Abstract
Bee populations are currently undergoing severe global declines driven by the interactive effects of a number of factors. Ongoing urbanisation has the potential to exacerbate bee declines, unless steps are taken to ensure appropriate floral resources are available. Sown wildflower strips are one way in which floral resources can be provided to urban bees. However, the use of these strips by pollinators in urban environments remains little studied. Here, we employ pollen metabarcoding of the rbcL gene to compare the foraging patterns of different bee species observed using urban sown wildflower strips in July 2016, with a goal of identifying which plant species are most important for bees. We also demonstrate the use of a non-destructive method of pollen collection. Bees were found to forage on a wide variety of plant genera and families, including a diverse range of plants from outside the wildflower plots, suggesting that foragers visiting sown wildflower strips also utilize other urban habitats. Particular plants within the wildflower strips dominated metabarcoding data, particularly Papaver rhoeas and Phacelia tanacetifolia. Overall, we demonstrate that pollinators observed in sown wildflower strips use certain sown foodplants as part of a larger urban matrix.
Keywords: Metabarcoding, Plant–pollinator interactions, DNA barcoding, rbcL, Conservation, Second-generation sequencing, Bumblebees, Halictidae, eDNA, Sown wildflower strips
Introduction
Over the last century, wild bee populations have suffered widespread declines in the form of substantial range contractions (Kerr et al., 2015) alongside local decreases in the abundance and species richness of hoverfly and bee communities (Biesmeijer et al., 2006; Potts et al., 2010; Bommarco et al., 2011; Cameron et al., 2011; Dupont, Damgaard & Simonsen, 2011; Bartomeus et al., 2013). Wild bee declines likely result from the interactive effects of multiple factors (Goulson et al., 2015), including habitat loss and fragmentation (e.g. due to urbanisation; Garibaldi et al., 2011; Gerard et al., 2010; Hendrickx et al., 2007; NEA, 2011), climate change (Kerr et al., 2015) and parasite and pathogen spread (Otterstatter & Thomson, 2008). Bee declines are of economic concern due to the value of pollination services to agriculture (Klein et al., 2007; NEA, 2011). While the global number of managed honeybee colonies has increased (Aizen & Harder, 2009), this is unlikely to be sufficient to compensate for wild pollinator losses: the rate of increase currently does not match the rate of increase in the need for pollinator services (Aizen & Harder, 2009) and certain regions are experiencing a reduction in numbers of beekeepers and managed honeybee colonies (National Research Council, 2007; Potts et al., 2010).
Urbanisation is increasing both within the UK and globally, with increasing housing density and population (Gerard et al., 2010; NEA, 2011; Seto et al., 2011). Continuing urbanisation, with associated displacement of semi-natural and agricultural habitats (Gerard et al., 2010; NEA, 2011), will potentially have further negative effects on bee populations. Compared to rural environments, urban areas may have lower bee species richness (Hernandez, Frankie & Thorp, 2009; Bates et al., 2011; Deguines et al., 2012), fewer plant–pollinator interactions (Geslin et al., 2013), and a lower abundance of pollinators (Bates et al., 2011). Conversely, other studies suggest neutral or even positive effects of urbanisation on pollinator species richness (Banaszak-Cibicka & Zmihorski, 2012; Baldock et al., 2015). The degree to which urban areas are able to support rich and abundant pollinator communities is related to the ability of these areas to provide the resources required to support wild bees and hoverflies, particularly floral resources (nectar and pollen) (McFrederick & LeBuhn 2006; Bates et al., 2011; Fortel et al., 2014; Hülsmann et al., 2015).
One method commonly advocated to enhance urban habitats for pollinators is the provision of sown wildflower plots. These plots provide significantly greater nectar resources than amenity grasslands (Hicks et al., 2016), and consequently attract significantly higher rates of pollinator visitation (Blackmore & Goulson, 2014). However, the plant species in wildflower seed mixtures vary greatly in their ability to provide nectar and pollen resources to foraging insects (Hicks et al., 2016), and this is reflected in differences in visitation rates by insects to these plots (Ahrné, Bengtsson & Elmqvist, 2009). Similarly, different wildflower mixes sown in agricultural margins support different communities of pollinators (Williams et al., 2015; Warzecha et al., 2018). Therefore, taxon specific knowledge of sown resource utilisation in urban areas will allow more specific recommendations for mixes to promote the abundance and diversity of each taxon of wild bees.
A number of studies have used DNA metabarcoding to study honeybee foraging choices by identifying pollen taken from honey samples (De Vere et al., 2017; Hawkins et al., 2015; Richardson et al., 2015a, 2015b) and from pollen traps placed at the entrances to beehives (Keller et al., 2015). More recently, it has been shown that pollen samples taken directly from the bodies of pollinators can give an indication of foraging behaviour at the level of individual insects (Bell et al., 2017; Lucas et al., 2018a, 2018b), although this requires killing the individuals sampled and thus may be problematic when sampling threatened species. The number of sequences obtained for a given plant species can offer a semi-quantitative picture of plant–pollinator interactions (Pornon et al., 2016). Sequencing-based identification of pollen is able to identify a greater number of taxa with better taxonomic resolution than morphological identification, and additionally reduces the requirement for highly specialised taxonomic expertise (Keller et al., 2015; Smart et al., 2017).
Here, we investigate foraging preferences of bees feeding in sown wildflower strips using observational approaches coupled with metabarcoding of rbcL, a chloroplast gene. We aim to investigate (i) how the use of sown wildflower strips fits within use of the wider urban landscape, (ii) whether particular sown species are used preferentially over others, and (iii) whether the former two questions are affected by bee species identity. Additionally, the study aims to ascertain the effectiveness of non-destructive pollen sampling from individual bees as an alternative to killing sampled individuals when conducting pollen metabarcoding studies.
Methods
Field sampling
Floral cover assessment, pollinator sampling and pollinator observations were carried out across 10 pollinator planting strip sites managed by Bournemouth Borough Council in July 2016 (Tables S1 and S2; Fig. S1). All data was collected between 9 am and 5 pm on dry days where the wind speed was less than 5 on the Beaufort scale and the temperature was above 15 °C.
At each site three 1 m2 quadrats were placed by selecting the patches with the highest density of open flowers. The total floral cover as a percentage of each plant species was measured in these quadrats (vegetative growth was not recorded). At each of the three quadrats, a single 10 min pollinator count was carried out by recording all pollinators to enter the quadrat. Pollinators were identified by observation only, without netting. Honey bees and bumblebees were identified to species level, while other bees were identified to family level where possible and recorded as ‘other solitary bees’ if not.
On the same day as observational data was collected, pollinators were sampled whilst they were visiting flowers within wildflower strips. As abundances were not being measured, sampling continued until 15 individuals had been caught, regardless of how long this took. Pollen was non-destructively collected from pollinators by confining pollinators in sterile microcentrifuge tubes: 1.5 ml (for A. mellifera and Bombus spp.) or 0.2 ml (for Halictidae spp., hoverflies and beetles). Pollinators were contained in the tubes for 5 min each, in a cool place, allowing pollen to be deposited on the tube walls by insect movement, and then released. Pollinators were captured whilst feeding, and the species of flower visited was also recorded. Bombus lucorum and B. terrestris could not be distinguished in the non-destructive field during sampling, so they have been grouped under the name B. terrestris in this study. Similarly, individuals from the Halicidae could not be reliably identified in the field so individuals are classified at the family level.
DNA extraction
DNA extraction was carried out following the method described by Hawkins et al. (2015). Pollen was resuspended in 400 μl of buffer AP1 from a DNeasy Plant Mini Kit (Qiagen, Venlo, Netherlands), to which 80 μl of proteinase K (one mg ml−1; Thermo Fisher Scientific, Waltham, MA, USA) was added alongside one μl of RNase A (100 mg ml−1; Qiagen, Venlo, Netherlands). Next, the samples were disrupted by shaking for four minutes at a speed of 30 1/s in a Retsch MM200 bead mill with custom adapter. Subsequent steps of the DNeasy Plant Mini Kit were carried out following manufacturer’s instructions, with the omission of the QIAshredder column. Following extraction, DNA was stored at −20 °C.
To test whether the non-destructive method of sampling provided the same information as destructive sampling, 10 honeybees (Apis mellifera) were collected from outside hives in Dorchester (latitude: 50.719°, longitude: −2.419°) and six bumblebees (Bombus terrestris) individuals were collected on Bournemouth University campus (latitude: 50.741°, longitude: −1.894°). Collection was carried out on the 11th of November 2016 for honeybees, and 13th of February 2017 for bumblebees. Each of these individuals was both destructively and non-destructively sampled in a paired test: first, pollen was non-destructively collected from each individual as detailed under ‘Field Sampling’, and then the whole insect was transferred to a fresh tube and frozen at −20 °C prior to further processing. Pollen was ‘washed’ from each insect following the method employed by Lucas et al. (2018a, 2018b): one ml of 1% sodium dodecyl sulphate (SDS) and 2% poly-vinyl pyrrolidinone (PVP) solution in water was added to the insect in each tube. Tubes were vigorously shaken by hand for 1 min, allowed to stand at room temperature for 5 min, and finally shaken vigorously by hand for a further 20 s. Next, the insect was removed and the tube containing pollen and the SDS-PVP solution was centrifuged at 13,000 rpm. Finally, the supernatant was removed and discarded, and DNA extraction was carried out as described above. Each extraction was tested by PCR amplification.
To prevent contamination, all DNA extractions were carried out in a laminar flow hood. Prior to each DNA extraction, surfaces within the hood were cleaned with 10% bleach followed by 95% ethanol, then all reagents and tools were placed within the hood and irradiated with UV light for at least 15 min. The hood was UV irradiated for 1 h every night. A negative control was included with each batch of extractions.
Library preparation and sequencing
A section of the rbcL gene was amplified and prepared for sequencing following the protocol of De Vere et al. (2017), adapted from that described by Illumina for the V4 region of 16S rRNA genes in bacteria (Illumina, 2013). The rbcL gene was chosen because a complete rbcL database has been created for native plants within Wales (De Vere et al., 2012), containing the majority of plants found in the UK as a whole. This protocol involves two PCR amplification steps: one to amplify the region of interest, and a second to add index and adapter sequences for sequencing. Following each PCR, samples are purified using AMPure beads. The final step involves library quantification, normalisation, and pooling. Only samples which produced a visible band in the first PCR step were carried through to further library preparation steps.
First, the rbcL gene was amplified using primers described in Table S3. Each reaction was at a final volume of 20 μl, and contained two μl of template DNA, Phusion High-Fidelity Master Mix at 1X concentration (New England Biolabs, Ipswich, MA, USA) and primers at 0.2 mM each. Thermal cycling conditions were as follows: 95 °C for 2 min; 35 cycles of 95 °C for 30 s, 50 °C for 1 min 30 s, 72 °C for 40 s (40 cycles); and 72 °C for 5 min. PCR clean-up was carried out using AMPure XP beads (Beckman Coulter, Brea, CA, USA) according to manufacturer’s instructions. The second stage PCR was carried out at a final volume of 25 μl, with each reaction containing 12.5 μl Phusion High-Fidelity Master Mix, 2.5 μl Nexera XT Index Primer 1 (N7XX), 2.5 μl Nextera XT Index Primer 2 (S5XX), five μl water and 2.5 μl of purified product from the first PCR. Thermal cycling conditions were as follows: 95 °C for 3 min; eight cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s; and 72 °C for 5 min. A second PCR clean-up was carried out as described above. The product was quantified using a Qubit Fluorometer and Qubit dsDNA HS Assay Kit according to manufacturer’s instructions, and pooled at equal concentrations to generate the final library pool. Prior to sequencing, the library was again quantified by Qubit and adjusted to 10 nM concentration with 0.1M Tris-HCl/0.01% Tween 20 solution, prior to denaturing and loading onto an Illumina MiSeq (Illumina, San Diego, CA, USA) following manufacturer’s instructions.
Two negative controls were included in the sequence run: one containing a randomly chosen DNA extraction negative control, and one containing purified water.
Data analysis
Sequencing data was analysed using a workflow previously described by De Vere et al. (2017) and available at https://github.com/colford/nbgw-plant-illumina-pipeline. Adapters and low-quality bases were trimmed using Trimmomatic (Bolger, Lohse & Usadel, 2014), then paired-end reads were merged using FLASH (Magoc & Salzberg, 2011). Singleton reads and merged sequences less than 450 bp in length were removed. Next, megablast (McGinnis & Madden, 2004) was used to search unique sequences against a custom BLAST database which consisted of all sequences from the Barcode Wales project (De Vere et al., 2012) alongside selected other sequences downloaded from GenBank (Benson et al., 2012). Results were manually filtered to remove plants that do not occur in the UK, based on Stace (2010), and Cubey & Merrick (2014).
All further analyses were carried out in R (R Computing Team, 2017). Rarefaction curves were generated using the R package ‘vegan’ (Oksanen et al., 2017), and rank-abundance curves in BiodiversityR. Bipartite pollinator–plant networks were drawn in R package ‘bipartite’ (Dormann, Gruber & Fruend, 2008). To test for significant differences in the number of reads and genus diversity between species and sites, generalized linear models were fitted with poisson (where no overdispersion was detected) or quasipoisson distributions using function ‘glm’. Post hoc Tukey comparisons were carried out using package ‘lsmeans’ (Lenth, 2016).
Results
Of the 152 DNA extractions carried out on pollen taken from insects collected on urban pollinator strips, 41 produced a visible band after forty cycles of PCR and were sent for sequencing. A number of pollinators other than bees were collected, but none of these produced a band following PCR (Table 1). Of the insects collected for comparison of destructive and non-destructive sampling methods, five of six B. terrestris and one of 10 A. mellifera samples amplified successfully for both methods.
Table 1. Number of individuals of each taxa collected and sequenced per site.
| Site number | Number Collected (Number sequenced in brackets) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 18 | 21 | 22 | 23 | 24 | 28 | 29 | 30 | 31 | 33 | Total | |
| Andrenidae | 2 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (0) |
| Apis mellifera | 5 (1) | 4 (1) | 3 (1) | 7 (1) | 9 (2) | 3 (0) | 0 (0) | 4 (2) | 2 (1) | 2 (0) | 39 (9) |
| Coleoptera | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (0) | 0 (0) | 3 (0) | 0 (0) | 0 (0) | 3 (0) | 8 (0) |
| Bombus hypnorum | 1 (1) | 2 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (1) |
| Bombus lapidarius | 2 (0) | 2 (0) | 0 (0) | 2 (0) | 0 (0) | 0 (0) | 3 (0) | 4 (1) | 2 (0) | 2 (0) | 17 (1) |
| Bombus pascorum | 1 (1) | 1 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1) | 0 (0) | 5 (2) |
| Bombus terrestris | 1 (0) | 6 (2) | 4 (4) | 4 (3) | 1 (0) | 4 (1) | 2 (2) | 6 (4) | 4 (3) | 4 (2) | 36 (21) |
| Diptera: Syrphidae | 1 (0) | 0 (0) | 2 (0) | 0 (0) | 0 (0) | 3 (0) | 0 (0) | 1 (0) | 2 (0) | 1 (0) | 10 (0) |
| Halictidae | 2 (1) | 0 (0) | 6 (2) | 1 (0) | 3 (1) | 5 (1) | 7 (1) | 0 (0) | 3 (0) | 5 (1) | 32 (7) |
Note:
The number collected is given first, followed by the number sequenced in brackets.
Sequencing yielded a total of 81,168,508 read pairs. Of these, 1,357,981 read pairs passed initial quality control and 98,985 were able to be paired. No reads from either negative control sample passed initial quality control, and no negative control produced a visible band following PCR amplification. Following manual filtering and removal of singleton reads, a mean of 1,131.0 (±178.0) reads per sample remained. Three samples yielded fewer than 100 reads and were excluded from all further analyses: these comprised two A. mellifera and one B. terrestris individual.
Rarefaction indicated that the number of reads required in order to detect the majority of genera varied greatly between samples (Fig. S2). However, rarefaction analysis indicated that sampling effort was sufficient to detect the majority of genera in most samples, despite variation in the number of reads. In most cases, a few plant genera made up the vast majority of reads with a longer ‘tail’ of genera that were only present at abundances of 5% or lower (Fig. S3). Based on this, a threshold of 5% of reads was considered to indicate ‘major’ food sources.
Comparison of destructive and non-destructive sampling
To compare destructive and non-destructive sampling methodologies, DNA was collected using both methods from ten A. mellifera and six B. terrestris individuals. A single A. mellifera individual and four B. terrestris individuals produced visible bands following PCR amplification, and were thus sequenced. For destructive sampling, two A. mellifera and six B. terrestris individuals produced visible bands.
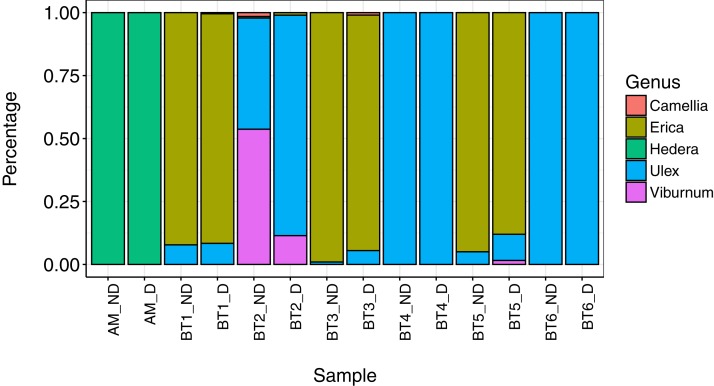
Overall, five genera were detected across all individuals collected for this part of the study: Camellia, Erica, Hedera, Ulex, and Viburnum. Hedera was the sole genus detected on A. mellifera, while Camellia, Erica, Ulex, and Viburnum were detected on B. terrestris. A minimum of 98% of genera in all pairs of destructively and non-destructively collected samples were shared between both samples (Table 2), and overall community composition was broadly similar between methods (Fig. 1). In all but one case, non-destructive and destructive sampling detected the same ‘major’ plant genera (defined as genera making up more than 5% of reads): the only exception to this was bee ‘Bt3’, where Ulex was detected in both samples but made up less than 1% of reads in the sample collected using non-destructive methods.
Table 2. Number of families detected using destructive and non-destructive sequencing, and the percentage of reads belonging to families that were detected using both sequencing methodologies.
| Insect | Method | Total genera | >5% Reads | % Reads in genera detected by both methods |
|---|---|---|---|---|
| AM | Non-destructive | 1 | 1 | 100 |
| Destructive | 1 | 1 | 100 | |
| BT1 | Non-destructive | 2 | 2 | 100 |
| Destructive | 3 | 2 | 99 | |
| BT2 | Non-destructive | 4 | 2 | 98 |
| Destructive | 3 | 2 | 100 | |
| BT3 | Non-destructive | 2 | 1 | 100 |
| Destructive | 3 | 2 | 99 | |
| BT4 | Non-destructive | 1 | 1 | 100 |
| Destructive | 1 | 1 | 100 | |
| BT5 | Non-destructive | 2 | 2 | 100 |
| Destructive | 3 | 2 | 98 | |
| BT6 | Non-destructive | 1 | 1 | 100 |
| Destructive | 1 | 1 | 100 |
Note:
AM, A. mellifera; BT, B. terrestris.
Figure 1. Composition of pollen collected using destructive (‘D’) and non-destructive sampling methods (‘ND’).
The single A. mellifera individual that was successfully sampled using both approaches is named ‘AM’, while the six B. terrestris individuals are labelled BT1–BR6.
Variation between bee species
The sequenced samples comprised 21 B. terrestris individuals, one individual each of B. hypnorum and B. lapidarius, two B. pascuorum individuals, nine A. mellifera individuals, and seven belonging to family Halictidae. Both hoverflies (Diptera: Syrphidae) and beetles (Coleoptera) were collected, but did not yield useable DNA. The numbers of pollinators collected and sequenced at each site are described in Table 1.
The three most abundant pollinator taxa in the sequencing dataset (A. mellifera, B. terrestris and Halictidae spp.; Table 1) were chosen for intra-species and intra-site comparisons. Reads from B. terrestris belonged to the broadest range of plant genera, followed by Halictidae and then A. mellifera (Table 3). Of the plant genera which individual bees were collected from, metabarcoding detected five of seven plant genera visited by B. terrestris, two of the four genera visited by A. mellifera, and four of the seven genera visited by Halictidae spp. Additionally, metabarcoding detected a number of plant genera which were not present in wildflower plots (Table 3).
Table 3. List of plant genera detected in sequenced pollen from each of the three most abundant pollinator species.
| B. terrestris | A. mellifera | Halictidae | |||
|---|---|---|---|---|---|
| Observed | Metabarcoding | Observed | Metabarcoding | Observed | Metabarcoding |
| Achillea | Achillea | Achillea | |||
| Anthemis | Anthemis | ||||
| Borago | |||||
| Brassica | |||||
| Buddleja | Buddleja | ||||
| Campanula | |||||
| Centaurea | Centaurea | Centaurea | Centaurea | Centaurea | |
| Chelidonium | |||||
| Chrysanth. | Chrysanth. | Chrysanth. | Chrysanth. | Chrysanth. | |
| Cirsium | |||||
| Cosmos | |||||
| Crataegus | |||||
| Echium | Echium | Echium | Echium | ||
| Escholzia | Escholzia | Escholzia | Escholzia | Escholzia | |
| Fallopia/Polygonum | |||||
| Fuchsia | |||||
| Hydrangea | Hydrangea | Hydrangea | |||
| Hypericum | |||||
| Lactuca | Lactuca | ||||
| Leucanthemum | |||||
| Ligustrum | Ligustrum | Ligustrum | |||
| Linaria | |||||
| Lupinus | Lupinus | ||||
| Malva | Malva | ||||
| Meconopsis | |||||
| Myosotis | |||||
| Oenothera | |||||
| Papaver | Papaver | Papaver | Papaver | Papaver | |
| Pentaglottis | Pentaglottis | ||||
| Phacelia | Phacelia | Phacelia | Phacelia | ||
| Plantago | |||||
| Rosa | Rosa | ||||
| Rubus | Rubus | ||||
| Salvia | |||||
| Sambucus | |||||
| Symphytum | |||||
| Taraxacum | |||||
| Trachelium | |||||
| Trifolium | Trifolium | ||||
Notes:
‘Observed’ interactions refers to all plant–pollinator interactions observed across three 10-min observation periods. Genera underlined were present in pollinator strips; bolded plant genera are unique to a single pollinator species.
Chyrsanth., Chyrsanthemum.
There were significant differences in genus richness of pollen found on individual insects of different species (resid. dev. = 36.1, df = 27, p = 0.02) and at different sites (resid. dev. = 47.1, df = 34, p = 0.03). In particular, pollen from B. terrestris individuals contained significantly more plant genera than that from A. mellifera (z = 2.5, p = 0.04). The mean number of plant genera per individual (±S.E.) was 4.2 (±0.4) for B. terrestris, 2.7 (±0.5) for A. mellifera, and 4.1 (±1.5) for Halictidae. The minimum number of genera detected on an individual was one for all bee species, with a maximum of seven for B. terrestris, five for A. mellifera, and 12 for Halictidae.
Use of sown wildflower strips by pollinators
Metabarcoding of pollen collected from the bodies of bees detected pollen from a wide variety of plant families, many of which were not present in the wildflower plots (Fig. 2; Table 3).
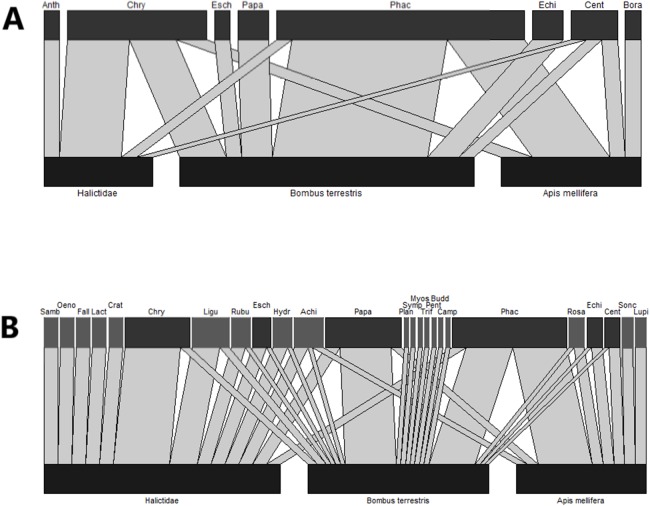
Figure 2. Bipartite network diagrams based on (A) observational data, that is, the number of bees captured on each plant genera and (B) the proportion of insects on which pollen from each plant genera was detected by metabarcoding.
Each plant genus was only counted as present on a given insect if it made up >5% of metabarcoding reads. In (A), only visitation data from bees whose pollen loads were sequenced is displayed. In (B), plant taxa which were present in the wildflower plots are coloured in dark grey, while other plant families are pale grey. Anth, Anthemis; Chry, Chrysanthemum; Esch, Escholzia; Papa, Papaver; Phac, Phacelia; Echi, Echium; Cent, Centaurea; Bora, Borago; Samb, Sambucus; Oeno, Oenothera; Fall, Fallopia; Lact, Lactuca; Crat, Crataegus; Ligu, Ligustrum; Rubu, Rubus; Hydr, Hydrangea; Achi, Achillea; Plan, Plantago; Symp, Symphytum; Myos, Myosotis; Camp, Campanula; Budd, Buddleja; Pent, Pentaglottis; Trif, Trifolium; Rosa, Rosa; Sonc, Sonchus; Lupi, Lupinus.
In order to allow comparison between the observational and metabarcoding datasets, only data for bees which were sequenced is shown in Fig. 2A. Bees were collected from the flowers of nine different plant genera, all of which were flowering within wildflower strips at the time of sampling. The largest numbers of insects were collected on Phacelia, followed by Centaurea and Chrysanthemum (Fig. 2A). Conversely, while both Phacelia and Chrysanthemum were abundant in DNA metabarcoding data, Papaver made up a larger proportion of metabarcoding reads than of available floral resources (Fig. 2B) and Centaurea was not detected by DNA metabarcoding. In addition, a number of plant genera were detected on bee bodies, but were not detected in floral surveys: particularly abundant were Ligustrum, Rosa, and Achillea (Fig. 2B). These genera were often found on only a small subset of bees.
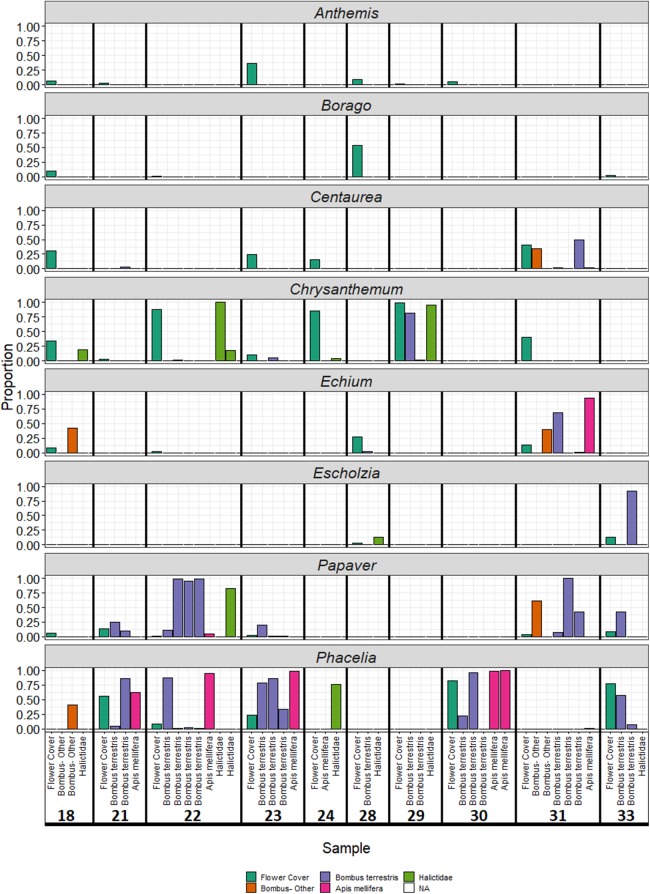
Plant genera present in wildflower plots accounted for the majority of reads (Fig. 3B), making up 69% of reads overall. Particularly well represented plant genera were Papaver and Phacelia. Conversely, the three members of the Asteraceae (Anthemis, Centaurea, and Chrysanthemum) found in sown wildflower plots were typically poorly represented amongst sequencing reads relative to the proportion of flowers available at each site: an exception to this was site 29, which was dominated by Chrysanthemum (Fig. 3) in the sequence reads. For three individuals, all pollen was assigned to a single genus that was not present in the wildflower strips: instead, all sequences were assigned to Achillea, Sonchus, or Rubus. Other insects appeared to mix genera found within the wildflower plots with genera from external sources.
Figure 3. Proportion of reads assigned to each plant species sown in wildflower strips by sample.
Colour of bars represents sample origin (floral survey or bee species).
Discussion
In this study, we used high-throughput sequencing of the rbcL gene to characterise pollen collected from wild bees which were captured while foraging in sown wildflower strips within urban areas during July 2016, demonstrating the applicability of recently developed ‘metabarcoding’ techniques to assessing the effectiveness of conservation methods. The majority of sequencing reads belonged to plant genera present in the wildflower strips, with particularly high abundances of Phacelia and Papaver. However, even though the individuals sampled were collected foraging in sown wildflower strips, bees were found to utilise a wide range of plant families and genera, including some which were not present in the strips. This indicates that wildflower strips are only providing a proportion of the resources used by urban bee species.
Comparison of destructive and non-destructive sampling
Previous studies have employed destructive sampling methods to collect pollen from individual insects (Pornon et al., 2016; Lucas et al., 2018a, 2018b), in which bees are killed prior to pollen removal. While effective, this approach may be problematic under certain circumstances, for example, sampling of rare non-colony-forming pollinators, such as threatened species of hoverfly (Ball & Morris, 2014) or the sampling of bumblebee workers early in the season, which has lasting effects on colony reproductive success (Muller & Schmid-Hempel, 1992). The non-destructive sampling method employed in the current study involved trapping individual pollinators in Eppendorf tubes for long enough for pollen to fall off, then releasing the insect to continue foraging. However, using this approach the number of individuals yielding sufficient pollen for analysis was considerably lower, particularly for hoverflies and solitary bees, meaning that the utility of non-destructive sampling methods may be limited unless refinements can be made to improve insect contact with the collection tube.
Overall, where sufficient DNA was obtained for amplification with both methods, both destructive and non-destructive sampling of pollen gave broadly similar results. In all sample pairs, the vast majority of reads belonged to genera present in both samples. Additionally, in all but one case, the number of plant genera detected was the same in both destructively and non-destructively collected samples once rare genera were excluded. The proportion of reads assigned to each species was broadly similar between both methods, although some differences did exist: in particular, where multiple genera were present in a sample Ulex typically made up a greater proportion of reads when destructive sampling was used rather than non-destructive. It is possible that this is due to differences in the effectiveness of non-destructive pollen sampling in removing different pollen species, for example due to species-specific differences in pollen attachment (Pacini & Hesse, 2005). An additional factor to consider when comparing destructive and non-destructive sampling methodologies is that it is not possible to separate pollen stored in the scopa (pollen storage structure) from pollen found on the rest of the body. Pollen load analysis is commonly carried out on isolated scopal pollen in order to only include deliberately collected pollen rather than pollen accidentally collected when foraging for nectar (Wood, Holland & Goulson, 2016), or alternatively may be carried out with the scopa removed in order to only include pollen available for pollination (Pornon et al., 2016).
Variation between bee species
Each bee species carried a unique range of plant genera (Table 3), similar to previous studies which have identified differences in floral preferences between pollinator species (Geslin et al., 2013; Kells, Holland & Goulson, 2001; Leonhardt & Blüthgen, 2012). The largest number of plant genera were detected on B. terrestris and the smallest number on Halictidae spp., although it should be noted that this pattern reflects the number of individuals sampled for each species. There were also differences in the choice of flowers used by each bee species: in observational data, Halictidae spp. were primarily observed feeding on Asteraceae such as Chrysanthemum and Anthemis, while B. terrestris and A. mellifera were observed to use Phacelia. Metabarcoding data detected similar species-specific patterns of plant use: Chrysanthemum was detected on the highest proportion of Halictidae individuals, Papaver on B. terrestris, and Phacelia on B. terrestris and A. mellifera. This pattern reflects differences in tongue length: B. terrestris has a longer tongue than A. mellifera, which in turn has a longer tongue than Halictidae spp. (Prys-Jones, 1982; Williams, 1997). Halictidae individuals were found to feed primarily on open, brush and composite flowers, whose pollen and nectar is easier for short-tongued pollinators to access than tube-shaped flowers, for example, those of Phacelia (Inouye, 1980; Willmer, 2011). While there were very few observations and opportunities to sample mid- or long-tongued length bumblebees, the two B. pascuorum individuals (a longer-tongued species) which were caught with sufficient pollen loads for analysis were both found to be feeding predominantly on the tube-shaped flowers of the genera Phacelia and Echium.
The number of genera detected on individual bees by metabarcoding was unexpectedly high, particularly for A. mellifera. Earlier work suggests that A. mellifera foragers typically concentrate on a single plant species within a given foraging trip, while Bombus spp. and Halictidae feed on multiple species per foraging trip (Beil, Horn & Schwabe, 2008; reviewed by Grant, 1950; Leonhardt & Blüthgen, 2012; Wood, Holland & Goulson, 2016; but c.f. Brodschneider et al., 2018). Conversely, in the current study A. mellifera individuals carried pollen from an average of 2.7 plant genera. However, in many samples diversity was increased by plant genera which made up only a small proportion of reads: while metabarcoding data is only semi-quantitative (Elbrecht & Leese, 2015; Richardson et al., 2015a), the low abundance of reads from these genera raises the possibility that they do not represent genuine food plants, and are instead a result of pollen grains left over from previous foraging trips or transferred from the bodies of other bees in the hive or nest (Free & Williams, 1972). Alternatively, these plants may represent secondary, or ‘minor’, food sources, which may become more important as some pollinators exhibit more generalist foraging strategies in urban habitats (Geslin et al., 2013).
Use of sown wildflower strips by pollinators
All species of bee included in the study were found to utilise a range of plant genera which were not present in pollinator strips, demonstrating the ability of pollen identification to detect plant–pollinator interactions on a broader spatial scale then could be easily achieved with observation alone, especially given that honeybees, solitary bees and bumblebees can all forage over long distances (Beekman & Ratnieks, 2000; Knight et al., 2005; Zurbuchen et al., 2010). Overall, five of the 21 B. terrestris pollen loads were dominated by non-sown genera (>50% of reads): this is comparable to B. terrestris individuals foraging in sown wildflower strips on arable land (Carvell et al., 2006). Outside of wildflower strips, the majority of plant genera detected contained native members: for example, Rubus and Rosa. Previous work has found that native species are often preferred by pollinators (Corbet et al., 2001; Pardee & Philpott, 2014; Salisbury et al., 2015). Most of the plant genera originating from outside wildflower strips are commonly found in parks and gardens, either cultivated (e.g. Lupinus, Hydrangea, Buddleja, Ligustrum) or as wild plants (e.g. Rubus, Sonchus, Lactuca). Gardens provide a large proportion of urban green space (Loram et al., 2007; Thompson et al., 2003) and contain diverse plant species (Gaston et al., 2005). Alongside previous studies (Matteson & Langellotto, 2009; Osborne et al., 2008), the presence of garden species in the current study highlights the importance of gardens for urban bees. The abundance of species from outside the wildflower strips suggests that while wildflower strips alone are not enough to provision nearby bees adequately, bees are able to flexibly utilise a wide range of plant taxa and urban landscape features in order to obtain adequate floral resources.
Despite the fact that all the insects studied here were collected in urban pollinator strips, there was a relatively weak correspondence between the floral composition of the strips (i.e. which plants were flowering in strips at the time of sampling) and the composition of pollen collected from bees. The plant genera detected in the highest proportion of pollen samples were Chrysanthemum, Papaver, and Phacelia, suggesting that these plants may be valuable contributors to wildflower mixes sown to support urban bees. Each of these genera established well and produced large quantities of flowers at the time of sampling. Phacelia in particular is a common component of wildflower mixes sown in agricultural margins, and is often a significant component of foraging on these margins, particularly for B. terrestris (Carreck & Williams, 1997; Kells, Holland & Goulson, 2001; Pywell et al., 2005). Members of family Asteraceae are the plants most visited by small bees in agricultural margins (Wood, Holland & Goulson, 2017). In the current study, similar patterns were found: A. mellifera and B. terrestris disproportionately carried pollen from Phacelia and Papaver, while Halictidae spp. carried pollen from Chrysanthemum. Therefore, a mixture of these species appears to provide floral resources for the range of Hymenoptera studied. Phacelia and Papaver both contain high levels of protein and essential amino acids (Hanley et al., 2008; Roulston, Cane & Buchmann, 2000; Weiner et al., 2010), although this is not the case for Chrysanthemum (Roulston, Cane & Buchmann, 2000). However, it should be noted that all sampling was carried out in July, and it is likely that the relative contribution of different plant species to bee foraging varies over the course of the season.
In several cases, bees were observed to feed on plant genera within the wildflower strips that were not present in metabarcoding data: examples include Borago, Linaria, Leucanthenum, and Anthemis. Only DNA extractions which produced a band following PCR amplification were processed for sequencing, and this may have biased sequencing towards samples which were taken from pollen-foraging individuals since these individuals carry more pollen grains and thus are more likely to yield adequate DNA for amplification. In A. mellifera, individual bees are specialised for either pollen or nectar collection (Robinson & Page, 1989). Similarly, while Halictidae and B. terrestris females do not show individual specialisation, they may forage exclusively for either nectar or pollen on separate trips (Batra, 1964; Delph & Lively, 1992; Konzmann & Lunau, 2014). In the current study, pollen stored in the scopa was not separated from pollen on the rest of the body, and so it is not possible to distinguish deliberately collected pollen from pollen accidentally collected while foraging for nectar. However, previous studies show that pollen-feeding insects may make different foraging choices to nectar-foraging individuals: in particular, Asteraceae and Boraginaceae (families containing Anthemis, Leucathernum, and Borago, which were represented in observational but not metabarcoding data) are heavily used for nectar but not for pollen by bumblebees (Goulson et al., 2005), although it should be noted that small quantities of each family are found in bumblebee pollen loads (Kleijn & Raemakers, 2008).
However, detection may have been inhibited by methodology: while family Boraginaceae is well-detected by the primers chosen (De Vere et al., 2012), the amplification efficiency of different species in mixed samples is variable (Pornon et al., 2016). Additionally, rbcL may offer only poor discrimination for Asteraceae due to low levels of interspecific divergence (Gao et al., 2010), which were highly represented in wildflower plots. While at least one member of family Asteraceae (Chrysanthemum) was confidently identified to genus level in sequence datasets, a large number of reads could only be assigned to Asteraceae at the family level and may originate from species that were observed in floral surveys but not metabarcoding data (e.g. Anthemis).
Conclusions
Despite the fact that all bees sampled were collected in wildflower strips, a number of them were found to utilise species not present in wildflower strips, highlighting the role that gardens play in providing adequate floral resources for urban bees. Within wildflower strips, both DNA metabarcoding data and observational data suggested that Phacelia and Chrysanthemum were particularly important genera for bees at the time of sampling, while metabarcoding additionally suggested that Papaver was also an important source of pollen for insects. Different bee species used different plant genera, highlighting the importance of including a range of plants in foraging strips: at the time of sampling, Papaver was used by the highest proportion of B. terrestris individuals, Phacelia by both A. mellifera and B. terrestris, and Chrysanthemum by Halictidae spp. However, all samples were collected in July and it is likely that other plants become more important during other times of the year. Finally, we show that non-destructive sampling coupled with DNA metabarcoding can be used to evaluate the ways in which pollinators interact with sown wildflower strips in urban environments, although it produces fewer successful samples compared to destructive methods.
Supplemental Information
For each sample, random subsampling was carried out at intervals of 1.
Only samples collected from wildflower strips in July are shown.
Unlike Figure 5, plant genera were counted as present if at least two reads were detected.
Both seed mixes contained a variety of wild seeds, with Papaver rhoas and Phacelia tanacetifolia being the dominant species by weight, followed by Rudbeckia, Leucanthemum and Chrysanthemum spp. Mixture 4 additionally contained Fagopyrum esculentum, Cichorium intybus and Helianthus annuus. Full details are contained in Table S2.
The amount of each seed is expressed as percentage of total weight. Each mix contained three mixes from Moles Seeds: “summer picking mix” contained Zinnia , Calendula, Centaurea cyanus, Malope, Cosmos, Malva, Centaurea, Tagetes erecta, Aster and Gypsophila; “medieval carpet mix” contained Calendula, Linum rubrum, Malope, Centaurea, Echium, Borago and Nigella; and “express summer mix” contained Linum rubrum, Saponaria vaccaria, Malope, Papaver, Calendula, Nigella, Tagates, Pulsatilla, and Centaurea cyanus. Information was not available on the proportion of each seed in the three Moles Seeds mixes.
Primers are shown in 5’ to 3’ orientation.
Values represent species abundances as a proportion of total high-quality reads. Columns represent either categories of metadata or species, and rows represent samples.
Acknowledgments
Thanks to Robert Potter and Mark Holloway of Bournemouth Borough Council for provision of wildflower strips, details of seed mixes and permission to collect samples. Thanks to Arne Loth and Kimberley Tickner for assistance in liaising with the council and locating and mapping the sown wildflower areas.
Funding Statement
This work was supported by a Bournemouth University Higher Education Innovation Fund Grant to Dr Elizabeth Franklin. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Natasha de Vere is an Academic Editor for PeerJ.
Author Contributions
Caitlin Potter performed the experiments, analysed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Natasha de Vere conceived and designed the experiments, analysed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Laura E. Jones performed the experiments, analysed the data, approved the final draft.
Col R. Ford analysed the data, contributed reagents/materials/analysis tools, approved the final draft.
Matthew J. Hegarty conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, approved the final draft.
Kathy H. Hodder conceived and designed the experiments, performed the experiments, approved the final draft.
Anita Diaz conceived and designed the experiments, performed the experiments, approved the final draft.
Elizabeth L. Franklin conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Sequencing data was analysed using a workflow available at https://github.com/colford/nbgw-plant-illumina-pipeline.
Raw sequence data is available on the Sequence Read Archive (SRA) at PRJNA481887
Potter, Caitlin (2019): Raw fastq files. figshare. Fileset. https://doi.org/10.6084/m9.figshare.6893501.v1. Potter, Caitlin (2019): Metadata. figshare. Dataset. https://doi.org/10.6084/m9.figshare.6930857.v1.
References
- Ahrné, Bengtsson & Elmqvist (2009).Ahrné K, Bengtsson J, Elmqvist T. Bumble bees (Bombus spp.) along a gradient of increasing urbanization. PLOS ONE. 2009;4(5):e5574. doi: 10.1371/journal.pone.0005574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizen & Harder (2009).Aizen MA, Harder LD. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Current Biology. 2009;19(11):915–918. doi: 10.1016/j.cub.2009.03.071. [DOI] [PubMed] [Google Scholar]
- Baldock et al. (2015).Baldock KC, Goddard MA, Hicks DM, Kunin WE, Mitschunas N, Osgathorpe LM, Potts SG, Robertson KM, Scott AV, Stone GN, Vaughan IP, Memmot J. Where is the UK’s pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proceedings of the Royal Society B: Biological Sciences. 2015;282(1803):20142849. doi: 10.1098/rspb.2014.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball & Morris (2014).Ball SG, Morris RKA. Peterborough: Joint Nature Conservation Committee; 2014. A review of the scarce and threatened flies of Great Britain. Part 6: Syrphidae. Species Status 9. Technical report. [Google Scholar]
- Banaszak-Cibicka & Zmihorski (2012).Banaszak-Cibicka W, Zmihorski M. Wild bees along an urban gradient: winners and losers. Journal of Insect Conservation. 2012;16(3):331–343. doi: 10.1007/s10841-011-9419-2. [DOI] [Google Scholar]
- Bartomeus et al. (2013).Bartomeus I, Ascher JS, Gibbs J, Danforth BN, Wagner DL, Hedtke SM, Winfree R. Historical changes in northeastern US bee pollinators related to shared ecological traits. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(12):4656–4660. doi: 10.1073/pnas.1218503110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates et al. (2011).Bates AJ, Sadler JP, Fairbrass AJ, Falk SJ, Hale JD, Matthews TJ. Changing bee and hoverfly pollinator assemblages along an urban-rural gradient. PLOS ONE. 2011;6(8):e23459. doi: 10.1371/journal.pone.0023459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra (1964).Batra SW. Behavior of the social bee, Lasioglossum zephyrum, within the nest (Hymenoptera: Halictidae) Insectes Sociaux. 1964;11(2):159–185. doi: 10.1007/bf02222935. [DOI] [Google Scholar]
- Beekman & Ratnieks (2000).Beekman M, Ratnieks F. Long-range foraging by the honey-bee, Apis mellifera L. Functional Ecology. 2000;14(4):490–496. doi: 10.1046/j.1365-2435.2000.00443.x. [DOI] [Google Scholar]
- Beil, Horn & Schwabe (2008).Beil M, Horn H, Schwabe A. Analysis of pollen loads in a wild bee community (Hymenoptera: Apidae)- a method for elucidating habitat use and foraging distances. Apidologie. 2008;39(4):456–467. doi: 10.1051/apido:2008021. [DOI] [Google Scholar]
- Bell et al. (2017).Bell KL, Fowler J, Burgess KS, Dobbs EK, Gruenewald D, Lawley B, Morozumi C, Brosi BJ. Applying pollen DNA metabarcoding to the study of plant-pollinator interactions. Applications in Plant Sciences. 2017;5(6):1600124. doi: 10.3732/apps.1600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson et al. (2012).Benson D, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Research. 2012;41(Database):D36–D42. doi: 10.1093/nar/gks1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesmeijer et al. (2006).Biesmeijer JC, Roberts SPM, Reemer M, Ohlemuller R, Edwards M, Peeters T, Schaffers AP, Potts SG, Kleukers R, Thomas CD, Settele J, Kunin WE. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313(5785):351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- Blackmore & Goulson (2014).Blackmore LM, Goulson D. Evaluating the effectiveness of wildflower seed mixes for boosting floral diversity and bumblebee and hoverfly abundance in urban areas. Insect Conservation and Diversity. 2014;7(5):480–484. doi: 10.1111/icad.12071. [DOI] [Google Scholar]
- Bolger, Lohse & Usadel (2014).Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommarco et al. (2011).Bommarco R, Lundin O, Smith HG, Rundlöf M. Drastic historic shifts in bumble-bee community composition in Sweden. Proceedings of the Royal Society B: Biological Sciences. 2011;279(1727):309–315. doi: 10.1098/rspb.2011.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodschneider et al. (2018).Brodschneider R, Gratzer K, Heigl H, Auer W, Moosbeckhofer R, Crailssheim K. What we can (or cannot) learn from multifloral pollen pellets. Bee World. 2018;95(3):78–80. doi: 10.1080/0005772x.2018.1483057. [DOI] [Google Scholar]
- Cameron et al. (2011).Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, Griswold TL. Patterns of widespread decline in North American bumble bees. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(2):662–667. doi: 10.1073/pnas.1014743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreck & Williams (1997).Carreck N, Williams IH. Observations on two commercial flower mixtures as food sources for beneficial insects in the UK. Journal of Agricultural Science. 1997;128(4):397–403. doi: 10.1017/s0021859697004279. [DOI] [Google Scholar]
- Carvell et al. (2006).Carvell C, Westrich P, Meek WR, Pywell RF, Nowakowski M. Assessing the value of annual and perennial forage mixtures for bumblebees by direct observation and pollen analysis. Apidologie. 2006;37(3):326–340. doi: 10.1051/apido:2006002. [DOI] [Google Scholar]
- Corbet et al. (2001).Corbet SA, Bee J, Dasmahapatra K, Gale S, Gorringe E, La Fera B, Moorhouse T, Trevail A, Van Bergen Y, Vorontsova M. Native or exotic? Double or single? Evaluating plants for pollinator-friendly gardens. Annals of Botany. 2001;87(2):219–232. doi: 10.1006/anbo.2000.1322. [DOI] [PubMed] [Google Scholar]
- Cubey & Merrick (2014).Cubey J, Edwards D, Lancaster N. RHS Plant Finder 2014. London: Royal Horticultural Society; 2014. [Google Scholar]
- Deguines et al. (2012).Deguines N, Juilliard R, De Flores M, Fontaine C. The whereabouts of flower visitors: contrasting land-use preferences revealed by a country-wide survey based on citizen science. PLOS ONE. 2012;7(9):e45822. doi: 10.1371/journal.pone.0045822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vere et al. (2017).De Vere N, Jones LE, Gilmore T, Moscrop J, Lowe A, Smith D, Hegarty MJ, Creer S, Ford CR. Using DNA metabarcoding to investigate honey bee foraging reveals limited flower use despite high floral availability. Scientific Reports. 2017;7(1):42838. doi: 10.1038/srep42838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vere et al. (2012).De Vere N, Rich TC, Ford CR, Trinder SA, Long C, Moor CW, Satterthwaite D, Davies H, Allainguillaume J, Ronca S, Tatarinova T, Garbett H, Walker K, Wilkinson MJ. DNA barcoding the native flowering plants and conifers of Wales. PLOS ONE. 2012;7(6):e37945. doi: 10.1371/journal.pone.0037945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delph & Lively (1992).Delph LF, Lively CM. Pollinator visitation, floral display, and nectar production of the sexual morphs of a gynodioecious shrub. Oikos. 1992;63(2):161–170. doi: 10.2307/3545374. [DOI] [Google Scholar]
- Dormann, Gruber & Fruend (2008).Dormann CF, Gruber B, Fruend J. Introducing the bipartite package: Analysing Ecological Networks. R News. 2008;8(2):7–24. [Google Scholar]
- Dupont, Damgaard & Simonsen (2011).Dupont YL, Damgaard C, Simonsen V. Quantitative historical change in bumblebee (Bombus spp.) assemblages of red clover fields. PLOS ONE. 2011;6(9):e25172. doi: 10.1371/journal.pone.0025172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbrecht & Leese (2015).Elbrecht V, Leese F. Can DNA-based ecosystem assessments quantify species abundance? Testing primer bias and biomass- sequence relationships with an innovative metabarcoding protocol. PLOS ONE. 2015;10(7):e0130324. doi: 10.1371/journal.pone.0130324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortel et al. (2014).Fortel L, Henry M, Guilbaud L, Guirao AL, Kuhlmann M, Mouret H, Rollin O, Vaissiere BE. Decreasing abundance, increasing diversity and changing structure of the wild bee community (Hymenoptera: Anthophila) along an urbanization gradient. PLOS ONE. 2014;9(8):e104679. doi: 10.1371/journal.pone.0104679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free & Williams (1972).Free J, Williams IH. The transport of pollen on the body hairs of honeybees (Apis mellifera L.) and bumblebees (Bombus spp. L.) Journal of Applied Ecology. 1972;9(2):609–615. doi: 10.2307/2402458. [DOI] [Google Scholar]
- Gao et al. (2010).Gao T, Yao H, Song J, Zhu Y, Liu C, Chen S. Evaluating the feasibility of using candidate DNA barcodes in discriminating species of the large Asteraceae family. BMC Evolutionary Biology. 2010;10:324. doi: 10.1186/1471-2148-10-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibaldi et al. (2011).Garibaldi LA, Steffan-Dewenter I, Kremen C, Morales JM, Bommarco R, Cunningham SA, Carvalheiro LG, Chacoff NP, Dudenhoffer JH, Greenleaf SS, Holzschuh A, Isaacs R, Krewenka K, Mandelik Y, Mayfield MM, Morandin LA, Potts SG, Ricketts TH, Szentgyorgyi H, Viana BF, Westphal C, Winfree R, Klein AM. Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecology Letters. 2011;14:1062–1072. doi: 10.1111/j.1461-0248.2011.01669.x. [DOI] [PubMed] [Google Scholar]
- Gaston et al. (2005).Gaston KJ, Warren PH, Thompson K, Smith RM. Urban domestic gardens (IV): the extent of the resource and its associated features. Biodiversity and Conservation. 2005;14(14):3327–3349. doi: 10.1007/s10531-004-9513-9. [DOI] [Google Scholar]
- Gerard et al. (2010).Gerard F, Petit S, Smith G, Thomson A, Brown N, Manchester S, Wadsworth R, Bugar G, Halada L, Bezak P, Boltizar M, De Badts E, Halabuk A, Mojses M, Petrovic F, Gregor M, Hazeu G, Mucher CA, Wachowicz M, Huitu H, Tuominen S, Kohler R, Olschofsky K, Ziese H, Kolar J, Sustera J, Luque S, Pino J, Pons X, Roda F, Roscher M, Feranec J. Land cover change in Europe between 1950 and 2000 determined employing aerial photography. Progress in Physical Geography. 2010;34(2):183–205. doi: 10.1177/0309133309360141. [DOI] [Google Scholar]
- Geslin et al. (2013).Geslin B, Gauzens B, Thébault E, Dajoz I. Plant pollinator networks along a gradient of urbanisation. PLOS ONE. 2013;8(5):e63421. doi: 10.1371/journal.pone.0063421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson et al. (2005).Goulson D, Hanley ME, Darvill B, Ellis JS, Knight ME. Causes of rarity in bumblebees. Biological Conservation. 2005;122(1):1–8. doi: 10.1016/j.biocon.2004.06.017. [DOI] [Google Scholar]
- Goulson et al. (2015).Goulson D, Nicholls E, Botias C, Rotheray EL. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015;347(6229):1255957. doi: 10.1126/science.1255957. [DOI] [PubMed] [Google Scholar]
- Grant (1950).Grant V. The flower constancy of bees. Botanical Review. 1950;16(7):379–398. doi: 10.1007/bf02869992. [DOI] [Google Scholar]
- Hanley et al. (2008).Hanley ME, Franco M, Pichon S, Darvill B, Goulson D. Breeding system, pollinator choice and variation in pollen quality in British herbaceous plants. Functional Ecology. 2008;22(4):592–598. doi: 10.1111/j.1365-2435.2008.01415.x. [DOI] [Google Scholar]
- Hawkins et al. (2015).Hawkins J, De Vere N, Griffith A, Ford CR, Allainguillaume J, Hegarty MJ, Baillie L, Adams-Groom B. Using DNA metabarcoding to identify the floral composition of honey: a new tool for investigating honey bee foraging preferences. PLOS ONE. 2015;10(8):e0134735. doi: 10.1371/journal.pone.0134735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx et al. (2007).Hendrickx F, Maelfait J-P, Van Wingerden W, Schweiger O, Speelmans M, Aviron S, Augenstein I, Billeter R, Bailey D, Bukacek R, Burel F, Diekotter T, Dirksen J, Herzog F, Liira J, Roubalova M, Vandomme V, Bugter R. How landscape structure, land-use intensity and habitat diversity affect components of total arthropod diversity in agricultural landscapes. Journal of Applied Ecology. 2007;44(2):340–351. doi: 10.1111/j.1365-2664.2006.01270.x. [DOI] [Google Scholar]
- Hernandez, Frankie & Thorp (2009).Hernandez JL, Frankie GW, Thorp RW. Ecology of urban bees: a review of current knowledge and directions for future study. Cities and the Environment. 2009;2(1):1–15. doi: 10.15365/cate.2132009. [DOI] [Google Scholar]
- Hicks et al. (2016).Hicks DM, Ouvrard P, Baldock KC, Baude M, Goddard MA, Kunin WE, Mitschunas N, Memmot J, Morse H, Nikolitsi M, Osgathorpe LM, Potts SG, Roberts KM, Scott AV, Sinclair F, Westbury DB, Stone GN. Food for pollinators: quantifying the nectar and pollen resources of urban flower meadows. PLOS ONE. 2016;11(6):e0158117. doi: 10.1371/journal.pone.0158117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülsmann et al. (2015).Hülsmann M, Von Wehrden H, Klein A-M, Leonhardt SD. Plant diversity and composition compensate for negative effects of urbanization on foraging bumble bees. Apidologie. 2015;46(6):760–770. doi: 10.1007/s13592-015-0366-x. [DOI] [Google Scholar]
- Illumina (2013).Illumina 16S Sample Preparation Guide. 2013. https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf Illumina.
- Inouye (1980).Inouye DW. The effect of proboscis and corolla tube lengths on patterns and rates of flower visitation by bumblebees. Oecologia. 1980;45(2):197–201. doi: 10.1007/bf00346460. [DOI] [PubMed] [Google Scholar]
- Keller et al. (2015).Keller A, Danner N, Grimmer G, Ankenbrand M, Von Der Ohe K, Von Der Ohe W, Rost S, Hartel S, Steffan-Dewenter I. Evaluating multiplexed next-generation sequencing as a method in palynology for mixed pollen samples. Plant Biology. 2015;17(2):558–566. doi: 10.1111/plb.12251. [DOI] [PubMed] [Google Scholar]
- Kells, Holland & Goulson (2001).Kells AR, Holland JM, Goulson D. The value of uncropped field margins for foraging bumblebees. Journal of Insect Conservation. 2001;5(4):283–291. doi: 10.1023/a:1013307822575. [DOI] [Google Scholar]
- Kerr et al. (2015).Kerr JT, Pindar A, Galpern P, Packer L, Potts SG, Roberts SM, Rasmont P, Schweiger O, Colla SR, Richardson LL, Wagner DL, Gall LF, Sikes DS, Pantoja A. Climate change impacts on bumblebees converge across continents. Science. 2015;349(6244):177–180. doi: 10.1126/science.aaa7031. [DOI] [PubMed] [Google Scholar]
- Kleijn & Raemakers (2008).Kleijn D, Raemakers I. A retrospective analysis of pollen host plant use by stable and declining bumble bee species. Ecology. 2008;89(7):1811–1823. doi: 10.1890/07-1275.1. [DOI] [PubMed] [Google Scholar]
- Klein et al. (2007).Klein A-M, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. Importance of pollinators in changing landscapes for world crops. Proceedings of the Royal Society B: Biological Sciences. 2007;274(1608):303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight et al. (2005).Knight ME, Martin AP, Bishop S, Osborne JL, Hale RJ, Sanderson RA, Goulson D. An interspecific comparison of foraging range and nest density of four bumblebee (Bombus) species. Molecular Ecology. 2005;14(6):1811–1820. doi: 10.1111/j.1365-294x.2005.02540.x. [DOI] [PubMed] [Google Scholar]
- Konzmann & Lunau (2014).Konzmann S, Lunau K. Divergent rules for pollen and nectar foraging bumblebees- a laboratory study with artificial flowers offering diluted nectar substitute and pollen surrogate. PLOS ONE. 2014;9(3):e91900. doi: 10.1371/journal.pone.0091900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth (2016).Lenth RV. Least-squares means: the R Package lsmeans. Journal of Statistical Software. 2016;69:1–33. [Google Scholar]
- Leonhardt & Blüthgen (2012).Leonhardt SD, Blüthgen N. The same, but different: pollen foraging in honeybee and bumblebee colonies. Apidologie. 2012;43(4):449–464. doi: 10.1007/s13592-011-0112-y. [DOI] [Google Scholar]
- Loram et al. (2007).Loram A, Tratalos J, Warren PH, Gaston KJ. Urban domestic gardens (X): the extent & structure of the resource in five major cities. Landscape Ecology. 2007;22(4):601–615. doi: 10.1007/s10980-006-9051-9. [DOI] [Google Scholar]
- Lucas et al. (2018b).Lucas A, Bodger O, Brosi BJ, Ford CR, Forman D, Greig C, Hegarty M, Jones L, Neyland PJ, De Vere N. Floral resource partitioning by individuals within generalised hoverfly pollination networks revealed by DNA metabarcoding. Scientific reports. 2018b;8(1):5133. doi: 10.1038/s41598-018-23103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas et al. (2018a).Lucas A, Bodger O, Brosi BJ, Ford CR, Forman DW, Greig C, Hegarty M, Neyland PJ, De Vere N. Generalisation and specialisation in hoverfly (Syrphidae) grassland pollen transport networks revealed by DNA metabarcoding. Journal of Animal Ecology. 2018a;87(4):1008–1021. doi: 10.1111/1365-2656.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoc & Salzberg (2011).Magoc T, Salzberg SL. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson & Langellotto (2009).Matteson KC, Langellotto GA. Bumble bee abundance in New York City community gardens: implications for urban agriculture. Cities and the Environment. 2009;2(1):1–12. doi: 10.15365/cate.2152009. [DOI] [Google Scholar]
- McFrederick & LeBuhn (2006).McFrederick QS, LeBuhn G. Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)? Biological Conservation. 2006;129(3):372–382. doi: 10.1016/j.biocon.2005.11.004. [DOI] [Google Scholar]
- McGinnis & Madden (2004).McGinnis S, Madden TL. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Research. 2004;32(Webserver):W20–W25. doi: 10.1093/nar/gkh435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller & Schmid-Hempel (1992).Muller C, Schmid-Hempel P. Variation in life-history pattern in relation to worker mortality in the bumble-bee, Bombus lucorum. Functional Ecology. 1992;6(1):48–56. doi: 10.2307/2389770. [DOI] [Google Scholar]
- National Research Council (2007).National Research Council . Status of pollinators in North America. Washington D.C.: National Academies Press; 2007. [Google Scholar]
- NEA (2011).NEA UK. The UK national ecosystem assessment: Synthesis of the key findings. Cambridge: UNEP-WCMC; 2011. [Google Scholar]
- Oksanen et al. (2017).Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H. Vegan: Community ecology package; 2017. [Google Scholar]
- Osborne et al. (2008).Osborne JL, Martin AP, Shortall CR, Todd AD, Goulson D, Knight ME, Hale RJ, Sanderson RA. Quantifying and comparing bumblebee nest densities in gardens and countryside habitats. Journal of Applied Ecology. 2008;45(3):784–792. doi: 10.1111/j.1365-2664.2007.01359.x. [DOI] [Google Scholar]
- Otterstatter & Thomson (2008).Otterstatter MC, Thomson JD. Does pathogen spillover from commercially reared bumble bees threaten wild pollinators? PLOS ONE. 2008;3(7):e2771. doi: 10.1371/journal.pone.0002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini & Hesse (2005).Pacini E, Hesse M. Pollenkitt- its composition, forms and functions. Flora-Morphology, Distribution, Functional Ecology of Plants. 2005;200(5):399–415. doi: 10.1016/j.flora.2005.02.006. [DOI] [Google Scholar]
- Pardee & Philpott (2014).Pardee GL, Philpott SM. Native plants are the bee’s knees: local and landscape predictors of bee richness and abundance in backyard gardens. Urban Ecosystems. 2014;17(3):641–659. doi: 10.1007/s11252-014-0349-0. [DOI] [Google Scholar]
- Pornon et al. (2016).Pornon A, Escaravage N, Burrus MaH, Holota H, Khimoun A, Mariette J, Pellizzari C, Iribar A, Etienne R, Taberlet P, Vidal M, Winterton P, Zinger L, Andalo C. Using metabarcoding to reveal and quantify plant-pollinator interactions. Scientific Reports. 2016;6(1):27282. doi: 10.1038/srep27282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts et al. (2010).Potts SG, Roberts SPM, Dean R, Marris G, Brown MA, Jones R, Neumann P, Settele J. Declines of managed honey bees and beekeepers in Europe. Journal of Agricultural Research. 2010;49(1):15–22. doi: 10.3896/ibra.1.49.1.02. [DOI] [Google Scholar]
- Prys-Jones (1982).Prys-Jones O. Ecological studies of foraging and life history in bumblebees. Cambridge: University of Cambridge; 1982. [Google Scholar]
- Pywell et al. (2005).Pywell RF, Warman EA, Carvell C, Sparks TH, Dicks LV, Bennett D, Wright A, Critchley CNR, Sherwood A. Providing foraging resources for bumblebees in intensively farmed landscapes. Biological Conservation. 2005;121(4):479–494. doi: 10.1016/j.biocon.2004.05.020. [DOI] [Google Scholar]
- Richardson et al. (2015a).Richardson RT, Lin C-H, Quijia JO, Riusech NS, Goodell K, Johnson RM. Rank-based characterization of pollen assemblages collected by honey bees using a multi-locus metabarcoding approach. Applications in Plant Sciences. 2015a;3(11):1500043. doi: 10.3732/apps.1500043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson et al. (2015b).Richardson RT, Lin C-H, Sponsler DB, Quijia JO, Goodell K, Johnson RM. Application of ITS2 metabarcoding to determine the provenance of pollen collected by honey bees in an agroecosystem. Applications in Plant Sciences. 2015b;3(1):1400066. doi: 10.3732/apps.1400066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson & Page (1989).Robinson GE, Page RE. Genetic determination of nectar foraging, pollen foraging, and nest-site scouting in honey bee colonies. Behavioral Ecology and Sociobiology. 1989;24(5):317–323. doi: 10.1007/bf00290908. [DOI] [Google Scholar]
- Roulston, Cane & Buchmann (2000).Roulston TH, Cane JH, Buchmann SL. What governs protein content of pollen: pollinator preferences, pollen-pistil interactions, or phylogeny? Ecological Monographs. 2000;70(4):617–643. doi: 10.2307/2657188. [DOI] [Google Scholar]
- Salisbury et al. (2015).Salisbury A, Armitage J, Bostock H, Perry J, Tatchell M, Thompson K. Enhancing gardens as habitats for flower-visiting aerial insects (pollinators): should we plant native or exotic species? Journal of Applied Ecology. 2015;52(5):1156–1164. doi: 10.1111/1365-2664.12499. [DOI] [Google Scholar]
- Seto et al. (2011).Seto KC, Fragkias M, Guneralp B, Reilly MK. A meta-analysis of global urban land expansion. PLOS ONE. 2011;6(8):e23777. doi: 10.1371/journal.pone.0023777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart et al. (2017).Smart M, Cornman RS, Iwanowicz DD, McDermott-Kubeczko M, Pettis JS, Spivak MS, Otto CRV. A comparison of honey bee-collected pollen from working agricultural lands using light microscopy and ITS metabarcoding. Environmental Entomology. 2017;46(1):38–49. doi: 10.1093/ee/nvw159. [DOI] [PubMed] [Google Scholar]
- Stace (2010).Stace C. New flora of the British Isles. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- R Computing Team (2017).R Computing Team . Vienna: R Foundation for Statistical Computing; 2017. R: a language and environment for statistical computing. [Google Scholar]
- Thompson et al. (2003).Thompson K, Austin KC, Smith RM, Warren PH, Angold PG, Gaston KJ. Urban domestic gardens (I): putting small-scale plant diversity in context. Journal of Vegetation Science. 2003;14(1):71–78. doi: 10.1111/j.1654-1103.2003.tb02129.x. [DOI] [Google Scholar]
- Warzecha et al. (2018).Warzecha D, Diekotter T, Wolters V, Jauker F. Attractiveness of wildflower mixtures for wild bees and hoverflies depends on some key plant species. Insect Conservation and Diversity. 2018;11(1):32–41. doi: 10.1111/icad.12264. [DOI] [Google Scholar]
- Weiner et al. (2010).Weiner CN, Hilpert A, Werner M, Linsenmair KE, Blüthgen N. Pollen amino acids and flower specialisation in solitary bees. Apidologie. 2010;41(4):476–487. doi: 10.1051/apido/2009083. [DOI] [Google Scholar]
- Williams (1997).Williams C. Foraging ecology of nectar-collecting bumblebees and honeybees. Cambridge: University of Cambridge; 1997. [Google Scholar]
- Williams et al. (2015).Williams NM, Ward KL, Pope N, Isaacs R, Wilson J, May EA, Ellis J, Daniels J, Akers P, Ullmann K, Peters J. Native wildflower plantings support wild bee abundance and diversity in agricultural landscapes across the United States. Ecological Applications. 2015;25(8):2119–2131. doi: 10.1890/14-1748.1. [DOI] [PubMed] [Google Scholar]
- Willmer (2011).Willmer P. Pollination and Floral Ecology. Princeton: Princeton University Press; 2011. [Google Scholar]
- Wood, Holland & Goulson (2016).Wood T, Holland JM, Goulson D. Diet characterisation of solitary bees on farmland: dietary specialisation predicts rarity. Biodiversity and Conservation. 2016;25(13):2655–2671. doi: 10.1007/s10531-016-1191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, Holland & Goulson (2017).Wood TJ, Holland JM, Goulson D. Providing foraging resources for solitary bees on farmland: current schemes for pollinators benefit a limited suite of species. Journal of Applied Ecology. 2017;54(1):323–333. doi: 10.1111/1365-2664.12718. [DOI] [Google Scholar]
- Zurbuchen et al. (2010).Zurbuchen A, Landert L, Klaiber J, Muller A, Hein S, Dorn S. Maximum foraging ranges in solitary bees: only few individuals have the capability to cover long foraging distances. Biological Conservation. 2010;143(3):669–676. doi: 10.1016/j.biocon.2009.12.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For each sample, random subsampling was carried out at intervals of 1.
Only samples collected from wildflower strips in July are shown.
Unlike Figure 5, plant genera were counted as present if at least two reads were detected.
Both seed mixes contained a variety of wild seeds, with Papaver rhoas and Phacelia tanacetifolia being the dominant species by weight, followed by Rudbeckia, Leucanthemum and Chrysanthemum spp. Mixture 4 additionally contained Fagopyrum esculentum, Cichorium intybus and Helianthus annuus. Full details are contained in Table S2.
The amount of each seed is expressed as percentage of total weight. Each mix contained three mixes from Moles Seeds: “summer picking mix” contained Zinnia , Calendula, Centaurea cyanus, Malope, Cosmos, Malva, Centaurea, Tagetes erecta, Aster and Gypsophila; “medieval carpet mix” contained Calendula, Linum rubrum, Malope, Centaurea, Echium, Borago and Nigella; and “express summer mix” contained Linum rubrum, Saponaria vaccaria, Malope, Papaver, Calendula, Nigella, Tagates, Pulsatilla, and Centaurea cyanus. Information was not available on the proportion of each seed in the three Moles Seeds mixes.
Primers are shown in 5’ to 3’ orientation.
Values represent species abundances as a proportion of total high-quality reads. Columns represent either categories of metadata or species, and rows represent samples.
Data Availability Statement
The following information was supplied regarding data availability:
Sequencing data was analysed using a workflow available at https://github.com/colford/nbgw-plant-illumina-pipeline.
Raw sequence data is available on the Sequence Read Archive (SRA) at PRJNA481887
Potter, Caitlin (2019): Raw fastq files. figshare. Fileset. https://doi.org/10.6084/m9.figshare.6893501.v1. Potter, Caitlin (2019): Metadata. figshare. Dataset. https://doi.org/10.6084/m9.figshare.6930857.v1.