Abstract
OBJECTIVE
Whether hyperglycemia in utero less than overt diabetes is associated with altered childhood glucose metabolism is unknown. We examined associations of gestational diabetes mellitus (GDM) not confounded by treatment with childhood glycemia in the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) cohort.
RESEARCH DESIGN AND METHODS
HAPO Follow-up Study (FUS) included 4,160 children ages 10–14 years who completed all or part of an oral glucose tolerance test (OGTT) and whose mothers had a 75-g OGTT at ∼28 weeks of gestation with blinded glucose values. The primary predictor was GDM by World Health Organization criteria. Child outcomes were impaired fasting glucose (IFG), impaired glucose tolerance (IGT), and type 2 diabetes. Additional measures included insulin sensitivity and secretion and oral disposition index.
RESULTS
For mothers with GDM, 10.6% of children had IGT compared with 5.0% of children of mothers without GDM; IFG frequencies were 9.2% and 7.4%, respectively. Type 2 diabetes cases were too few for analysis. Odds ratios (95% CI) adjusted for family history of diabetes, maternal BMI, and child BMI z score were 1.09 (0.78–1.52) for IFG and 1.96 (1.41–2.73) for IGT. GDM was positively associated with child’s 30-min, 1-h, and 2-h but not fasting glucose and inversely associated with insulin sensitivity and oral disposition index (adjusted mean difference −76.3 [95% CI −130.3 to −22.4] and −0.12 [−0.17 to −0.064]), respectively, but not insulinogenic index.
CONCLUSIONS
Offspring exposed to untreated GDM in utero are insulin resistant with limited β-cell compensation compared with offspring of mothers without GDM. GDM is significantly and independently associated with childhood IGT.
Introduction
The incidence of type 2 diabetes among youth is rising, due, in part, to increasing prevalence of childhood obesity (1–3). Worldwide, it is estimated that type 2 diabetes in children will continue to increase, posing a significant public health and financial burden (4). In addition to childhood obesity, intrauterine exposure to maternal pre-existing diabetes or gestational diabetes mellitus (GDM) is associated with a higher risk for offspring abnormal glucose metabolism (5). Early evidence for these associations came from two long-term studies: a longitudinal study of Pima Indians (6) and cohort study of a mixed ethnic population in Chicago (7). However, these studies focused on women with a high prevalence of diabetes, making it unclear whether these results could be extrapolated to other populations. More recently, maternal treatment during pregnancy has confounded studies examining the impact of the intrauterine milieu on offspring risk of hyperglycemia (8). Studies have also not adequately addressed whether hyperglycemia in utero less than overt diabetes is associated with altered glucose metabolism in childhood.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Follow-up Study (FUS) offered a unique opportunity to examine associations of maternal glycemia during pregnancy, less than overt diabetes and not confounded by maternal treatment, with childhood glucose metabolism. The HAPO study, an observational epidemiological study that recruited a large, multinational, racially and ethnically diverse cohort, demonstrated that glucose levels below those diagnostic of diabetes were associated with adverse pregnancy outcomes (9). Based on these results and others, new diagnostic criteria for GDM were proposed by the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) (10) and adopted by the World Health Organization (WHO) and others (11). These IADPSG/WHO criteria, based on newborn outcomes, reduced both the glucose levels and required number of abnormal values (from two to one) to diagnose GDM compared with Carpenter-Coustan criteria (12). This report from the HAPO FUS examines whether in utero exposure to untreated GDM, defined post hoc by IADPSG/WHO criteria, is associated with prespecified glucose outcomes (impaired fasting glucose [IFG], impaired glucose tolerance [IGT], or type 2 diabetes) in children ages 10–14 years.
Research Design and Methods
HAPO was a population-based study in which women underwent a 75-g oral glucose tolerance test (OGTT) at ∼28 weeks of gestation (9). Fasting plasma glucose (FPG) and 1-h and 2-h plasma glucose (PG) were measured at a central laboratory (9). OGTT results remained blinded to caregivers and participants unless FPG >5.8 mmol/L and/or 2-h PG >11.1 mmol/L, either measure was <2.5 mmol/L, or random PG at 34–37 weeks of gestation was >8.9 mmol/L (9). Using these criteria, 427 (1.8%) participants were unblinded based on FPG and/or 2-h PG. Blinded participants were untreated. Height, weight, and blood pressure were measured using standardized procedures. Demographic and lifestyle characteristics, including age, self-reported race/ethnicity, and any smoking or alcohol use during pregnancy, were collected via questionnaire and parity by medical record abstraction.
Participants
HAPO FUS participants were recruited during 2013–2016 from 10 of 15 HAPO field centers based on feasibility to recruit participants. Eligibility criteria for HAPO FUS included caregivers and participants being blinded to HAPO OGTT results, gestational age at delivery ≥37 weeks, and no major neonatal malformations or fetal/neonatal death. This yielded 15,812 eligible mother-child pairs. The recruitment target was 7,000 pairs, based on the primary childhood outcome of overweight/obesity (13). Multiple attempts were made to contact all eligible participants through institutional review board (IRB)–approved means. Of the 15,812 eligible mother-child pairs, 6,490 could not be contacted and 4,488 declined participation (Supplementary Fig. 1). A total of 4,834 children completed all or part of the HAPO FUS visit. OGTT completion was not required for participation. One child was excluded due to inadequate fasting and a second for lack of cooperation. Of the remaining 4,832 children, data were analyzed from 4,160 who had an FPG and at least one other timed OGTT measurement or who reported diabetes on treatment and were not excluded as having type 1 diabetes by antibody testing (see below).
The protocol was approved by each center’s IRB. All mothers gave written informed consent for their child, and children assented where required by the local IRB. There was an external Observational Study Monitoring Board.
Study Visit
Height was measured twice without shoes to the nearest 0.5 cm with a stadiometer and again if results differed by >1.0 cm. Weight was measured twice to the nearest 0.1 kg and again if results differed by >0.5 kg. Participants underwent a 2-h OGTT with a glucose load of 1.75 g/kg body wt (maximum 75 g) after an 8-h overnight fast with samples drawn for glucose and C-peptide at fasting and 30 min, 1 h, and 2 h. If the child had self-reported diabetes on drug treatment, only a nonfasting blood sample was collected. All samples were processed at the field center laboratory and stored at −80°C until shipment to the Central Laboratory.
Skinfolds (triceps, subscapular, and suprailiac) were measured twice with calibrated calipers (Harpenden, London, U.K.) to the nearest 0.1 mm and again if results differed by >1.0 mm. Percent fat was measured by air displacement plethysmography (BOD POD; COSMED, Rome, Italy). Tanner staging was performed by trained individuals using breast/areolar development for girls and testicular volume (Prader orchidometer) for boys. Child’s age, first-degree family history of diabetes, and menstrual history for girls were collected from the mother via questionnaire.
Laboratory Measurements
Glucose was measured by hexokinase in the Clinical Chemistry Laboratory of Northwestern Memorial Hospital on a Beckman Coulter SYNCHRON LX analyzer. Blinded duplicate samples were assayed several weeks apart and coefficients of variation (CVs) calculated within pairs for a random 10% subset; mean CV was 1.5% for FPG and 1-h and 2-h PG and 1.3% for 30-min PG. C-peptide was measured in the Comprehensive Metabolic Core at Northwestern using the electrochemiluminescence immunoassay method on a Roche cobas e 411 immunoassay analyzer (14). Mean CVs were 2.8% for fasting, 2.9% for 30-min, 3.0% for 1-h, and 3.2% for 2-h C-peptide. To diagnose type 1 diabetes, serum anti-GAD65, -insulin, -ZnT8, and –IA-2 antibodies were measured at the Barbara Davis Center for Diabetes (Aurora, CO) for children reported to have diabetes on treatment (n = 9) and for children with OGTT values indicative of incident diabetes (n = 5). Of these 14 children, 4 had positive antibody results and were excluded from analyses.
Outcomes and Predictors
Dichotomous childhood outcomes were as follows: IGT (2-h glucose 7.8–11.0 mmol/L); IFG by American Diabetes Association (ADA) and International Society for Pediatric and Adolescent Diabetes criteria (IFG-ADA, FPG 5.6–6.9 mmol/L); IFG by WHO criteria (IFG-WHO, FPG 6.1–6.9 mmol/L); and type 2 diabetes (FPG ≥7.0 mmol/L and/or 2-h PG ≥11.1 mmol/L). Continuous outcomes included FPG and 30-min, 1-h, and 2-h PG from the OGTT at individual time points. The sum of individual glucose z scores, an integrated measure that gives equal weight to each of the four glucose values during the OGTT, was also examined. This involved calculating z scores at each OGTT time point by subtracting the mean glucose level from all observed values at that time point, dividing by the SD of the glucose values at that time point, and summing the four individual z scores.
Matsuda, insulinogenic, and disposition indices were examined as continuous outcomes. The Matsuda index estimates insulin sensitivity using glucose and insulin levels from an OGTT (15). A modified Matsuda index was calculated using OGTT glucose and C-peptide levels (15). The insulinogenic index was calculated using C-peptide levels and defined as the ΔC-peptide (0–30 min, nmol/L)/Δglucose (0–30 min, mmol/L) (16). The disposition index was calculated as the product of the Matsuda index and insulinogenic index and then log transformed (17).
The primary predictor for child glucose outcomes was mother’s GDM status during the HAPO pregnancy using IADPSG/WHO criteria where one or more glucose values from a 75-g OGTT equaled or exceeded the following thresholds: FPG 5.1 mmol/L, 1-h PG 10.0 mmol/L, and 2-h PG 8.5 mmol/L.
Exploratory analyses were conducted by removing women from the data set who met Carpenter-Coustan GDM criteria, defined in this study as two abnormal glucose values from a 2-h 75-g OGTT that equaled or exceeded the following thresholds: FPG 5.3 mmol/L, 1-h PG 10.0 mmol/L, and 2-h PG 8.6 mmol/L. A predictor for mothers who only met IADPSG/WHO GDM criteria and not Carpenter-Coustan criteria, versus mothers without GDM, was then evaluated.
Statistical Analyses
Data were summarized using frequencies and counts for categorical variables and means and SDs for continuous variables. Histograms and box plots were examined to assess distributions and identify potential outliers. Multiple logistic regression was used to estimate odds ratios (ORs) and 95% CIs for dichotomous outcomes, and modified least squares regression with Huber-White robust SE was used to estimate risk differences with 95% CIs (18). Multiple linear regression was used for continuous outcomes to estimate adjusted mean differences with 95% CIs. Two-sided P < 0.05 was used for evaluating statistical significance. All statistical analyses were conducted in R (3.4.1) (19).
Multiple models were considered for all outcomes, with variables identified according to study design, known potential confounders, and adjustments used in HAPO analyses (9). Covariate adjustments were as follows: model 1: field center (proxy for race/ancestry since most centers were predominantly one race/ancestry group), child age, sex, and pubertal status (Tanner stage 1, 2/3, or 4/5) with sex by Tanner stage interaction term, maternal variables at pregnancy OGTT (age, height, mean arterial pressure, parity [0 or 1+], smoking [yes/no], drinking alcohol [yes/no], and gestational age), and child’s family history of diabetes in first-degree relatives; model 2: model 1 + maternal BMI at pregnancy OGTT; model 3: model 1 + child’s BMI z score; and model 4: model 1 + maternal BMI at pregnancy OGTT + child’s BMI z score. Child BMI z scores were calculated according to skewness-median-coefficient of variation curves used by the International Obesity Task Force (20). Adjustments for other child adiposity variables were also explored, substituting child’s percent body fat in models 5 and 6 and child’s sum of skinfolds in models 7 and 8 for child’s BMI z score. Although the study was not powered to evaluate Tanner stage–specific associations, an interaction term between GDM status and Tanner stage was evaluated to explore potential effect modification by Tanner stage. Exploratory analyses were also performed within Tanner stage 1, 2/3, and 4/5 groups, using model covariates just listed but removing main effects for Tanner stage and sex × Tanner stage interactions. Multiple imputation was used to account for missing data using a “missing at random” assumption after confirming findings varied little under “missing not at random” (13). Logistic regression model fit was measured using C-statistics and confirmed by Hosmer-Lemeshow goodness-of-fit tests (21). Colinearity of model predictors was evaluated using pairwise correlations.
Results
Participants
Characteristics of participating children (mean age 11.4 years) and their mothers during the HAPO study, overall and by GDM status, are shown in Table 1. At the HAPO OGTT, mothers with GDM were on average older with higher weight, BMI, and mean arterial pressure. Among mothers of children who did and did not participate, mean age and frequency of GDM were 30.0 years and 14.9% and 29.1 years and 16.9%, respectively (weighted summaries) (Supplementary Table 1). Mean maternal BMI, FPG, and 1-h and 2-h PG during the HAPO OGTT and race/ethnicity were similar.
Table 1.
Characteristics of mothers during HAPO pregnancy OGTT and their children at follow-up according to mother’s GDM status
| Overall |
GDM* |
No GDM |
|
|---|---|---|---|
| During HAPO pregnancy |
n = 4,160 |
n = 589 |
n = 3,571 |
| Characteristics (mothers) | Mean (SD) | Mean (SD) | Mean (SD) |
| Age at OGTT (years) | 29.9 (5.7) | 31.8 (5.3) | 29.6 (5.7) |
| Gestational age at OGTT (weeks) | 27.6 (1.7) | 27.9 (1.7) | 27.6 (1.7) |
| Height (cm) | 161.7 (6.8) | 160.9 (7.0) | 161.8 (6.8) |
| Weight (kg) | 71.8 (14.1) | 77.2 (15.9) | 70.9 (13.6) |
| BMI (kg/m2) | 27.4 (4.9) | 29.7 (5.4) | 27.0 (4.7) |
| Mean arterial pressure (mmHg) | 80.2 (7.9) | 83.3 (7.7) | 79.7 (7.8) |
| FPG (mmol/L) | 4.5 (0.4) | 4.9 (0.4) | 4.4 (0.3) |
| 1-h PG (mmol/L) | 7.4 (1.7) | 9.6 (1.6) | 7.0 (1.4) |
| 2-h PG (mmol/L) | 6.1 (1.3) | 7.7 (1.5) | 5.9 (1.1) |
| n (%) | n (%) | n (%) | |
| Race/ethnicity | |||
| White, non-Hispanic | 1,778 (42.7) | 215 (36.5) | 1,563 (43.8) |
| Hispanic | 465 (11.2) | 104 (17.7) | 361 (10.1)) |
| Black, non-Hispanic | 719 (17.3) | 71 (12.1) | 648 (18.1) |
| Asian | 1,125 (27.0) | 184 (31.2) | 941 (26.4) |
| Other | 73 (1.8) | 15 (2.5) | 58 (1.6) |
| Any prenatal smoking | 177 (4.3) | 33 (5.6) | 144 (4.0) |
| Any prenatal alcohol use | 283 (6.8) | 39 (6.6) | 244 (6.8) |
| Parity (any prior delivery ≥20 weeks) | 2,146 (51.6) | 334 (56.7) | 1,812 (50.7) |
| Characteristics (children), at follow-up | Mean (SD) | Mean (SD) | Mean (SD) |
| Age (years) | 11.4 (1.2) | 11.4 (1.3) | 11.3 (1.2) |
| Height (cm) | 148.5 (10.4) | 149.4 (9.9) | 148.4 (10.4) |
| Weight (kg) | 43.2 (13.6) | 45.9 (14.5) | 42.7 (13.4) |
| BMI z score | 0.47 (1.25) | 0.73 (1.31) | 0.43 (1.24) |
| Sum of skinfolds (mm) | 38.8 (21.3) | 43.6 (23.8) | 38.1 (20.8) |
| n (%) | n (%) | n (%) | |
| Sex (female) | 2,036 (48.9) | 272 (46.2) | 1,764 (49.4) |
| Tanner stage (girls) | |||
| 1 | 340 (19.0) | 39 (16.0) | 301 (19.5) |
| 2/3 | 761 (42.6) | 108 (44.4) | 653 (42.3) |
| 4/5 | 686 (38.4) | 96 (39.5) | 590 (38.2) |
| Tanner stage (boys) | |||
| 1 | 523 (35.8) | 66 (31.4) | 457 (36.6) |
| 2/3 | 673 (46.1) | 100 (47.6) | 573 (45.9) |
| 4/5 | 263 (18.0) | 44 (21.0) | 219 (17.5) |
| Family history of diabetes | 1,899 (45.7) | 358 (60.8) | 1,541 (43.2) |
| Outcomes (children) | Mean (SD) | Mean (SD) | Mean (SD) |
| Fasting glucose (mmol/L) | 5.0 (0.4) | 5.1 (0.4) | 5.0 (0.4) |
| 30-min glucose (mmol/L) | 7.8 (1.3) | 8.2 (1.4) | 7.7 (1.3) |
| 1-h glucose (mmol/L) | 6.8 (1.7) | 7.3 (1.8) | 6.8 (1.7) |
| 2-h glucose (mmol/L) | 6.0 (1.2) | 6.2 (1.2) | 5.9 (1.1) |
| Sum of glucose z scores | −0.01 (2.8) | 0.9 (2.9) | −0.2 (2.8) |
| Matsuda index | 1,810.9 (747.3) | 1,620.0 (681.8) | 1,838.4 (753.3) |
| Insulinogenic index | 0.82 (0.69) | 0.78 (0.64) | 0.82 (0.70) |
| Disposition index | 7.02 (0.58) | 6.88 (0.59) | 7.05 (0.57) |
| n (%) | n (%) | n (%) | |
| IFG-ADA | 316 (7.6) | 54 (9.2) | 262 (7.4) |
| IFG-WHO | 23 (0.6) | 3 (0.5) | 20 (0.6) |
| IGT | 238 (5.8) | 61 (10.6) | 177 (5.0) |
| Type 2 diabetes | 10 (0.2) | 2 (0.3) | 8 (0.2) |
Type 2 diabetes (FPG ≥7.0 and/or 2-h PG ≥11.0 mmol/L). Disposition index is reported on a log scale.
*GDM is defined by IADPSG/WHO criteria (one or more glucose values from a 75-g OGTT equals or exceeds FPG 5.1 mmol/L, 1-h 10.0 mmol/L, 2-h 8.5 mmol/L).
Offspring of mothers with and without GDM had similar age and height. Offspring of mothers with GDM were heavier and had a higher sum of skinfolds, more frequent family history of diabetes in first-degree relatives, as well as lower insulin sensitivity (Matsuda index), insulin secretion (insulinogenic index), and β-cell compensation for insulin resistance (disposition index), compared with offspring of mothers without GDM.
IFG-ADA was present in 54 (9.2%) of offspring of mothers with GDM compared with 262 (7.4%) offspring of mothers without GDM, and 61 (10.6%) offspring of mothers with GDM had IGT compared with 177 (5.0%) offspring of mothers without GDM (Table 1). IFG-WHO was present in 3 (0.5%) offspring of mothers with GDM and 20 (0.6%) offspring of mothers without GDM. Type 2 diabetes was present in 2 (0.3%) offspring of mothers with GDM and 8 (0.2%) offspring of mothers without GDM.
Model Diagnostics
Hosmer-Lemeshow P values for logistic regression models ranged 0.59–0.97 for all outcomes, indicating reasonable model fit. C-statistics for logistic regression models ranged 0.70–0.78 and changed very little for each outcome for models 1–4. R2 values ranged 0.071–0.23 for continuous outcomes and changed very little across models 1–4. Colinearity was not a concern with pairwise correlations ranging from 0 to 0.20 for model covariates. Visual inspection of residual plots confirmed linear modeling assumptions, and DFBETA statistics indicated no observations of undue influence.
Association of Maternal GDM Status With Childhood Glucose Levels
Offspring of mothers with GDM had higher 30-min, 1-h, and 2-h PG during their OGTT and sum of glucose z scores compared with offspring of mothers without GDM. GDM was positively associated with child 30-min, 1-h, and 2-h PG and sum of glucose z scores but not child FPG (Table 2). For the above associations, adjusting for maternal BMI at the time of the pregnancy OGTT (model 2), child’s BMI z score (model 3), or both maternal BMI and child’s BMI z score (model 4) had little effect. Results were similar after adjusting for either child percent body fat or sum of skinfolds at follow-up instead of child’s BMI z score (Supplementary Table 2), demonstrating independence of the associations from maternal and child BMI and adiposity.
Table 2.
Association of maternal GDM status during pregnancy with child glucose outcomes
| Continuous child metabolic outcomes | Model 1 |
Model 2 |
Model 3 |
Model 4 |
|---|---|---|---|---|
| β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
|
| P value, R2 | P value, R2 | P value, R2 | P value, R2 | |
| Fasting glucose (mmol/L) | 0.026 (−0.0056 to 0.056) | 0.024 (−0.0072 to 0.055) | 0.021 (−0.0094 to 0.052) | 0.022 (−0.0083 to 0.053) |
| P = 0.11, 0.23 | P = 0.13, 0.23 | P = 0.18, 0.23 | P = 0.16, 0.23 | |
| 30-min glucose (mmol/L) | 0.27 (0.16–0.39) | 0.28 (0.17–0.40) | 0.27 (0.15–0.38) | 0.28 (0.16–0.40) |
| P < 0.0001, 0.12 | P < 0.0001, 0.12 | P < 0.0001, 0.12 | P < 0.0001, 0.12 | |
| 1-h glucose (mmol/L) | 0.34 (0.19–0.49) | 0.35 (0.20–0.50) | 0.33 (0.18–0.48) | 0.35 (0.20–0.50) |
| P < 0.0001, 0.071 | P < 0.0001, 0.072 | P < 0.0001, 0.072 | P < 0.0001, 0.072 | |
| 2-h glucose (mmol/L) | 0.20 (0.10–0.30) | 0.20 (0.10–0.30) | 0.18 (0.08–0.28) | 0.20 (0.10–0.30) |
| P < 0.0001, 0.079 | P < 0.0001, 0.079 | P = 0.0004, 0.095 | P = 0.0001, 0.096 | |
| Sum of glucose z scores | 0.65 (0.41–0.88) | 0.66 (0.42–0.90) | 0.61 (0.37–0.84) | 0.65 (0.41–0.88) |
| P < 0.0001, 0.17 | P < 0.0001, 0.17 | P < 0.0001, 0.17 | P < 0.0001, 0.17 | |
| Matsuda index | −114.5 (−174.5 to −54.5) | −91.6 (−151.6 to −31.6) | −70.9 (−124.4 to −17.5) | −76.3 (−130.3 to −22.4) |
| P = 0.0002, 0.22 | P = 0.0031, 0.23 | P = 0.010, 0.38 | P = 0.0063, 0.38 | |
| Insulinogenic index | −0.034 (−0.10 to 0.029) | −0.053 (−0.12 to 0.010) | −0.052 (−0.11 to 0.010) | −0.060 (−0.120 to 0.0030) |
| P = 0.28, 0.052 | P = 0.10, 0.058 | P = 0.10, 0.080 | P = 0.061, 0.081 | |
| Disposition index | −0.12 (−0.17 to −0.06) | −0.12 (−0.18 to −0.065) | −0.11 (−0.17 to −0.057) | −0.12 (−0.17 to −0.064) |
| P < 0.0001, 0.071 | P < 0.0001, 0.071 | P < 0.0001, 0.073 | P < 0.0001, 0.074 |
Model 1 was adjusted for field center + child age, sex, and pubertal status (Tanner stage 1, 2/3, 4/5, and sex × Tanner stage interaction) + maternal variables at pregnancy OGTT (age, height, mean arterial pressure, parity [0 or 1+], smoking [yes/no], drinking [yes/no], and gestational age), and child’s family history of diabetes in first-degree relatives. Model 2 was model 1 + maternal BMI at pregnancy OGTT. Model 3 was model 1 + child’s BMI z score. Model 4 was model 1 + maternal BMI at pregnancy OGTT + child’s BMI z score.
Association of Maternal GDM Status With Childhood Dichotomous Glucose Outcomes
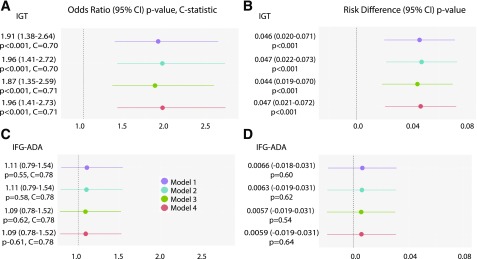
GDM was associated with offspring IGT. After model 1 adjustments, the OR (CI) and risk difference (CI) for IGT in offspring were 1.91 (1.38–2.64) and 0.046 (0.020–0.071), respectively. Adjusting for maternal BMI and/or child’s BMI z score (models 2–4) did not attenuate associations (ORs 1.87–1.96 and risk differences 0.044–0.047) (Fig. 1 and Table 3). In contrast, GDM was not associated with a higher risk for IFG-ADA (ORs 1.09–1.11 and risk differences 0.0057–0.0066). The ORs and risk differences for IGT and IFG-ADA were similar after adjusting for child percent body fat or sum of skinfolds instead of child BMI z score (models 5–8) (Supplementary Table 3). Due to small sample sizes for IFG-WHO and type 2 diabetes, associations of GDM with these outcomes were not analyzed.
Figure 1.
ORs (95% CIs), P values, and C-statistics from logistic regression analyses and risk differences (95% CIs) and P values for the association of maternal GDM during pregnancy with outcomes in HAPO FUS children. A and B: IGT. C and D: IFG-ADA. Results are presented for each outcome for model 1 (purple), model 2 (blue), model 3 (green), and model 4 (orange) covariates.
Table 3.
Association of maternal GDM status during pregnancy with dichotomous child metabolic outcomes
| Dichotomous child glucose outcome | Model 1 |
Model 2 |
Model 3 |
Model 4 |
||||
|---|---|---|---|---|---|---|---|---|
| IFG-ADA | OR 1.11 | Risk difference 0.0066 | OR 1.11 | Risk difference 0.0063 | OR 1.09 | Risk difference 0.005 | OR 1.09 | Risk difference 0.005 |
| 95% CI 0.79–1.54 | 95% CI −0.018 to 0.031 | 95% CI 0.79–1.54 | 95% CI −0.019 to 0.031 | 95% CI 0.78–1.52 | 95% CI −0.019 to 0.031 | 95% CI 0.78–1.52 | 95% CI −0.019 to 0.031 | |
| P = 0.55 | P = 0.60 | P = 0.58 | P = 0.62 | P = 0.62 | P = 0.54 | P = 0.61 | P = 0.64 | |
| C-statistic = 0.78 | C-statistic = 0.78 | C-statistic = 0.78 | C-statistic = 0.78 | |||||
| IGT | OR 1.91 | Risk difference 0.046 | OR 1.96 | Risk difference 0.047 | OR 1.87 | Risk difference 0.044 | OR 1.96 | Risk difference 0.047 |
| 95% CI 1.38–2.64 | 95% CI 0.020–0.071 | 95% CI 1.41–2.72 | 95% CI 0.022–0.073 | 95% CI 1.35–2.59 | 95% CI 0.019–0.070 | 95% CI 1.41–2.73 | 95% CI 0.021–0.072 | |
| P < 0.0001 | P = 0.0004 | P < 0.0001 | P = 0.0003 | P = 0.0001 | P = 0.0007 | P < 0.0001 | P = 0.0004 | |
| C-statistic = 0.70 | C-statistic = 0.70 | C-statistic = 0.71 | C-statistic = 0.71 | |||||
Model 1 was adjusted for field center + child age, sex, and pubertal status (Tanner stage 1, 2/3, 4/5, and sex × Tanner stage interaction) + maternal variables at pregnancy OGTT (age, height, mean arterial pressure, parity [0 or 1+], smoking [yes/no], drinking [yes/no], and gestational age), and child’s family history of diabetes in first-degree relatives. Model 2 was model 1 + maternal BMI at pregnancy OGTT. Model 3 was model 1 + child’s BMI z score. Model 4 was model 1 + maternal BMI at pregnancy OGTT + child’s BMI z score.
Association of Maternal GDM Status With Childhood Insulin Sensitivity and Secretion
GDM was inversely associated with child insulin sensitivity after adjusting for covariates in model 1. This association was attenuated by adjusting for maternal BMI (model 2) and/or child BMI z score (models 3 and 4) but remained significant, demonstrating independence from these two measures. Adjusting for either child percent body fat or sum of skinfolds at follow-up also attenuated the associations, but they remained significant (Supplementary Table 2). GDM was not associated with the insulinogenic index except for a borderline significant inverse association after adjusting for both maternal BMI and child BMI z score (P = 0.06). Results were comparable after adjusting for child percent body fat or sum of skinfolds (Supplementary Table 2). GDM was significantly inversely associated with the disposition index in all models, including after adjusting for child percent body fat and sum of skinfolds (Table 2 and Supplementary Table 2).
Puberty affects insulin sensitivity and glucose metabolism (22). Thus, additional exploratory analyses were conducted separately for Tanner stages 1, 2/3, and 4/5. These analyses suggested that some of the associations described above may be strongest after the onset of puberty (model 4 results in Supplementary Table 4). However, for all childhood outcomes, statistical interaction terms between GDM and Tanner stage were not significant.
Association of Maternal GDM Status According to IADPSG/WHO but Not Carpenter-Coustan Criteria With Childhood Glucose Outcomes
The diagnosis of GDM using Carpenter-Coustan criteria requires two abnormal glucose values: FPG >5.3 mmol/L, 1-h PG >10.0 mmol/L, 2-h PG >8.6 mmol/L, or 3-h PG >7.8 mmol/L. The current study was not powered to assess differences in childhood glucose outcomes for women with Carpenter-Coustan GDM, GDM by IADPSG/WHO criteria only, and no GDM. However, exploratory analyses were conducted by examining childhood dichotomous outcome frequencies and childhood continuous outcome means across the three groups. In addition, fully adjusted model 4 logistic and linear regression results were examined after removing women meeting Carpenter-Coustan GDM criteria to evaluate associations with childhood outcomes for mothers with GDM by IADPSG/WHO criteria only compared with those without GDM. In general, childhood dichotomous outcome frequencies and continuous outcome means for mothers with GDM by IADPSG/WHO criteria only were midway between those for mothers with GDM by Carpenter-Coustan criteria and mothers without GDM, except for FPG and insulinogenic index (Supplementary Table 5). As observed for analyses including mothers with Carpenter-Coustan GDM, GDM according to IADPSG/WHO criteria only was not associated with IFG or FPG in the fully adjusted model 4. However, GDM according to IADPSG/WHO criteria only was associated with IGT, 30-min, 1-h, and 2-h PG, glucose sum of z scores, and disposition index. ORs and adjusted mean differences were attenuated compared with analyses including mothers with Carpenter-Coustan GDM, but results still indicate statistically significant higher risks of adverse metabolic outcomes for children of mothers with GDM according to IADPSG/WHO criteria alone.
Conclusions
This study demonstrated that offspring of mothers with untreated GDM by IADPSG/WHO criteria are at high risk for IGT 10–14 years postpartum. In the U.S., over a time frame similar to HAPO, GDM prevalence using Carpenter-Coustan criteria was 7.4% (23). In contrast, IADPSG/WHO criteria, which require only one abnormal glucose value during a 2-h OGTT, identify a larger group of mothers, e.g., 17.8% of women in the HAPO cohort (24). Thus, offspring of women meeting IADPSG/WHO criteria for GDM represent a sizable group at risk for IGT during childhood, which is important from a public health perspective, given the risk for progression to type 2 diabetes in children with IGT (25). The frequency of offspring type 2 diabetes in the current study was low, which precluded analyses of association of offspring type 2 diabetes with GDM.
Previous studies have examined the association between GDM or pre-existing diabetes in utero and later risk of altered offspring glucose metabolism (5,26–28). Associations of preexisting maternal type 2 diabetes or GDM with offspring glycemic outcomes have been inconsistent, relating, in part, to the size of the studies and failure to adjust for maternal BMI (29). A recent meta-analysis of studies performed prior to the development of IADPSG/WHO criteria examined associations of GDM with glucose outcomes in children from prepuberty through young adulthood (30). Among 5,850 children, GDM was not associated with child FPG, whereas association of GDM with child 2-h glucose was demonstrated in a group of 890 children. The current study also demonstrated no association of GDM with child FPG but did demonstrate that after adjustment for both maternal and child BMI, GDM was associated with not only child 2-h glucose but also with 30-min and 1-h PG.
We have demonstrated associations of GDM according to IADPSG/WHO criteria with offspring obesity and adiposity in the HAPO FUS cohort (13). However, despite the known impact of childhood obesity on glucose metabolism (25), a singular feature of the current report is that association of GDM with childhood IGT was not attenuated by adjusting for the child’s BMI z score or other measures of adiposity or maternal BMI during pregnancy. Compared with mothers without GDM, mothers with GDM more frequently have a family history of type 2 diabetes and are at higher risk for developing type 2 diabetes (31,32). Offspring of mothers with GDM in this study more frequently had a family history of diabetes in first-degree relatives; however, the association of maternal GDM with child IGT was independent of the child’s family history of diabetes. Together, these data demonstrate that GDM is associated with an independent risk for offspring IGT, suggesting that fetal programming may contribute to the observed associations, although additional factors, e.g., shared environmental factors and shared genetics not captured by family history of diabetes, may also contribute. Importantly, this study also demonstrated that the risk of IGT in offspring of mothers with GDM occurred with lesser degrees of hyperglycemia during pregnancy compared with GDM diagnostic thresholds used in earlier studies. Consistent with that, exploratory analyses that removed women with Carpenter-Coustan GDM showed that GDM according to IADPSG/WHO criteria was significantly associated with child IGT and other measures of glucose metabolism compared with mothers without GDM.
Previous studies have demonstrated the association of maternal diabetes with offspring insulin resistance during childhood (25,33,34). In the current study, insulin sensitivity was also lower in offspring of mothers with GDM compared with those without GDM. Adjusting for child BMI or adiposity attenuated the association of GDM with insulin sensitivity, but the association remained significant, suggesting that it was, in part, independent of child BMI and adiposity. Insulin resistance is an early abnormality in children with abnormal glucose metabolism and a major driving force behind dysglycemia in adolescents (35). Progression to type 2 diabetes in children occurs, in part, secondary to a rapid decline in β-cell function (36–38). We found that offspring of mothers with GDM had, on average, a lower insulinogenic index, but the association was borderline significant only after adjusting for both maternal BMI during pregnancy and child’s BMI z score. However, the oral disposition index in offspring of mothers with GDM was consistently lower than in offspring of mothers without GDM. This is consistent with a β-cell defect in these children, manifest as limited β-cell compensation for the underlying insulin resistance. Greater insulin resistance together with lower disposition index is seen in individuals who tend to progress to type 2 diabetes (39,40) and likely contributes to the higher risk for IGT in offspring of mothers with GDM.
Of particular note in this study was the positive association of GDM with the child’s 30 min, 1-h, and 2-h PG but not FPG. Similarly, GDM was associated with a higher risk of IGT but not IFG-ADA. IFG and IGT are considered to be distinct metabolic conditions with differing pathophysiologies (41,42). Isolated IFG is thought to reflect high hepatic insulin resistance with relatively normal insulin sensitivity in skeletal muscle, whereas those with isolated IGT typically exhibit insulin resistance in skeletal muscle (41). Thus, GDM may preferentially impact offspring skeletal muscle as opposed to liver.
Strengths of the study include that HAPO was a blinded observational study in which both caregivers and mothers were unaware of maternal glucose levels; thus, child outcomes were not confounded by treatment of maternal hyperglycemia. Also, HAPO included participants from multiple races and ethnicities from field centers around the world, making the results broadly applicable.
This study had some limitations. First, the proportion of participants who met IADPSG/WHO criteria and participated in the HAPO FUS (weighted estimate 14.9%) is lower than in all eligible participants (16.2%). Second, we were unable to diagnose IGT in all participants due to some missing 2-h PG measurements. Participants with a normal FPG but missing 2-h PG value (n = 54) were defined as having normal glucose metabolism; thus, the number of individuals with IGT may have been underestimated. Third, 1.8% of HAPO participants with OGTT values higher than predefined thresholds were unblinded and excluded from HAPO primary analyses and this study (24). This subgroup would likely have included children at highest risk for IGT. Fourth, the HAPO FUS was not powered to formally examine Tanner stage–specific associations. Fifth, paternal BMI data were not available. Finally, postnatal factors that might influence child glucose outcomes were not available for analyses.
In summary, against a background of limited data and conflicting results, the present large, racially and ethnically diverse study demonstrates that maternal hyperglycemia during pregnancy is associated with a higher risk of IGT in childhood. In addition, this association is evident even when maternal GDM is defined by the less stringent IADPSG/WHO criteria. Importantly, this association persisted after adjustment for maternal BMI and childhood adiposity, which are also associated with GDM in this cohort (13). Given the risk for progression to type 2 diabetes in children with IGT (42), our findings have important public health implications. A review of trials examining the effect of GDM treatment on child metabolic outcomes reported no difference in offspring of treated versus nontreated mothers, but the overall strength of evidence was deemed insufficient (43). With the increasing prevalence of GDM and potential transgenerational impact of in utero exposure to GDM, future, well-powered interventional trials are needed to address the impact of prevention and treatment of GDM diagnosed using IADPSG/WHO criteria on subsequent childhood glucose outcomes.
Supplementary Material
Article Information
Acknowledgments. The HAPO FUS investigators are grateful for all mothers and children who participated in HAPO and HAPO FUS.
Funding. The HAPO FUS is funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1U01-DK-094830). The HAPO study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01-HD-34242 and R01-HD-34243). HAPO FUS data were collected and managed using REDCap electronic data capture tools hosted at the Northwestern University Feinberg School of Medicine (FSM). REDCap is supported at FSM by the Northwestern University Clinical and Translational Science Institute. Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences (UL1-TR-001422).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. W.L.L., L.P.L., and B.E.M. conceived and designed the study, researched the literature, assisted with data collection, interpreted the data, and wrote the manuscript. D.M.S. researched the literature; designed the study; acquired, analyzed, and interpreted the data; and wrote the manuscript and figures. A.K., M.N., and O.T. analyzed the data. B.L. designed the study, interpreted the data, and wrote the manuscript. J.M.L., Y.L., D.M., W.J.B., P.C., R.C.M., and W.H.T. acquired data and participated in writing the manuscript. J.H. researched the literature, acquired and interpreted the data, and wrote the manuscript. A.R.D. designed the study, acquired and interpreted the data, and wrote the manuscript. P.M.C. conceived and designed the study, acquired and interpreted the data, and wrote the manuscript. B.E.M. and D.M.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1646/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
*A complete list of the HAPO Follow-up Study Cooperative Research Group can be found in the Supplementary Data online.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article is part of a special article collection available at http://care.diabetesjournals.org/gdm-new-evidence.
Contributor Information
Collaborators: HAPO Follow-up Study Cooperative Research Group, Chaicharn Deerochanawong, Thadchanan Tanaphonpoonsuk, Sukeeta Binratkaew Uraiwan Chotigeat, Wanee Manyam, Martinette Forde, Andre Greenidge, Kathleen Neblett, Paula Michele Lashley, Desiree Walcott, Katie Corry, Loraine Francis, Jo-anne Irwin, Anne Langan, David R. McCance, Maureen Mousavi, Ian Young, Jennifer Gutierrez, Jennifer Jimenez, Jean M. Lawrence, David A. Sacks, Harpreet S. Takhar, Elizabeth Tanton, Wendy J. Brickman, Jennifer Howard, Jami L. Josefson, Lauren Miller, Jacqui Bjaloncik, Patrick M. Catalano, Ajuah Davis, Michaela Koontz, Larraine Presley, Shoi Smith, Amanda Tyhulski, Albert Martin Li, Ronald C. Ma, Risa Ozaki, Wing Hung Tam, Michelle Wong, Cindy Siu Man Yuen, Peter E. Clayton, Aysha Khan, Avni Vyas, Michael Maresh, Hadasse Benzaquen, Naama Glickman, Alona Hamou, Orna Hermon, Orit Horesh, Yael Keren, Yael Lebenthal, Shlomit Shalitin, Kristina Cordeiro, Jill Hamilton, Hahn Y. Nguyen, Shawna Steele, Fei Chen, Alan R. Dyer, Wenyu Huang, Alan Kuang, Maria Jimenez, Lynn P. Lowe, William L. Lowe, Jr, Boyd E. Metzger, Michael Nodzenski, Anna Reisetter, Denise Scholtens, Octavious Talbot, Paul Yim, David Dunger, Alicia Thomas, Mary Horlick, Barbara Linder, Aynur Unalp-Arida, and Gilman Grave
References
- 1.Viner R, White B, Christie D. Type 2 diabetes in adolescents: a severe phenotype posing major clinical challenges and public health burden. Lancet 2017;389:2252–2260 [DOI] [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017;390:2627–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Lawman HG, et al. . Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA 2016;315:2292–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imperatore G, Boyle JP, Thompson TJ, et al.; SEARCH for Diabetes in Youth Study Group . Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care 2012;35:2515–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol 2007;50:972–979 [DOI] [PubMed] [Google Scholar]
- 6.Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes 1988;37:622–628 [DOI] [PubMed] [Google Scholar]
- 7.Silverman BL, Metzger BE, Cho NH, Loeb CA. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care 1995;18:611–617 [DOI] [PubMed] [Google Scholar]
- 8.Landon MB, Rice MM, Varner MW, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network . Mild gestational diabetes mellitus and long-term child health. Diabetes Care 2015;38:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metzger BE, Lowe LP, Dyer AR, et al.; HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 10.Metzger BE, Gabbe SG, Persson B, et al.; International Association of Diabetes and Pregnancy Study Groups Consensus Panel . International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract 2014;103:341–363 [DOI] [PubMed] [Google Scholar]
- 12.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 1982;144:768–773 [DOI] [PubMed] [Google Scholar]
- 13.Lowe WL Jr., Scholtens DM, Lowe LP, et al.; HAPO Follow-up Study Cooperative Research Group . Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA 2018;320:1005–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manley SE, Stratton IM, Clark PM, Luzio SD. Comparison of 11 human insulin assays: implications for clinical investigation and research. Clin Chem 2007;53:922–932 [DOI] [PubMed] [Google Scholar]
- 15.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 16.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med 1994;11:286–292 [DOI] [PubMed] [Google Scholar]
- 17.Weiss R, Cali AM, Dziura J, Burgert TS, Tamborlane WV, Caprio S. Degree of obesity and glucose allostasis are major effectors of glucose tolerance dynamics in obese youth. Diabetes Care 2007;30:1845–1850 [DOI] [PubMed] [Google Scholar]
- 18.Cheung YB. A modified least-squares regression approach to the estimation of risk difference. Am J Epidemiol 2007;166:1337–1344 [DOI] [PubMed] [Google Scholar]
- 19.RC Team R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing, 2016 [Google Scholar]
- 20.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes 2012;7:284–294 [DOI] [PubMed] [Google Scholar]
- 21.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York, Wiley, 2013 [Google Scholar]
- 22.Kelly LA, Lane CJ, Weigensberg MJ, Toledo-Corral CM, Goran MI. Pubertal changes of insulin sensitivity, acute insulin response, and β-cell function in overweight Latino youth. J Pediatr 2011;158:442–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999-2005. Diabetes Care 2008;31:899–904 [DOI] [PubMed] [Google Scholar]
- 24.Sacks DA, Hadden DR, Maresh M, et al.; HAPO Study Cooperative Research Group . Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care 2012;35:526–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannini C, Dalla Man C, Groop L, et al. . Co-occurrence of risk alleles in or near genes modulating insulin secretion predisposes obese youth to prediabetes. Diabetes Care 2014;37:475–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holder T, Giannini C, Santoro N, et al. . A low disposition index in adolescent offspring of mothers with gestational diabetes: a risk marker for the development of impaired glucose tolerance in youth. Diabetologia 2014;57:2413–2420 [DOI] [PubMed] [Google Scholar]
- 27.Plagemann A, Harder T, Kohlhoff R, Rohde W, Dörner G. Glucose tolerance and insulin secretion in children of mothers with pregestational IDDM or gestational diabetes. Diabetologia 1997;40:1094–1100 [DOI] [PubMed] [Google Scholar]
- 28.Tam WH, Ma RCW, Ozaki R, et al. . In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care 2017;40:679–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donovan LE, Cundy T. Does exposure to hyperglycaemia in utero increase the risk of obesity and diabetes in the offspring? A critical reappraisal. Diabet Med 2015;32:295–304 [DOI] [PubMed] [Google Scholar]
- 30.Kawasaki M, Arata N, Miyazaki C, et al. . Obesity and abnormal glucose tolerance in offspring of diabetic mothers: a systematic review and meta-analysis. PLoS One 2018;13:e0190676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773–1779 [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep 2016;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bush NC, Chandler-Laney PC, Rouse DJ, Granger WM, Oster RA, Gower BA. Higher maternal gestational glucose concentration is associated with lower offspring insulin sensitivity and altered beta-cell function. J Clin Endocrinol Metab 2011;96:E803–E809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lacroix M, Kina E, Hivert MF. Maternal/fetal determinants of insulin resistance in women during pregnancy and in offspring over life. Curr Diab Rep 2013;13:238–244 [DOI] [PubMed] [Google Scholar]
- 35.Chen ME, Chandramouli AG, Considine RV, Hannon TS, Mather KJ. Comparison of β-cell function between overweight/obese adults and adolescents across the spectrum of glycemia. Diabetes Care 2017;41:318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bacha F, Gungor N, Lee S, Arslanian SA. Progressive deterioration of β-cell function in obese youth with type 2 diabetes. Pediatr Diabetes 2013;14:106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bacha F, Lee S, Gungor N, Arslanian SA. From pre-diabetes to type 2 diabetes in obese youth: pathophysiological characteristics along the spectrum of glucose dysregulation. Diabetes Care 2010;33:2225–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burns SF, Bacha F, Lee SJ, Tfayli H, Gungor N, Arslanian SA. Declining β-cell function relative to insulin sensitivity with escalating OGTT 2-h glucose concentrations in the nondiabetic through the diabetic range in overweight youth. Diabetes Care 2011;34:2033–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyssenko V, Almgren P, Anevski D, et al.; Botnia Study Group . Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 2005;54:166–174 [DOI] [PubMed] [Google Scholar]
- 40.Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care 2007;30:1544–1548 [DOI] [PubMed] [Google Scholar]
- 41.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379:2279–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss R, Santoro N, Giannini C, Galderisi A, Umano GR, Caprio S. Prediabetes in youth - mechanisms and biomarkers. Lancet Child Adolesc Health 2017;1:240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med 2013;159:123–129 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.