Abstract
B-cell non-Hodgkin lymphoma (NHL) is the most frequent hematologic malignancy. Despite the refinement of chemoimmunotherapy, a substantial number of patients experience chemorefractory disease. Anti-CD19 chimeric antigen receptor (CAR) T-cell therapy is considered the most promising and effective therapy to overcome chemorefractory B-cell NHL. Based on the promising results obtained from pivotal trials, the US Food and Drug Administration and European Medicines Agency approved anti-CD19 CAR T-cell therapy for relapsed/refractory diffuse large B-cell lymphoma. Nonetheless, there remain several controversial issues and problems awaiting solutions, including optimal management of toxicities, overcoming relapsed/refractory disease after CAR T-cell therapy, and improving CAR-T manufacturing platform. Definite unmet medical needs among patients with chemorefractory B-cell NHL still exist. CAR T-cell therapy might be a game changer that can defeat chemorefractory B-cell NHL, and further clinical development is warranted. In this review, we summarize the recent clinical developments, clinical implications, and perspectives of CAR T-cell therapy, focusing on B-cell NHL.
Keywords: axicabtagene ciloleucel, B-cell non-Hodgkin lymphoma, CAR-T, CD19, chimeric antigen receptor, diffuse large B-cell lymphoma, lisocabtagene maraleucel, tisagenlecleucel
Introduction
Non-Hodgkin lymphoma (NHL) is the most frequent hematologic malignancy. It is divided into approximately 70 histologic subtypes based on the revised WHO classification.1 Among them, diffuse large B-cell lymphoma (DLBCL) is the most frequent subtype and has an aggressive clinical course.
Refinement of chemotherapy regimens and introduction of the anti-CD20 monoclonal antibody, rituximab, have improved the prognosis of patients with DLBCL in the recent decades.2,3 Currently, approximately 50–60% of patients with newly diagnosed DLBCL are cured with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) therapy in combination with rituximab. However, once it becomes a refractory disease, it is difficult to manage the disease with conventional chemotherapies. According to data from Western countries, the median overall survival (OS) in patients with refractory DLBCL is only 6–7 months,4 similar to Asian countries.5
To overcome the chemorefractoriness of DLBCL, several novel agents are actively being developed (Table 1).6–15 Among them, anti-CD19 chimeric antigen receptor (CAR) T-cell therapy is considered the most promising and effective therapy for relapsed/refractory DLBCL.16 Several studies have shown its remarkable efficacy in patients with relapsed/refractory DLBCL. In 2017, the US Food and Drug Administration (FDA) approved the first anti-CD19 CAR T-cell therapy for patients with relapsed/refractory DLBCL after two or more lines of systemic therapy.17 Currently, two types of CAR T-cell therapies (tisagenlecleucel and axicabtagene-ciloleucel) have been available in the United States and Europe.
Table 1.
Selected new agent studies on relapsed/refractory DLBCL.
| Agents | Mechanism of action | Subtype | Phase | N | ORR (%) | %CR |
|---|---|---|---|---|---|---|
| Ibrutinib6 | BTK inhibitor | ABC type | I/II | 38 | 37 | 16 |
| Lenalidomide7 | IMiD | Non-GCB type | II/III | 28 | 29 | 14 |
| MOR208 + Lenalidomide8,9 | Anti-CD19 antibody | All subtypes | II | 44 | 53 | 32 |
| Polatuzumab vedotin10 | Anti-CD79b ADC | All subtypes | I | 25 | 56 | 15 |
| Tazemetostat11,12 | EZH2 inhibitor | EZH2 mutation (+) | II | 17 | 29 | 0 |
| Nivolumab13 | PD-1 blockade | All subtypes | II | 121 | 13 | 3 |
| Tisagenlecleucel14 | Anti-CD19 CAR-T | All subtypes | II | 93 | 52 | 40 |
| Axicabtagene ciloleucel15 | Anti-CD19 CAR-T | All subtypes | II | 101 | 82 | 54 |
ABC, activated B-cell; BTK, Bruton’s tyrosine kinase; CAR-T, chimeric antigen receptor T cell; CR, complete remission; DLBCL, diffuse large B-cell lymphoma; EZH2, enhancer of zeste homolog 2; GCB, germinal center B cell; IMiD, immunomodulatory drug; ORR, overall response rate; PD-1, programmed death-1.
In this review, we summarize the recent clinical developments, clinical implications, and perspectives of CAR T-cell therapy, focusing on B-cell NHL.
Structure of anti-CD19 CAR
CD19 is a B-cell receptor-associated protein expressed on B-cell surface. It is considered an optimal therapeutic target because it is uniformly expressed on the surface of B-cell malignancies and is solely expressed in the B-cell lineage and not in other lineages or tissues. The majority of currently developed CAR T cells for B-cell NHL utilize CD19 as a therapeutic target.
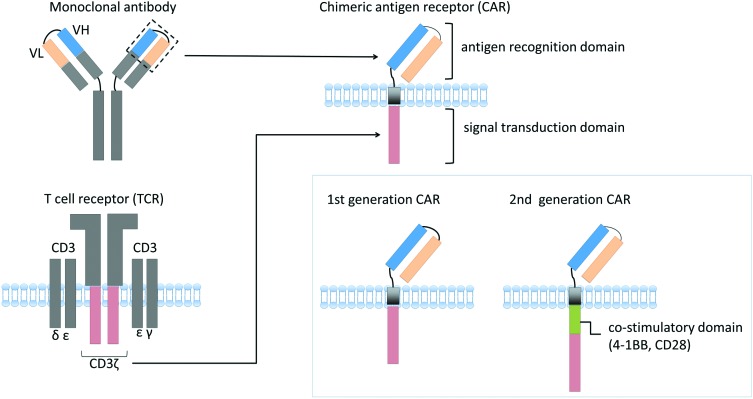
Anti-CD19 CAR is a recombinant molecule consisting of three parts: (1) an antigen recognition domain derived from a single-chain variable domain (scFv) of anti-CD19 monoclonal antibody; (2) a transmembrane domain; and (3) the signal transduction domain, CD3ζ, derived from T-cell receptor (TCR) (Figure 1).18 When a CAR-T recognizes a specific antigen, the cell is activated via the intracellular signal transduction domain resulting in the killing of target cells. Nonetheless, first-generation CAR-T showed limited expansion and antitumor efficacy. This is partly because first-generation CAR-T expansion was solely dependent on interleukin (IL)-2 production via TCR activation. In physiological conditions, T cells are activated not only via TCR, but also via several costimulatory receptors. Therefore, to improve CAR-T expansion capacity and antitumor activity, second-generation CAR contains a costimulatory domain, such as CD2819 or 4-1BB20,21 (Figure 1). Large-scale CAR-T trials that were conducted recently used these second-generation CARs.
Figure 1.
Schematic structure of a chimeric antigen receptor.
Chimeric antigen receptor (CAR) comprises a single-chain variable domain derived from a monoclonal antibody, a transmembrane domain, and a signal transduction domain of T-cell receptor (CD3ζ). To improve CAR T-cell expansion capacity, second-generation CAR that contains costimulatory domain is currently used in several clinical trials.
VH, heavy-chain variable region; VL, light-chain variable region.
An outline of CAR T-cell therapy
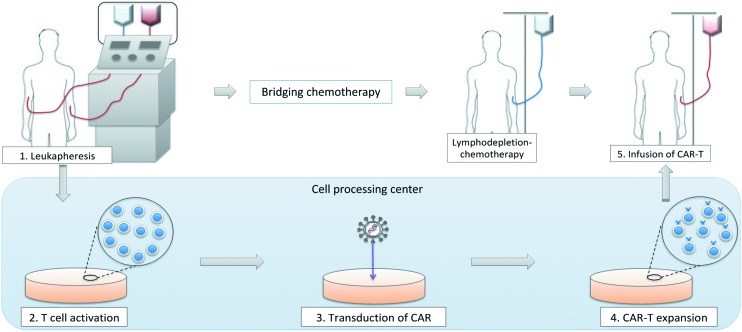
The outline of CAR T-cell therapy is shown in Figure 2. First, mononuclear cells are collected from peripheral blood of a patient using a blood cell separator. The patient’s mononuclear cells are transferred to a cell-processing center, and selected T cells are activated in a proliferative environment. CAR genes are transfected into T cells using retroviral or lentiviral vectors, and CAR T cells are generated. The expanded CAR T cells are sent back to the institution and are infused into the patient. This manufacturing process takes at least 2–3 weeks in general. Therefore, physicians often perform bridging chemotherapy to avoid rapid disease progression and to maintain patient’s general condition during the CAR-T production period.
Figure 2.
Outline of CAR T-cell therapy.
CAR, chimeric antigen receptor.
Before the infusion of CAR T cells, lymphodepleting (LD) chemotherapy is required. LD chemotherapy decreases the number of T cells in vivo, including regulatory T cells, and consequently upregulates cytokines such as IL-7 and IL-15.22 These cytokines promote T-cell expansion, including CAR T cells, and augment the antitumor activity of CAR T-cell therapy. Several regimens, including fludarabine plus cyclophosphamide (FC), cyclophosphamide plus etoposide, cyclophosphamide alone, or bendamustine, have been utilized as a LD chemotherapeutic regimen. Previous study conducted by Turtle and colleagues demonstrated that the patients who received FC-based LD chemotherapy showed higher complete response (CR) rate compared to that of patients who received fludarabine with or without etoposide (CR rate 50% versus 8%, p=0.02).23 Therefore, fludarabine-based LD chemotherapy is preferably used in recent CAR-T trials.
Clinical trials of anti-CD19 CAR T-cell therapy for B-cell NHL (focusing on DLBCL)
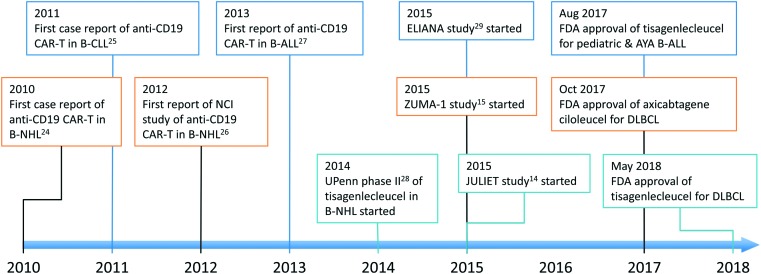
Since the first case report of anti-CD19 CAR-T therapy in 2010,24 it has been actively studied and received the first approval by the US FDA within 7 years (Figure 3).14,15,24–29 Currently, several anti-CD19 CAR T-cell therapies are being tested in B-cell malignancies. The details of selected CAR T cells are summarized in Table 2. Among these CAR-T, tisagenlecleucel (CTL019), axicabtagene-ciloleucel (KTE-C19, axi-cel), and lisocabtagene-maraleucel (JCAR017, liso-cel) are being tested in relatively larger scale clinical trials of patients with DLBCL (Table 3).
Figure 3.
Development of anti-CD19 CAR T-cell therapy.
AYA, adolescent and young adult; B-ALL, B-cell acute lymphoblastic leukemia; B-CLL, B-cell chronic lymphocytic leukemia; B-NHL, B-cell non-Hodgkin lymphoma; CAR-T, chimeric antigen receptor T-cell therapy; DLBCL, diffuse large B-cell lymphoma; FDA, Food and Drug Administration; UPenn, University of Pennsylvania.
Table 2.
Currently developing anti-CD19 CAR T-cell therapies.
| Type of CAR-T | Academic institute | Collaborating company | Hinge | Transmembrane | Costimulatory | Production starting cell population | Vector |
|---|---|---|---|---|---|---|---|
| Tisagenlecleucel | UPenn | Novartis | CD8 | CD8 | 4-1BB | PBMC | Lentivirus |
| Axi-cel | NCI | Kite/Gilead | CD28 | CD28 | CD28 | PBMC | Retrovirus |
| Liso-cel | FHCRC/SCRI | Juno/Celgene | IgG4 | IgG4 | 4-1BB | CD4+/CD8+ | Lentivirus |
| JCAR014 | FHCRC | Juno/Celgene | IgG4 | CD28 | 4-1BB | CD4+/CD8+CM | Lentivirun |
| Product of MDACC | MDACC | Ziopharma | IgG4 | CD28 | CD28 | PBMC | Transposon |
| UCART19 | UCL etc. | Cellectis/Servier/Pfizer | CD8 | CD8 | 4-1BB | PBMC of healthy donor | Lentivirus/Gene editing with TALEN |
Axi-cel, axicabtagene ciloleucel; CM, central memory; FHCRC, Fred Hutchinson Cancer Research Center; Liso-cel, lisocabtagene maraleucel; MDACC, MD Anderson Cancer Center; NCI, National Cancer Institute; PBMC, peripheral blood mononuclear cell; TALEN, transcription activator-like effector nucleases; UCL, University College London; UPenn, University of Pennsylvania.
Table 3.
Clinical trials of anti-CD19 CAR T-cell therapy for DLBCL.
| JULIET | ZUMA-1 | Transcend NHL001 | |
|---|---|---|---|
| CAR-T | Tisagenlecleucel | Axicabtagene ciloleucel | Lisocabtagene maraleucel |
| Leukapheresis pts | 165, 111 infused | 112, 108 infused | 140, 114 infused |
| Evaluable pts | 111 for safety 93 for response |
108 | 102 for safety 37 at DL2 for response |
| Histologies | DLBCL, t-FL | Cohort 1: DLBCL Cohort 2: PMBL, t-FL |
Aggressive B-cell NHL |
| CNS disease | Excluded | Excluded | Allowed |
| Prior allo-SCT | Excluded | Excluded | Allowed |
| LD chemo | CY 250 mg/m2, FLU 25 mg/m2, for 3 days or Bendamustine 90 mg/m2 |
CY 500 mg/m2, FLU 30 mg/m2, for 3 days | CY 300 mg/m2, FLU 30 mg/m2, for 3 days |
| Production failure | 6.1% (9/147) -> 3% | 1% (110/111) | 1% (2/134) |
| CD4:CD8 | Not specified | Not specified | CD4:CD8=1:1 |
| CAR-T cell dose | 1–5×108 cells | 2×106 cells/kg | DL1: 5×107 cells DL2: 1×108 cells |
| Median follow-up duration | 14 months | 27.1 months | 8 months |
| ORR/CR rate | 33%/29% (at 6M) | 39%/37% (at 27.1M) | 49%/46% (DL2 DLBCL) |
| Grade≥3 CRS | 22% | 11% | 1% |
| Grade≥3 Neurotoxicity | 12% | 32% | 13% |
| Received tocilizumab | 14% | 43% | 17% |
| Received steroid | 10% | 27% | 21% |
allo-SCT, allogeneic stem cell transplantation; CAR-T, chimeric antigen receptor T cell; CR, complete response; CRS, cytokine-release syndrome; CY, cyclophosphamide; DL, dose level; DLBCL, diffuse large B-cell lymphoma; FLU, fludarabine; LD, lymphodepletion; NHL, non-Hodgkin lymphoma; ORR, overall response rate; PMBL, primary mediastinal large B-cell lymphoma; t-FL, transformed follicular lymphoma.
Tisagenlecleucel
Tisagenlecleucel is a second-generation CAR-T utilizing 4-1BB as a costimulatory domain. It is developed by the investigators of the University of Pennsylvania (UPenn) in collaboration with Novartis. It is the first CAR T cell to obtain the FDA approval for pediatric B-cell acute lymphoblastic leukemia (B-ALL) in 2017.30
A pilot study of tisagenlecleucel in patients with B-cell lymphoma was conducted by the investigators of the UPenn.28 The primary endpoint was the overall response rate (ORR) at 3 months. In total, 38 patients with relapsed/refractory B-cell NHL (DLBCL, n=23; follicular lymphoma [FL], n=15) were enrolled. Only 28 of 38 patients (73%) received CAR T-cell infusion. The remaining 10 patients were unable to receive the infusion because of rapid disease progression (n=4), manufacturing failure due to low lymphocyte count (n=5), and withdrawal of consent (n=1). In the 28 evaluable patients (DLBCL, n=14; FL, n=14), the ORR was 64% (18 of 28), with 57% (16 of 28) achieving CR. The ORR of each histologic subtype at 3 months after infusion was 50% (7 of 14) in DLBCL and 79% (11 of 14) in FL. Moreover, 16 patients (DLBCL, n=6; FL n=10) who achieved CR at 6 months after infusion obtained durable responses with a median follow-up duration of 29.3 months. Notably, 4 patients (DLBCL, n=1; FL, n=3) who achieved partial response (PR) at 3 months converted into CR at 6 months after the infusion. These findings suggest that the best response to tisagenlecleucel can be observed later in selected patients compared with conventional chemotherapy.
Cytokine-release syndrome (CRS) was observed in 16 patients (53%), and most of them were of grade 2 (grade 2, n=14; grade 3, n=4; grade 4, n=1).28 The prevalence of severe CRS was relatively lower than that in patients with B-ALL receiving CAR T-cell therapy. Neurotoxicity was observed in 11 patients and 8 of them had grade 1–2 neurotoxicity. The remaining 3 patients experienced grade 3 or higher neurotoxicity. Moreover, 1 patient died from encephalopathy associated with neurotoxicity, while the neurotoxicities of other 2 patients recovered within a week.
Subsequently, an international phase II trial of tisagenlecleucel for relapsed/refractory DLBCL was conducted (JULIET; NCT02445248).14,31 JULIET is the global CAR-T trial for DLBCL and 27 sites across 10 countries participated. Adult patients (≥18 years of age) with two or more prior treatments were eligible, and active central nervous system (CNS) involvement was excluded. The primary endpoint was the best ORR, and the null hypothesis was 20% or lower.
In total, 165 patients were enrolled in this study. However, only 111 patients were infused with CAR-T cells: 4 patients were awaiting infusion at data cut off, 12 were production failure, 16 died before infusion, 16 had their treating physician decide against further participation, 3 had an adverse event, 2 decided against further participation, and 1 had a protocol deviation. Reasons for discontinuation such as death, physician’s decision, and patient’s decision were mainly related to disease progression during the manufacturing period. These patients tended to have a lower performance status than those who received an infusion, and a greater proportion of the patients who did not receive an infusion were refractory to the most recent prior therapy. Among the 111 infused patients, 54 patients (49%) relapsed after autologous stem cell transplantation. Double- or triple-hit lymphomas were also included (19 of 70 evaluable patients; 27%). Among the 93 evaluable patients, the best ORR of tisagenlecleucel was 52% (48/93) with 40% CR (37/93) and the study met the primary endpoint. Subgroup analyses showed no difference in ORR based on prior treatment or other risk factors, such as cell of origin and MYC and BCL2 rearrangement. The common adverse events were CRS (all grade, 58% [64/111]; grade 3, 14% [15/111]; grade 4, 8% [9/111]), neurotoxicity (all grade, 21% [23/111], grade 3, 7% [8/111]; grade 4, 5% [5/111]), prolonged cytopenia (32%), and infections (34% [38/111]). There were no deaths associated with CAR-T-related toxicities.
CRS occurred at a median of 3 days (range 1–9) after infusion, and 28% of patients experienced hypotension that required intervention. In total, 15% of patients received tocilizumab for IL-6 blockade.
Although the median progression-free survival (PFS) was only 2.9 months in all patients, it has not been reached in patients with CR. The estimated PFS rate at 12 months was 83% in patients with CR or PR at 3 months. These data suggest that the response status at month 3 might be an indicator of durable responses. A longer follow-up data was presented at the 60th Annual Meeting of the American Society of Hematology (ASH) in 2018.32 The median duration of response and OS for patients in CR were not reached within the median follow-up duration of 19.3 months. Relapse-free survival rate among all responders was 64% both at 12 months and at 18 months.
Based on these promising results, the US FDA approved tisagenlecleucel for relapsed/refractory DLBCL in May 2018. It was also approved by the European Medicines Agency (EMA) in 2018.
Axicabtagene-ciloleucel (Axi-cel)
Axi-cel is a second-generation CAR-T utilizing a CD28 costimulatory domain.
It was initially developed by the investigators of the National Cancer Institute (NCI). This construct is now being developed by Kite Pharma, Gilead Sciences, and Daiichi Sankyo. They conducted a pivotal phase I/II study of axi-cel in patients with R/R DLBCL called ZUMA-1 trial (NCT02348216).15,33,34
In the phase I part, 7 patients with refractory DLBCL received axi-cel infusion at a target dose of 2×106 cells/kg.33 Five of the 7 patients (71%) achieved objective response within a month after the infusion, with 4 of 7 patients (57%) achieving CR; 6 of 7 patients experienced CRS, 71% (5/7) experienced grade 1–2 CRS, and 14% (1/7) experienced grade 4 CRS, which was a dose-limiting toxicity (DLT). All evaluable patients experienced at least one neurotoxicity, with 43% (3/7) having maximum grade 3 and 14% (1/7) a maximum grade 4 (occurring in the same patient with a DLT). Except for 1 patient with DLT, CRS and neurotoxicity were reversible (median duration was 7–8 days).
Based on these results, the subsequent pivotal phase II part was conducted.15,34 Different from other CAR-T studies, bridging chemotherapy was not allowed in this trial. In total, 111 patients with relapsed/refractory DLBCL were enrolled and 101 patients received axi-cel. Axi-cel was successfully manufactured in 99% of the patients enrolled. The median turnaround time from leukapheresis to the delivery of axi-cel to the treatment facility was 17 days, which is a relatively short period when compared to other trials. This is partly because the NCI group developed a new rapid cell expansion procedure for axi-cel, making it possible to implement a 6–8 day process of manufacturing.35
Among 101 evaluable patients, the best ORR was 82% (83/101) with 54% (55/101) CR and 28% (28/101) PR. The best ORR, a primary endpoint of this study, was significantly higher than that in the historical control of refractory DLBCL who are treated with conventional chemotherapies.4 Recently, long-term follow-up data have been published.34 With a median follow-up of 27.1 months, the median duration of response was 11.1 months, and the median PFS was 5.9 months. The median PFS of patients with CR/PR at 3 months was not reached, whereas the median PFS of stable disease (SD) was only 7.3 months.34 Notably, 11 of 33 patients with PR at 1 month, and 11 of 24 patients with SD at 1 month, subsequently converted into CR. The most conversions occurred by 6 months after infusion. These findings are similar to those of other studies.28
Grade 3 or higher CRS occurred in 11% (12/108) of patients and grade 3 or higher neurotoxicity was observed in 32% (35/108), which was relatively higher than that of other studies. Moreover, 4 patients died in this study: 2 of them had cardiac arrest associated with severe CRS, while the remaining 2 patients died of reasons not related to axi-cel treatment.
Based on these results, the US FDA approved axi-cel for relapsed refractory DLBCL after at least two lines of therapy in October 2017, and EMA approved in June 2018.
Lisocabtagene-maraleucel
Investigators at Fred Hutchinson Cancer Research Center (FHCRC), the Memorial Sloan Kettering Cancer Center, and Seattle Children’s Research Institute founded a venture, Juno Therapeutics, and conducted several clinical trials of CD19 CAR-T products: JCAR014, JCAR015, JCAR017, JCAR021, and others. Among them, JCAR017 (lisocabtagene-maraleucel, liso-cel) was investigated in later phase clinical trials in patients with B-cell NHL. Liso-cel is a second-generation anti-CD19 CAR T cell utilizing 4-1BB costimulatory domain and produced from separated subsets of CD4- and CD8-positive cells to make a CD4/8 ratio of 1:1 in CAR-T. It is based on the results of preclinical studies by the investigators at FHCRC. They reported that the CAR-T generated from a different subset of T cells show a different function in vivo.36 For example, CD8-positive central memory (CD8+CM)-CAR-T exerted a potent direct antitumor activity, and CD4+-CAR-T showed a milder activity than CD8+CM-CAR-T. CD4+-CAR-T produced several inflammatory cytokines, and after infusion of CD8+CM-CAR-T, synergistic enhancement of proliferation was observed. Based on these findings, it was implied that liso-cel was produced from separate subsets of CD4+ and CD8+T cells.
A single institute phase I study of second-generation CAR T cell containing 4-1BB costimulatory domain and with defined ratio of CD4/CD8+CM (JCAR014) in patients with relapsed/refractory B-cell NHL was conducted in FHCRC.23 In total, 32 patients were enrolled, and 30 patients were evaluable for response; 18 patients were treated with FC-based LD chemotherapy and remaining 12 patients received fludarabine with or without etoposide. The ORR and CR rate in patients who received FC-based LD chemotherapy were 72 and 50%, respectively. In contrast, the ORR of patients who received fludarabine with or without etoposide was 50%, and the CR rate was only 8%.
Currently, Juno therapeutics and Celgene are conducting a US multicenter phase I study of liso-cel (JCAR017) named Transcend NHL001. The study initially enrolled various subtypes of aggressive B-cell NHL and subsequent expansion cohort enrolling selected pivotal population (CORE cohort): DLBCL, double/triple-hit lymphoma, and transformed follicular lymphoma. The updated results of Transcend NHL001 in CORE cohort have been presented at the ASCO meeting in 2018.37 In total, 37 patients with relapsed/refractory DLBCL received the pivotal dose of liso-cel: single dose of 1×108 cells. The ORR at 6 months was 49% with 46% CR. Remarkably, the toxicities were well managed and only 1% of patients experienced grade 3 or higher CRS and 13% experienced grade 3 or 4 neurotoxicity. Based on these promising results, a multicenter international phase II study of liso-cel is ongoing in Europe and Japan.
Problems to be solved in CD19 CAR T-cell therapy
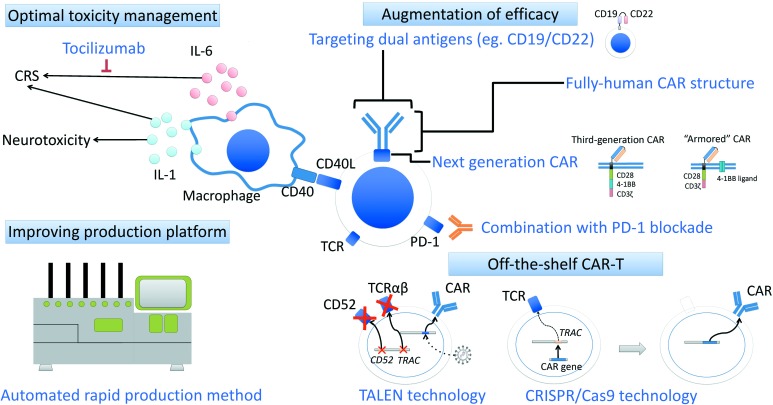
Second-generation anti-CD19 CAR T-cell therapies demonstrate substantial efficacy in patients with chemorefractory DLBCL (Table 3). However, several problems remain to be solved (Figure 4).
Figure 4.
Possible way to overcome the problems in the treatment of second-generation anti-CD19 CAR T-cell therapy.
There are several controversial issues and problems awaiting solutions, including optimal management of toxicities, overcoming relapsed/refractory disease after CAR T-cell therapy, and improving CAR-T manufacturing platform. The ways to overcome these problems are currently investigated.
CAR, chimeric antigen receptor; CRISPR/Cas9, clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9; CRS, cytokine-release syndrome; PD-1, programmed death 1; TALEN, transcription activator-like effector nuclease; TCR, T-cell receptor.
Toxicity management
One of the most important problems of CAT T-cell therapy is the management of CRS and neurotoxicity.38
CRS is a general term for adverse events related to immune activation, which occurs several hours to 14 days following CAR T-cell infusion. When a CAR T cell recognizes a specific antigen on the tumor cell, it is activated and secretes cytokines including IL-6, IL-10, IL-15, and tumor necrotic factor (TNF)-α.39 These cytokines promote CAR T-cell expansion and subsequent antitumor activity. However, too many cytokines lead to severe CRS. In other words, the immune response of CAR T-cell therapy is a double-edge sword. Recent studies suggested several possible risk factors of sever CRS such as; higher peak expansion level of CAR-T, tumor burden, baseline lactate dehydrogenase (LDH) level, early-onset CRS within 3 days of infusion, and elevations of selected cytokine level after infusion.39–42
IL-6 blockade, tocilizumab, is considered the most effective available agent in the management of CRS. In early clinical trials of CAR T-cell therapy, tocilizumab with or without steroid were carefully used because it might decrease the efficacy of CAR T-cell therapy. According to the ‘Penn scale’ grading system, only patients with severe CRS, such as patients with hypotension who do not respond to fluid resuscitation and require high-dose vasopressor, are treated with tocilizumab (Table 4).43 Nevertheless, the subgroup analysis of ZUMA-1 suggested that tocilizumab with or without steroid use did not impact the clinical activity of CAR T cells and similar results were observed in several studies.15 Therefore, grade 2 or higher CRS with hypotension or hypoxia are treated with tocilizumab regardless of the response to vasopressor. As a grading system, ‘Lee criteria’ is preferably utilized recently, which recommends early use of tocilizumab.38,44 In addition, there is a need of corticosteroids in severe CRS, because tocilizumab alone sometimes failed to control severe CRS. However, corticosteroids can destroy CAR-T and should be used with caution. In our institution, we preferably combine steroids with tocilizumab in selected patients such as patients with bulky mass lesion, and with CRS grade 2 or higher that occurs within 3 days after infusion.
Table 4.
Grading system and management of CRS.
| Penn grading scale43 | Lee criteria44 | |||
|---|---|---|---|---|
| Definition/Symptoms | Management | Definition/Symptoms | Management | |
| Grade 1 | Mild reaction |
|
Symptoms are not life-threatening and require symptomatic treatment only.(e.g. fever, nausea, fatigue, headache, myalgia, malaise) |
|
| Grade 2 | Moderate reaction:
|
|
Symptoms require and respond to moderate intervention.
|
|
| Grade 3 | More severe reaction:
|
|
Symptoms require and respond to aggressive intervention
|
|
| Grade 4 | Life-threatening complications:
|
|
Life-threatening symptoms:
|
|
The optimal use and timing of tocilizumab (IL-6 blockade) is the most important key point in the management of CRS. IL-6 blockade and steroid use are shown in bold.
Definition of high-dose vasopressor: norepinephrine (≥0.2 mcg/kg/min); dopamine (≥10 mcg/kg/min); phenylephrine (≥200 mcg/min); epinephrine (≥0.1 mcg/kg/min); If on vasopressin: norepinephrine equivalent‡ of ≥10 mcg/min; If combination vasopressors: norepinephrine equivalent of 20 mcg/min
Norepinephrine equivalent dose = [norepinephrine (mcg/min)] + [dopamine (mcg/kg/min) ÷ 2] + [epinephrine (mcg/min)] + [phenylephrine (mcg/min) ÷10].
FFP, fresh frozen plasma; LFT, liver function test.
Despite the dramatic response of tocilizumab against CRS, the management of neurotoxicity remains difficult. The early signs of neurotoxicity are aphasia, impaired handwriting, confusion, disorientation, agitation, and tremors. In severe cases, seizures, motor weakness, incontinence are observed and cerebral edema, which is sometimes fatal, can occur. Siddiqi and colleagues reported that the neurotoxicity was associated with higher baseline levels of ferritin, C-reactive protein, D-dimer, IL-6, IL-15, TNF-α, and macrophage inflammatory protein (MIP)-1α.39 Although most neurotoxicities are reversible without any treatment, 10–30% of patients experience severe neurotoxicity and effective therapy does not exist. Conventionally, severe neurotoxicity with CRS is treated using tocilizumab with or without steroids, and neurotoxicity without CRS is treated with corticosteroids alone. In ZUMA-1 safety management cohort, prophylactic tocilizumab and levetiracetam were tested to decrease the risk of CRS and neurotoxicity.45 However, this strategy did not reduce the risk of severe neurotoxicity, suggesting that a different approach rather than IL-6 blockade is required to manage neurotoxicity.
Recently, the Memorial Sloan Kettering Group and Italian group demonstrated that IL-1 produced by activated macrophages plays an important role in the pathophysiology of CAR-T-associated neurotoxicity in a mouse model.46–48 These data may shed light on future neurotoxicity management with IL-1 blockade and further investigation is warranted.
Augmentation of efficacy
Although anti-CD19 CAR T-cell therapy showed remarkable efficacy in patients with B-ALL, its efficacy in patients with DLBCL was somewhat lower. Around 50% or more patients with DLBCL who received second-generation CAR T-cell therapy could not be cured.
Two potential mechanisms of resistance after CAR T-cell infusion have been suggested: CD19 antigen loss and expression of immune checkpoint molecules. According to the preliminary analysis of biopsy specimen during disease progression in ZUMA-1 study, 33% were negative for CD19 and 62% were positive for programmed death ligand 1 (PD-L1) in immunohistochemistry.15,49
To overcome tumor immune evasion from CAR T cells, combination therapy of CAR-T and immune checkpoint inhibitors are being actively developed. For example, the UPenn group reported a case of DLBCL that progressed after therapy with tisagenlecleucel and obtained clinically significant response following programmed death 1 (PD-1) blockade.50 They are conducting a phase I/II trial to evaluate the feasibility and efficacy of an anti-PD-1 antibody, pembrolizumab, in patients with B-cell NHL (NCT02650999) failing to respond to (or relapsing after) tisagenlecleucel.51 According to the recently presented data at the 60th Annual Meeting of ASH in 2018, 12 patients who received tisagenlecleucel were enrolled with progressive disease (n=8) or relapse (n=4) after the CAR-T infusion. The median PFS after the infusion of tisagenlecleucel was 2.2 months. Patients received pembrolizumab 200 mg/day every 3 weeks until progression or occurrence of unacceptable toxicity. Among the 11 evaluable patients, the best ORR after pembrolizumab was 27% (3/11) including 2 patients with CR. Moreover, 9 of 12 patients showed a re-expansion of tisagenleculeucel with median days to re-expansion was 3 days. Responding patients had more than one re-expansion peak during pembrolizumab, while nonresponding patients had only one expansion peak or no expansion. Further evaluation in large number of patients is expected.
Kite Pharma is conducting Zuma-6 trial, a phase I/II study to explore axi-cel in combination with atezolizumab (an anti-PD-L1 antibody) in patients with relapsed/refractory DLBCL. The results of phase I part of ZUMA-6 trial have been presented at the 60th Annual Meeting of ASH in 2018.52 Atezolizumab was administered at 1200 mg/day, every 21 days for 4 doses. Three types of schedules were evaluated: starting on day 21 (Cohort 1), day 14 (Cohort 2), and day 1 (Cohort 3). In total, 12 patients have received axi-cel and one or more dose of atezolizumab (Cohort 1: n=3, Cohort 2: n=3, Cohort 3: n=6).
The ORR in 10 evaluable patients was 90% (9/10) with 6 patients with CR. The median follow-up duration was 4.4 months. The AUC of CAR T cell in the first 28 days was higher than that of ZUMA-1. One patient in Cohort 3 experienced DLT (prolonged thrombocytopenia and neutropenia, grade 4). Based on these results, the authors concluded that axi-cel in combination with atezolizumab has a manageable safety profile and a phase II portion of ZUMA-6 is ongoing with a Cohort 3 schedule. However, the more potent the CAR-T therapy, the more toxic it may become. In this study, 5 of 12 patients experienced encephalopathy, and 6 of 12 patients experienced grade 3 or higher neurotoxicity. Thus, combination strategy with PD-1 blockade should be carefully evaluated.
To overcome CD19 negative relapses, CAR T-cell therapy targeting CD22 (another pan B-cell marker) is actively being studied alternatively. The NCI group published the results of a phase I study of anti-CD22 CAR T-cell therapy in patients with B-ALL.53 In total, 21 patients were enrolled in this study, and 17 patients were previously treated with anti-CD19 immunotherapy. Among the 15 evaluable patients who received ≥1×106/kg CAR T cells, 11 patients achieved CR.
Although CD22 seems to be an attractive target, targeting only one antigen may result in antigen escape. Therefore, dual targeting CAR T-cell therapies are also investigated to decrease the risk of antigen escape. There are three strategies to target two antigens at once: (1) Using two types of CAR-T; (2) Transducing two CARs into the same T cell (dual-specific CAR-T); and (3) Coupling two different single-chain fragment variable domains into a single-CAR construct (bivalent CAR-T).54 The strategy using two types of CAR-T with different targets may not be an effective strategy, because only one of the CARs will expand and the other will die. Qin and colleagues demonstrated that simultaneous infusion of CD19-CAR-T and CD22-CAR-T resulted in the progression of CD19−CD22+ tumor cells in a preclinical model, suggesting that the CD19 CAR may dominate in functionally.55 Although dual-specific CAR-T was also tested, the cotransduction efficiency was consistently low. In addition, these two strategies require two types of vectors, which increase the cost, time, and effort. Therefore, bivalent CAR that is containing two different single-chain fragment variable domains was developed. In a preclinical study, Qin and colleagues tested various types of bivalent anti-CD19/CD22 CAR structures and demonstrated that bivalent anti-CD19/CD22 CAR with loop structure (so-called bicistronic CAR) is more effective compared to bivalent anti-CD19/CD22CAR with tandem structure.55 Based on these results, a phase I study of anti-CD19/CD22 bivalent loop CAR-T for relapsed/refractory B-cell malignancies is ongoing in the US (NCT03448393).
Insufficient persistence of CAR T cells in vivo may also limit the effectiveness of CAR T-cell therapy. Because the antigen recognition domain of CAR is usually derived from murine antibodies, it is believed that immune responses against CAR partly cause CAR T-cell elimination in the human body. Currently, fully human CARs are tested in several groups, but the clinical implications of fully human CAR remain unclear.56,57 The exhaustion of CAR-T partly caused by too much CAR-T activation is also associated with limited persistence of second-generation CAR-T. According to the preclinical study reported by Feucht and colleagues, decreasing the number of immunoreceptor tyrosine-based activation motif (ITAM) of CD3 zeta from 3 to 1, can achieve persistent expansion of CD28-based CAR-T without exhaustion.58 Their data shed light on the importance of CD3 zeta structure to design a CD28-based CAR with optimal function. Another solution for increasing efficacy and persistence of CAR T cells is utilizing a ‘next-generation’ CAR structure. Recently, a third-generation CAR that contains both 4-1BB and CD28 as a costimulatory domain was tested and demonstrated efficacy with modest toxicity profile in patients with B-cell malignancies.59 Furthermore, the Memorial Sloan Kettering group is conducting a first-in-human phase I/II study of the ‘armored’ CAR-T that expressing anti-CD19 CAR with CD28 costimulatory domain and 4-1BB ligand (4-1BBL) on the CAR T-cell surface.60 Preclinical study demonstrated that the binding of 4-1BBL to its cognate receptor in tumor microenvironment enhances T-cell proliferation, IL-2 secretion, and survival and cytolytic activity of the T cells compared to other second or third-generation CAR T cells.61 In the phase I study, 29 patients with B-cell malignancies including 9 patients with DLBCL received the Armored CAR-T infusion. Among the 28 evaluable patients, 23 patients (82%) achieved objective responses including 15 patients with CR. In 9 patients with DLBCL, 7 patients achieved CR and 1 patient obtained PR. Severe CRS was not seen and grade 3 neurotoxicity was observed in 10% (3/29) with no grade 4 neurotoxicity. Further evaluation in larger number of patients is expected.
Improving CAR-T platform
Improving the CAR-T production platform of CAR T-cell therapy is also an important issue to enable patients to access this treatment more easily. In the international study JULIET, only 70% of patients received CAR-T infusion. It is partly because of the relatively longer turnaround time (the median time from enrollment to infusion was 54 days at the JULIET study14), especially outside the United States. During CAR-T manufacturing, physicians must control chemorefractory DLBCL with conventional chemotherapies, sometimes for more than a month. Therefore, rapid production is essential for patients to receive CAR-T infusions. Previously, CAR-T manufacturing included several steps and open-tissue culture vessels were utilized with several manual steps. Recently, CliniMACS Prodigy achieved an automated rapid production system, which takes only 7–14 days from cell preparation to formulation.35,62
Another solution to reduce the production waiting time is off-the-shelf CAR-T bank. Patients and physicians must wait for CAR-T production because it is custom-made for each patient. Furthermore, there is a risk of production failure especially in heavily pretreated patients who do not have adequate healthy T cells. Cellectis, Servier, and Pfizer developed allogeneic off-the-shelf anti-CD19 CAR T cell named UCART19.63,64 They disrupted the T-cell receptor alpha constant (TRAC) gene with activator-like effector nuclease (TALEN®) technology to avoid the graft-versus-host disease. They also disrupted CD52 gene to utilize anti-CD52 antibody alemtuzumab in LD chemotherapy. Simultaneously, the CAR gene was transduced into cells with lentiviral vector. Currently, a phase I study of UCART19 in patients with B-ALL is ongoing (CALM study, NCT02746952). In addition, the Memorial Sloan Kettering group reported successful target insertion of CAR gene into TRAC locus using clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (CRISPR/Cas9) technology.65,66 These recent progresses of gene editing technology might result in the manufacture of perfect off-the-shelf allogeneic CAR T cells in the future.
Conclusions
Several trials have reported promising results of anti-CD19 CAR T-cell therapy in patients with relapsed/refractory B-cell NHL, especially in DLBCL. Although the median PFS with anti-CD19 CAR T-cell therapy is not high enough, there is a plateau phase on the survival curve suggesting that there are selected patients probably curable with this treatment. Nonetheless, there remain several controversial issues and problems awaiting solutions, including optimal management of toxicities, overcoming relapsed/refractory disease after CAR T-cell therapy, and improving CAR-T manufacturing platform. To determine the optimal protocol of CAR T-cell therapy, further research including larger-scale randomized phase III trial is necessary. Currently, Novartis and Celgene are conducting phase III trials for transplant-eligible relapsed/refractory DLBCL comparing CAR T-cell therapy and standard salvage therapy followed by autologous stem cell transplantation (NCT03570892; BELINDA by Novartis and NCT03575351; TRANSFORM by Celgene). The results of these large-scale trials might change our daily clinical practice in the future.
Because CAR T-cell therapy has more complex methodologies than conventional chemotherapy, it requires sufficient multidisciplinary support from intensive-care unit doctors, well-educated nurses, and technicians qualified to manipulate cells. Preparing these resources for CAR T-cell therapy is also necessary.
Definite unmet medical needs among patients with chemorefractory B-cell NHL still exist. CAR T-cell therapy might be a game changer that can defeat chemorefractory NHL, and further clinical development is warranted.
Acknowledgments
We thank members of Team CAR-T at the National Cancer Center Hospital (NCCH) for supporting the CAR-T therapy: Ms Moemi Kasane, Ms Saori Nakabayashi (Cell Preparation Technicians), Mr Hidekazu Fukumoto (Medical engineer), Ms Yukari Torihata, Mr Katsuyuki Ikarashi, and Ms Hiroko Takagi (Clinical Research Coordinators). We also thank Dr Takahiro Fujino, Dr Yo Saito (Fellows), Dr Akiko Miyagi Maeshima (Pathologist), Dr Tetsufumi Sato (ICU doctor), and Dr Yasuji Miyakita (Neurologist) for their great cooperation to the CAR-T trials. Lastly we thank Dr Koji Izutsu, Chief of the Department of Hematology, NCCH.
Footnotes
Contributions: SM wrote the initial draft of this manuscript. KI, SK, and KT assisted in the preparation of the manuscript. The final version of the manuscript was approved by all authors. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: SM, KI, and SK have nothing to disclose. KT received consulting fees and lecture fees from Zenyaku Kogyo, grant support and lecture fees from Eisai, Takeda, Mundipharma International, Janssen, Kyowa Hakko Kirin, Chugai Pharma, and Ono Pharmaceutical, grant support, consulting fees, and lecture fees from HUYA Bioscience International, and grant support from GlaxoSmithKline, Servier, and AbbVie. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors are available for download at http://www.drugsincontext.com/wp-content/uploads/2019/01/dic.212567-COI.pdf
Funding declaration: This work was supported in part by the National Cancer Center Research and Development Fund (26-A-4 and 27-A-2). Language editing was provided by Editage and was supported the internal fund of the National Cancer Center Hospital Tokyo, Japan.
Correct attribution: Copyright © 2019 Makita S, Imaizumi K, Kurosawa S, Tobinai K. https://doi.org/10.7573/dic.212567. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: invited; externally peer reviewed.
Peer review comments to author: 28 November 2018
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised Fourth Edition. Lyon: IARC Press; 2017. [Google Scholar]
- 2.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328(14):1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 4.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–1808. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki T, Maruyama D, Maeshima AM, et al. Impact of the double expression of MYC and BCL2 on outcomes of primary refractory diffuse large B-cell lymphoma following R-CHOP chemoimmunotherapy. [Accessed January 29, 2019.];Blood. 2017 130(Suppl 1):2846. [Abstract] http://www.bloodjournal.org/content/130/Suppl_1/2846. [Google Scholar]
- 6.Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21:922–926. doi: 10.1038/nm.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czuczman MS, Trněný M, Davies A, et al. A phase 2/3 multicenter, randomized, open-label study to compare the efficacy and safety of lenalidomide versus investigator’s choice in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res. 2017;23:4127–4137. doi: 10.1158/1078-0432.CCR-16-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salles G, Duell J, González-Barca E, et al. Single-arm phase II study of MOR208 combined with lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma: L-Mind. [Accessed January 29, 2019];Blood. 2017 130(Suppl 1):4123. [Abstract] http://www.bloodjournal.org/content/130/suppl_1/4123. [Google Scholar]
- 9.Makita S, Tobinai K. Antibody therapy targeting CD19 for B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2018;29:1086–1089. doi: 10.1093/annonc/mdy092. [DOI] [PubMed] [Google Scholar]
- 10.Palanca-Wessels MC, Czuczman M, Salles G, et al. Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol. 2015;16:704–715. doi: 10.1016/S1470-2045(15)70128-2. [DOI] [PubMed] [Google Scholar]
- 11.Morschhauser F, Salles G, McKay P, et al. Interim report from a phase 2 multicenter study of tazemetostat, an EZH2 inhibitor, in patients with relapsed or refractory B-cell non-Hodgkin lymphomas. Hematol Oncol. 2017;34(suppl 2):24–25. doi: 10.1002/hon.2437_3. [Abstract] [DOI] [Google Scholar]
- 12.Makita S, Tobinai K. Targeting EZH2 with tazemetostat. Lancet Oncol. 2018;19:586–587. doi: 10.1016/S1470-2045(18)30149-9. [DOI] [PubMed] [Google Scholar]
- 13.Study of nivolumab in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) that have either failed or are not eligible for autologous stem cell transplant (CheckMate 139) Clinical Trials.gov. [Accessed September 30, 2018]. https://clinicaltrials.gov/ct2/show/results/NCT02038933?sect=X970156#outcome3.
- 14.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 15.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makita S, Yoshimura K, Tobinai K. Clinical development of anti-CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. Cancer Sci. 2017;108:1109–1118. doi: 10.1111/cas.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Approved drugs. U.S. Food and Drug Administration; [Accessed September 30, 2018]. FDA approves axicabtagene ciloleucel for large B-cell lymphoma. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm581296.htm. [Google Scholar]
- 18.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long AH, Haso WM, Shern JF, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klebanoff CA, Khong HT, Antony PA, et al. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–117. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8:355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Approved drugs. U.S. Food and Drug Administration; [Accessed September 30, 2018]. FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm574154.htm. [Google Scholar]
- 31.Borchmann P, Tam CS, Jäger U, et al. An updated analysis of JULIET, a global pivotal phase 2 trial of tisagenlecleucel in adult patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL). Abstract S799. The 23rd Congress of European Hematology Association (EHA); Stockholm, Sweden. [Accessed September 30, 2018]. https://learningcenter.ehaweb.org/eha/2018/stockholm/214521/peter.borchmann.an.updated.analysis.of.juliet.a.global.pivotal.phase.2.trial.html. [Google Scholar]
- 32.Schuster SJ, Bishop MR, Tam C, et al. Sustained disease control for adult patients with relapsed or refractory diffuse large B-cell lymphoma: an updated analysis of Juliet, a global pivotal phase 2 trial of tisagenlecleucel. ASH annual meeting; San Diego. 2018; Dec 1–4; [Accessed January 8, 2019]. [abstract #1684]. https://ash.confex.com/ash/2018/webprogram/Paper115252.html. [Google Scholar]
- 33.Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25:285–295. doi: 10.1016/j.ymthe.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu TL, Pugach O, Somerville R, et al. A rapid cell expansion process for production of engineered autologous CAR-T cell therapies. Hum Gene Ther Methods. 2016;27:209–218. doi: 10.1089/hgtb.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sommermeyer D, Hudecek M, Kosasih PL, et al. Chimeric antigen receptor-modified T cells derived from defined CD8 + and CD4 + subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abramson JS, Gordon LI, Palomba ML, et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. 2018 American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, US. [Accessed September 30, 2018]. [Abstract 7505]. https://meetinglibrary.asco.org/record/159487/abstract. [Google Scholar]
- 38.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy – assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siddiqi T. Patient characteristics and pre-infusion biomarkers of Inflammation correlate with clinical outcomes after treatment with the defined composition, CD19-targeted CAR T cell product, JCAR017. Blood. 2017;130(Suppl 1):193. [Abstract] [Google Scholar]
- 40.Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frey N. Cytokine release syndrome: who is at risk and how to treat. Best Pract Res Clin Haematol. 2017;30:336–340. doi: 10.1016/j.beha.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8:958–971. doi: 10.1158/2159-8290.CD-17-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol. 2018;11:35. doi: 10.1186/s13045-018-0571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Locke FL, Neelapu SS, Bartlett NL, et al. Preliminary results of prophylactic tocilizumab after axicabtagene ciloleucel (axi-cel; KTE-C19) treatment for patients with refractory, aggressive non-Hodgkin lymphoma (NHL) [Accessed January 29, 2019];Blood. 2017 130(Suppl 1):1547. [Abstract] http://www.bloodjournal.org/content/130/Suppl_1/1547. [Google Scholar]
- 46.Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 47.Giavridis T, van der Stegen SJC, Eyquem J, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rooney C, Sauer T. Modeling cytokine release syndrome. Nat Med. 2018;24:705–706. doi: 10.1038/s41591-018-0068-9. [DOI] [PubMed] [Google Scholar]
- 49.Neelapu SS, Locke FL, Bartlett NL, et al. Long-term follow-up ZUMA-1: a pivotal trial of axicabtagene ciloleucel (axi-cel; KTE-C19) in patients with refractory aggressive non-Hodgkin lymphoma (NHL) [Accessed Janurary 29, 2019.];Blood. 2017 130(Suppl 1):578. [Abstract] http://www.bloodjournal.org/content/130/Suppl_1/578. [Google Scholar]
- 50.Chong EA, Melenhorst JJ, Lacey SF, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017;129:1039–1041. doi: 10.1182/blood-2016-09-738245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chong EA, Svoboda J, Nasta SD, et al. Sequential anti-CD19 directed chimeric antigen receptor modified T-cell therapy (CART19) and PD-1 blockade with pembrolizumab in patients with relapsed or refractory B-cell non-Hodgkin lymphomas. ASH annual meeting; San Diego. 2018; Dec 1–4; [Accessed January 15, 2019]. [abstract #4198]. https://ash.confex.com/ash/2018/webprogram/Paper119502.html. [Google Scholar]
- 52.Jacobson CA, Locke FL, Miklos DB, et al. End of phase 1 results from ZUMA-6: axicabtagene ciloleucel (axi-cel) in combination with atezolizumab for the treatment of patients with refractory diffuse large B cell lymphoma. ASH annual meeting; San Diego. 2018; Dec 1–4; [Accessed January 15, 2019]. [abstract #4192]. https://ash.confex.com/ash/2018/webprogram/Paper111523.html. [Google Scholar]
- 53.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruella M, Maus MV. Catch me if you can: leukemia escape after CD19-directed T cell immunotherapies. Comput Struct Biotechnol J. 2016;14:357–362. doi: 10.1016/j.csbj.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin H, Ramakrishna S, Nguyen S, et al. Preclinical development of bivalent chimeric antigen receptors targeting both CD19 and CD22. Mol Ther Oncolytics. 2018;11:127–137. doi: 10.1016/j.omto.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alabanza L, Pegues M, Geldres C, et al. Function of novel anti-CD19 chimeric antigen receptors with human variable regions is affected by hinge and transmembrane domains. Mol Ther. 2017;25:2452–2465. doi: 10.1016/j.ymthe.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sommermeyer D, Hill T, Shamah SM, et al. Fully human CD19-specific chimeric antigen receptors for T-cell therapy. Leukemia. 2017;31:2191–2199. doi: 10.1038/leu.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feucht J, Sun J, Eyquem J, et al. Calibration of CAR activation potential directs alternative T cell fates and theraputic potency. Nat Med. 2019;25:82–88. doi: 10.1038/s41591-018-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Enblad G, Karlsson H, Gammelgård G, et al. A phase I/IIa trial using CD19-targeted third-generation CAR T cells for lymphoma and leukemia. Clin Cancer Res. 2018;24:6185–6194. doi: 10.1158/1078-0432.CCR-18-0426. [DOI] [PubMed] [Google Scholar]
- 60.Park JH, Palomba ML, Batlevi CL, et al. A phase I first-in-human clinical trial of CD19-targeted 19-28z/4-1BBL “Armored” CAR T cells in patients with relapsed or refractory NHL and CLL including Richter’s transformation. ASH annual meeting; San Diego. 2018; Dec 1–4; [Accessed January 15, 2019]. [abstract #224]. https://ash.confex.com/ash/2018/webprogram/Paper117737.html. [Google Scholar]
- 61.Zhao Z, Condomines M, van der Stegen SJC, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mock U, Nickolay L, Philip B, et al. Automated manufacturing of chimeric antigen receptor T cells for adoptive immunotherapy using CliniMACS prodigy. Cytotherapy. 2016;18:1002–1011. doi: 10.1016/j.jcyt.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 63.Qasim W, Zhan H, Samarasinghe S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9(374) doi: 10.1126/scitranslmed.aaj2013. pii: eaaj2013. [DOI] [PubMed] [Google Scholar]
- 64.Qasim W, Ciocarlie O, Adams S, et al. Phase I study of UCART19, an allogeneic anti-CD19 CAR T-cell product, in high risk pediatric patients with CD19+relapsed/refractory (R/R) B-cell ALL: preliminary results of PALL study. Abstract PF175. The 23rd Congress of EHA; Stockholm, Sweden. [Accessed September 30, 2018]. https://learningcenter.ehaweb.org/eha/2018/stockholm/214671/waseem.qasim.phase.i.study.of.ucart19.an.allogeneic.anti-cd19.car.t-cell.html?f=menu=6*ce_id=1346*ot_id=19044*media=3. [Google Scholar]
- 65.Eyquem J, Mansilla-Soto J, Giavridis T, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maus MV. Immunology: T-cell tweaks to target tumours. Nature. 2017;543:48–49. doi: 10.1038/nature21506. [DOI] [PubMed] [Google Scholar]