389–1396 (2016). [DOI] [PubMed] [Google Scholar]
- 152.Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT & Vinolo MA Regulation of immune cell function by short-chain fatty acids. Clin. Transl Immunol 5, e73 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang L et al. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin-angiotensin system. J. Hypertens 35, 1899–1908 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang T et al. Shifts in the gut microbiota composition due to depleted bone marrow beta adrenergic signaling are associated with suppressed inflammatory transcriptional networks in the mouse colon. Front. Physiol 8, 220 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim S et al. Angiotensin II regulation of proliferation, differentiation, and engraftment of hematopoietic stem cells. Hypertension 67, 574–584 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zubcevic J et al. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension 63, 542–550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zubcevic J et al. A single angiotensin II hypertensive stimulus is associated with prolonged neuronal and immune system activation in Wistar-Kyoto rats. Front. Physiol 8, 592 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim S et al. Hypertensive patients exhibit gut microbial dysbiosis and an increase in TH17 cells [abstract]. J. Hypertension 33 (Suppl. 1), 6B.07 (2015). [Google Scholar]
- 159.Richards EM, Pepine CJ, Raizada MK & Kim S The gut, its microbiome, and hypertension. Curr. Hypertens. Rep 19, 36 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ramezani A et al. Role of the gut microbiome in uremia: a potential therapeutic target. Am. J. Kidney Dis 67, 483–498 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Afsar B et al. Brain-kidney cross-talk: definition and emerging evidence. Eur J. Intern. Med 36, 7–12 (2016). [DOI] [PubMed] [Google Scholar]
- 162.Kaur J, Young BE & Fadel PJ Sympathetic overactivity in chronic kidney disease: consequences and mechanisms. Int. J. Mol. Sci 18, 1682 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Johns EJ, Kopp UC & DiBona GF Neural control of renal function. Compr. Physiol 1, 731–767 (2011). [DOI] [PubMed] [Google Scholar]
- 164.Bigazzi R, Kogosov E & Campese VM Altered norepinephrine turnover in the brain of rats with chronic renal failure. J. Am. Soc. Nephrol 4, 1901–1907 (1994). [DOI] [PubMed] [Google Scholar]
- 165.Amann K et al. Effects of low dose sympathetic inhibition on glomerulosclerosis and albuminuria in subtotally nephrectomized rats. J. Am. Soc. Nephrol 11, 1469–1478 (2000). [DOI] [PubMed] [Google Scholar]
- 166.Hausberg M et al. Sympathetic nerve activity in end-stage renal disease. Circulation 106, 1974–1979 (2002). [DOI] [PubMed] [Google Scholar]
- 167.Pongratz G & Straub RH The sympathetic nervous response in inflammation. Arthritis Res. Ther 16, 504 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lorton D & Bellinger DL Molecular mechanisms underlying β-adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells. Int. J. Mol. Sci 16, 5635–5665 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Singh MV, Chapleau MW, Harwani SC & Abboud FM The immune system and hypertension. Immunol. Res 59, 243–253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Grassi G et al. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension 57, 846–851 (2011). [DOI] [PubMed] [Google Scholar]
- 171.Fisher JP, Young CN & Fadel PJ Central sympathetic overactivity: maladies and mechanisms. Auton. Neurosci 148, 5–15 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shi P et al. Direct pro-inflammatory effects of prorenin on microglia. PLoS ONE 9, e92937 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M & Demkow U Brain inflammation and hypertension: the chicken or the egg? J. Neuroinflamm 12, 85 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.de Kloet AD, Liu M, Rodriguez V, Krause EG & Sumners C Role of neurons and glia in the CNS actions of the renin-angiotensin system in cardiovascular control. Am. J. Physiol. Regul. Integr. Comp. Physiol 309, R444–R458 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Adesso S et al. Indoxyl sulfate affects glial function increasing oxidative stress and neuroinflammation in chronic kidney disease: interaction between astrocytes and microglia. Front. Pharmacol 8, 370 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nishihara M, Takesue K & Hirooka Y Renal denervation enhances GABA-ergic input into the PVN leading to blood pressure lowering in chronic kidney disease. Auton. Neurosci 204, 88–97 (2017). [DOI] [PubMed] [Google Scholar]
- 177.Kurella M, Yaffe K, Shlipak MG, Wenger NK & Chertow GM Chronic kidney disease and cognitive impairment in menopausal women. Am. J. Kidney Dis 45, 66–76 (2005). [DOI] [PubMed] [Google Scholar]
- 178.Kurella Tamura M et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study.Am. J. Kidney Dis 52, 227–234 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jassal SK, Kritz-Silverstein D & Barrett-Connor E A prospective study of albuminuria and cognitive function in older adults: the Rancho Bernardo study. Am. J. Epidemiol 171, 277–286 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Helmer C et al. Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology 77, 2043–2051 (2011). [DOI] [PubMed] [Google Scholar]
- 181.Kurella Tamura M et al. Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. Am. J. Kidney Dis 58, 756–763 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.De Deyn PP, Vanholder R, Eloot S & Glorieux G Guanidino compounds as uremic (neuro)toxins. Semin. Dial 22, 340–345 (2009). [DOI] [PubMed] [Google Scholar]
- 183.Goek ON et al. Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrol. Dial. Transplant 28, 2131–2138 (2013). [DOI] [PubMed] [Google Scholar]
- 184.Clarke G et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 18, 666–673 (2013). [DOI] [PubMed] [Google Scholar]
- 185.Orhan F et al. Tryptophan metabolism along the kynurenine pathway downstream of Toll-like receptor stimulation in peripheral monocytes. Scand. J. Immunol 84, 262–271 (2016). [DOI] [PubMed] [Google Scholar]
- 186.Davis I & Liu A What is the tryptophan kynurenine pathway and why is it important to neurotherapeutics? Expert Rev. Neurother 15, 719–721 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG & Keane RW Pattern recognition receptors and central nervous system repair.Exp. Neurol 258, 5–16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Maddison DC & Giorgini F The kynurenine pathway and neurodegenerative disease. Semin. Cell Dev. Biol 40, 134–141 (2015). [DOI] [PubMed] [Google Scholar]
- 189.van Koppen A et al. Healthy bone marrow cells reduce progression of kidney failure better than CKD bone marrow cells in rats with established chronic kidney disease. Cell Transplant 21, 2299–2312 (2012). [DOI] [PubMed] [Google Scholar]
- 190.Romano KA et al. Metabolic, Epigenetic, and Transgenerational Effects of Gut Bacterial Choline Consumption. Cell Host Microbe 22, 279–290.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Savidge TC Epigenetic regulation of enteric neurotransmission by gut bacteria. Front. Cell Neurosci 9, 503 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li L, Ma L & Fu P Gut microbiota-derived short-chain fatty acids and kidney diseases. Drug Des. Devel Ther 11, 3531–3542 S150825 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Paul B et al. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin. Epigenet 7, 112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang T, Owen JL, Lightfoot YL, Kladde MP & Mohamadzadeh M Microbiota impact on the epigenetic regulation of colorectal cancer. Trends Mol. Med 19, 714–725 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shiels PG, McGuinness D, Eriksson M,Kooman JP & Stenvinkel P The role of epigenetics in renal ageing. Nat. Rev. Nephrol 13, 471–482 (2017). [DOI] [PubMed] [Google Scholar]
- 196.Shi S et al. Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J. Am. Soc. Nephrol 19, 2159–2169 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ko YA et al. Cytosine methylation changes in enhancer regions of core pro-fibrotic genes characterize kidney fibrosis development. Genome Biol. 14, R108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mu S et al. Epigenetic modulation of the renal β-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat. Med 17, 573–580 (2011). [DOI] [PubMed] [Google Scholar]
- 199.Lee HA et al. Tissue-specific upregulation of angiotensin-converting enzyme 1 in spontaneously hypertensive rats through histone code modifications. Hypertension 59, 621–626 (2012). [DOI] [PubMed] [Google Scholar]
- 200.Hoban AE et al. Microbial regulation of microRNA expression in the amygdala and prefrontal cortex. Microbiome 5, 102 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Semenkovich NP et al. Impact of the gut microbiota on enhancer accessibility in gut intraepithelial lymphocytes. Proc. Natl Acad. Sci. USA 113, 14805–14810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mukerjee S et al. Perinatal exposure to Western diet programs autonomic dysfunction in the male offspring. Cell. Mol. Neurobiol 38, 233–242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kim S et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin. Sci 132, 701–718 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ponticelli C & Campise MR Neurological complications in kidney transplant recipients. J. Nephrol 18, 521–528 (2005). [PubMed] [Google Scholar]
- 205.Shi P et al. Brain microglial cytokines in neurogenic hypertension. Hypertension 56, 297–303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hering D et al. Effect of renal denervation on kidney function in patients with chronic kidney disease. Int. J. Cardiol 232, 93–97 (2017). [DOI] [PubMed] [Google Scholar]
- 207.Ott C et al. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J. Hypertens 33, 1261–1266 (2015). [DOI] [PubMed] [Google Scholar]
- 208.Clark A & Mach N Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. J. Int. Soc. Sports Nutr 13, 43 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Steinberg D, Bennett GG & Svetkey L The DASH diet, 20 years later. JAMA 317, 1529–1530 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jenkins DJ et al. Soluble fiber intake at a dose approved by the US Food and Drug Administration for a claim of health benefits: serum lipid risk factors for cardiovascular disease assessed in a randomized controlled crossover trial. Am. J. Clin. Nutr 75, 834–839 (2002). [DOI] [PubMed] [Google Scholar]
- 211.Pins JJ et al. Do whole-grain oat cereals reduce the need for antihypertensive medications and improve blood pressure control? J. Fam. Pract 51, 353–359 (2002). [PubMed] [Google Scholar]
- 212.Chiavaroli L, Mirrahimi A, Sievenpiper JL, Jenkins DJ & Darling PB Dietary fiber effects in chronic kidney disease: a systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr 69, 761–768 (2015). [DOI] [PubMed] [Google Scholar]
- 213.Lu L et al. Dietary fiber intake is associated with chronic kidney disease (CKD) progression and cardiovascular risk, but not protein nutritional status, in adults with CKD. Asia Pac. J. Clin. Nutr 26, 598–605 (2017). [DOI] [PubMed] [Google Scholar]
- 214.Rossi M et al. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): a randomized trial. Clin. J. Am. Soc. Nephrol 11, 223–231 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Vaziri ND et al. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS ONE 9, e114881 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Koppe L, Mafra D & Fouque D Probiotics and chronic kidney disease. Kidney Int. 88, 958–966 (2015).This review introduces basic concepts of gut and kidney communication, summarizes the current available probiotic treatments in animals and human patients with CKD and highlights the potential mechanisms of probiotics in the treatment of CKD.
- 217.Kieffer DA et al. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am. J. Physiol. Renal Physiol 310, F857–F871 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 74, 349–355 (1996). [DOI] [PubMed] [Google Scholar]
- 219.Eyler RF & Mueller BA Antibiotic pharmacokinetic and pharmacodynamic considerations in patients with kidney disease. Adv. Chron. Kidney Dis 17, 392–403 (2010). [DOI] [PubMed] [Google Scholar]
- 220.Kim GJ, Je NK, Kim DS & Lee S Adherence with renal dosing recommendations in outpatients undergoing haemodialysis. J. Clin. Pharm. Ther 41, 26–33 (2016). [DOI] [PubMed] [Google Scholar]
- 221.Smith KE et al. Antibiotic treatment of Escherichia coli O157 infection and the risk of hemolytic uremic syndrome, Minnesota. Pediatr.Infect. Dis. J 31, 37–41 (2012). [DOI] [PubMed] [Google Scholar]
- 222.Xu Y, Liu Y, Pei J, Yao S & Cheng C Bacteriophage therapy against Enterobacteriaceae. Virol. Sin 30, 11–18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hamdi S et al. Characterization of two polyvalent phages infecting Enterobacteriaceae. Sci. Rep 7, 40349 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Thongprayoon C, Cheungpasitporn W, Phatharacharukul P, Mahaparn P & Bruminhent J High mortality risk in chronic kidney disease and end stage kidney disease patients with Clostridium difficile infection: a systematic review and meta-analysis. J. Nat. Sci 1, e85 (2015). [PMC free article] [PubMed] [Google Scholar]
- 225.Youngster I et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 312, 1772–1778 (2014). [DOI] [PubMed] [Google Scholar]
- 226.Ott SJ et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile Infection. Gastroenterology 152, 799–811.e7 (2017). [DOI] [PubMed] [Google Scholar]
- 227.Itoh Y, Ezawa A, Kikuchi K, Tsuruta Y & Niwa T Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal. Bioanal. Chem 403, 1841–1850 (2012). [DOI] [PubMed] [Google Scholar]
- 228.Yamaguchi J, Tanaka T & Inagi R Effect of AST-120 in chronic kidney disease treatment: still a controversy? Nephron 135, 201–206 (2017). [DOI] [PubMed] [Google Scholar]
- 229.Nikolic SB, Sharman JE, Adams MJ & Edwards LM Metabolomics in hypertension. J. Hypertens 32, 1159–1169 (2014). [DOI] [PubMed] [Google Scholar]
- 230.Goek ON et al. Serum metabolite concentrations and decreased GFR in the general population. Am. J. Kidney Dis 60, 197–206 (2012). [DOI] [PubMed] [Google Scholar]
- 231.O’Mahony SM, Clarke G, Borre YE, Dinan TG & Cryan JF Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res 277, 32–48 (2015). [DOI] [PubMed] [Google Scholar]
- 232.Madeo F, Eisenberg T, Pietrocola F & Kroemer G Spermidine in health and disease. Science 359, eaan2788 (2018). [DOI] [PubMed] [Google Scholar]
- 233.Mazumder MK, Giri A, Kumar S & Borah A A highly reproducible mice model of chronic kidney disease: evidences of behavioural abnormalities and blood-brain barrier disruption. Life Sci. 1 61, 27–36 (2016). [DOI] [PubMed] [Google Scholar]
- 234.Lau WL, Kalantar-Zadeh K & Vaziri ND The gut as a source of inflammation in chronic kidney disease. Nephron 130, 92–98 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Vaziri ND, Zhao YY & Pahl MV Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol. Dial. Transplant 31, 737–746 (2016). [DOI] [PubMed] [Google Scholar]
- 236.Wester AL, Vatn MH & Fausa O Secondary amyloidosis in inflammatory bowel disease: a study of 18 patients admitted to Rikshospitalet University Hospital, Oslo, from 1962 to 1998. Inflamm. Bowel Dis 7, 295–300 (2001). [DOI] [PubMed] [Google Scholar]
- 237.McBryde FD, Guild SJ, Barrett CJ, Osborn JW & Malpas SC Angiotensin II-based hypertension and the sympathetic nervous system: the role of dose and increased dietary salt in rabbits. Exp. Physiol 92, 831–840 (2007). [DOI] [PubMed] [Google Scholar]