Abstract
OBJECTIVE
To examine for a legacy effect of early glycemic control on diabetic complications and death.
RESEARCH DESIGN AND METHODS
This cohort study of managed care patients with newly diagnosed type 2 diabetes and 10 years of survival (1997–2013, average follow-up 13.0 years, N = 34,737) examined associations between HbA1c <6.5% (<48 mmol/mol), 6.5% to <7.0% (48 to <53 mmol/mol), 7.0% to <8.0% (53 to <64 mmol/mol), 8.0% to <9.0% (64 to <75 mmol/mol), or ≥9.0% (≥75 mmol/mol) for various periods of early exposure (0–1, 0–2, 0–3, 0–4, 0–5, 0–6, and 0–7 years) and incident future microvascular (end-stage renal disease, advanced eye disease, amputation) and macrovascular (stroke, heart disease/failure, vascular disease) events and death, adjusting for demographics, risk factors, comorbidities, and later HbA1c.
RESULTS
Compared with HbA1c <6.5% (<48 mmol/mol) for the 0-to-1-year early exposure period, HbA1c levels ≥6.5% (≥48 mmol/mol) were associated with increased microvascular and macrovascular events (e.g., HbA1c 6.5% to <7.0% [48 to <53 mmol/mol] microvascular: hazard ratio 1.204 [95% CI 1.063–1.365]), and HbA1c levels ≥7.0% (≥53 mmol/mol) were associated with increased mortality (e.g., HbA1c 7.0% to <8.0% [53 to <64 mmol/mol]: 1.290 [1.104–1.507]). Longer periods of exposure to HbA1c levels ≥8.0% (≥64 mmol/mol) were associated with increasing microvascular event and mortality risk.
CONCLUSIONS
Among patients with newly diagnosed diabetes and 10 years of survival, HbA1c levels ≥6.5% (≥48 mmol/mol) for the 1st year after diagnosis were associated with worse outcomes. Immediate, intensive treatment for newly diagnosed patients may be necessary to avoid irremediable long-term risk for diabetic complications and mortality.
Introduction
In the U.S., an estimated 1.4 million adults are newly diagnosed with diabetes every year and present an important intervention opportunity for health care systems. In patients newly diagnosed with type 2 diabetes, the benefits of maintaining an HbA1c <7.0% (<53 mmol/mol) are well established. The UK Prospective Diabetes Study (UKPDS) found that a mean HbA1c of 7.0% (53 mmol/mol) lowers the risk of diabetes-related end points by 12–32% compared with a mean HbA1c of 7.9% (63 mmol/mol) (1,2). Long-term observational follow-up of this trial revealed that this early glycemic control has durable effects: Reductions in microvascular events persisted, reductions in cardiovascular events and mortality were observed 10 years after the trial ended, and HbA1c values converged (1). Similar findings were observed in the Diabetes Control and Complications Trial (DCCT) in patients with type 1 diabetes (2–4). These posttrial observations have been called legacy effects (also metabolic memory) (5), and they suggest the importance of early glycemic control for the prevention of future complications of diabetes. Although these clinical trial long-term follow-up studies demonstrated legacy effects, whether legacy effects exist in real-world populations, how soon after diabetes diagnosis legacy effects may begin, or for what level of glycemic control legacy effects may exist are not known.
In a previous retrospective cohort study, we found that patients with newly diagnosed diabetes and an initial 10-year HbA1c trajectory that was unstable (i.e., changed substantially over time) had an increased risk for future microvascular events, even after adjusting for HbA1c exposure (6). In the same cohort population, this study evaluates associations between the duration and intensity of glycemic control immediately after diagnosis and the long-term incidence of future diabetic complications and mortality. We hypothesized that a glycemic legacy effect exists in real-world populations, begins as early as the 1st year after diabetes diagnosis, and depends on the level of glycemic exposure.
Research Design and Methods
Source Population
Since 1993, the Kaiser Permanente Northern California (KPNC) Diabetes Registry has maintained a cohort of patients with diabetes (7,8). Patients with diabetes are identified through an algorithm that is based on any of the following: 1) inpatient diagnosis (principal diagnosis of ICD-9 code 250), 2) outpatient diagnosis (two or more diagnoses with ICD-9 code 250, excluding diagnoses collected in the emergency, optometry, or ophthalmology departments), 3) two abnormal outpatient laboratory results (fasting glucose ≥126 mg/dL, random or postchallenge [75-g] glucose ≥200 mg/dL, HbA1c ≥6.5% [≥48 mmol/mol] tested on separate days within a 3-year period), or 4) pharmacy use (prescription for insulin or oral antihyperglycemic medications) (9). This algorithm has been compared internally with chart review, and its sensitivity was 96% for identifying patients with diabetes (8). This study, a substudy of the National Institute of Diabetes and Digestive and Kidney Diseases–funded Diabetes & Aging Study, used KPNC Diabetes Registry data to examine the epidemiology of diabetes across the life course (6,10–13). The study received institutional review board approval from the Kaiser Foundation Research Institute (Oakland, CA) and The University of Chicago (Chicago, IL).
The current study is a follow-up to our previous analysis describing associations between 10-year HbA1c trajectories and risk of diabetic complications in patients with newly diagnosed diabetes (6). We used the same initial cohort, defined as patients diagnosed with type 2 diabetes between 1997 and 2003 who had continuous membership in KPNC for at least 2 years before diagnosis and at least 10 years of survival after diagnosis (Supplementary Fig. 1). It was necessary to include patients who survived for at least 10 years to study the effects of various periods of early HbA1c exposure on outcomes. All patients received standard diabetes care from KPNC while enrolled in the health system. We excluded patients with anemia during the study period because of potential inaccuracies of HbA1c in assessing glycemic exposure in these patients (14–16). We also excluded patients who did not have any HbA1c results during the first 3 months after diagnosis and who did not have a second HbA1c result during the first 2 years after diagnosis because we were unable to classify their levels of early HbA1c exposure. In addition, we excluded patients who had missing HbA1c results for more than one-half of the follow-up years after year 3 because patients with frequent missing HbA1c values may have been receiving care outside of Kaiser and may be missing clinical outcome data.
Design
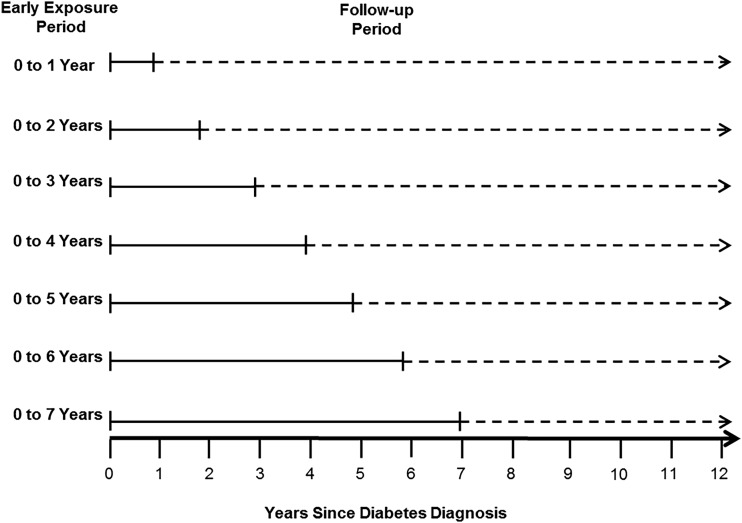
This study used survival analysis methods to examine associations of various periods of early glycemic exposure and various levels of glycemic exposure with the risks for subsequent diabetic complications and death. Figure 1 defines the seven early exposure periods and subsequent follow-up periods. In total, 21 Cox proportional hazards models (seven early exposure periods multiplied by three outcomes of interest) were used to analyze these associations. For each outcome, because the seven models differed in the number of years of early glycemic exposure, differences in hazard ratios (HRs) across models reflect additional hazards as a result of longer durations of early HbA1c exposure compared with an HbA1c <6.5% (<48 mmol/mol) for the same duration of exposure.
Figure 1.
Definitions of early exposure periods and subsequent follow-up periods for the research design.
Exposure
To explore the effects of various periods of early glycemic exposure, we defined a family of seven early exposure periods (0–1, 0–2, 0–3, 0–4, 0–5, 0–6, and 0–7 years), beginning with 3 months after the first measured HbA1c result (Fig. 1). The mean HbA1c value was calculated for each early exposure period by using all HbA1c results except those measured within 3 months after diagnosis. These initial values were excluded because they reflect control before treatment was initiated, and the glycemic legacy effect has been demonstrated only in populations receiving diabetes treatment.
To explore the effects of various levels of glycemic exposure, the mean HbA1c value for each of the seven early exposure periods was categorized into either HbA1c <6.5% (<48 mmol/mol), 6.5% to <7.0% (48 to <53 mmol/mol), 7.0% to <8.0% (53 to <64 mmol/mol), 8.0% to <9.0% (64 to <75 mmol/mol), or ≥9.0% (≥75 mmol/mol) or as missing if no values were available. All HbA1c assays were conducted at KPNC’s centralized laboratory, which is certified by the NGSP (16).
Outcomes
The outcomes of interest were incident future advanced microvascular events, macrovascular events, and death. Advanced microvascular events included end-stage renal disease, diabetic eye disease, and lower-extremity amputation; macrovascular events included cerebrovascular disease, heart disease, heart failure, and vascular disease. Events were ascertained on the basis of a combination of outpatient, emergency department, or inpatient primary diagnostic or procedure codes (Supplementary Table 1). Advanced diabetic eye disease was identified by diagnostic codes for proliferative diabetic retinopathy, diabetic macular edema, or blindness/low vision and procedure codes for destruction of a localized retinal lesion, destruction or treatment of extensive/progressive retinopathy, or photocoagulation. We included only the first occurrence of microvascular and macrovascular events. Mortality data were obtained from the California state mortality file, social security death records, and Kaiser administrative records.
Covariates
We adjusted for potentially confounding variables. Models adjusted for sex, race/ethnicity, age at diagnosis, and year of diagnosis as well as for cardiovascular risk factors (total and HDL cholesterol, BMI, blood pressure, smoking status), using the last observed value for each of the seven early exposure periods. When there were issues with missing data, the last observation was carried backward. Certain cardiovascular risk factors were more likely to be missing than other variables, reflecting systematic differences in how variables were collected in clinical practice during the study period. For example, BMI and smoking data were available in only one-half of patients in years 4 and 6 after diabetes diagnosis, respectively, whereas cholesterol and blood pressure data were available in 85% of patients in years 1 and 3 after diabetes diagnosis.
In addition, models adjusted for the Charlson comorbidity index (17). We calculated the Charlson comorbidity index using several years of data because of concerns about missing diagnostic codes in the administrative database. The Charlson comorbidity index for the 0-to-1- and 0-to-2-year early exposure periods was calculated using 3 years of data, starting with data from 2 years before diagnosis. For the 0-to-3- and 0-to-4-year early exposure periods, the Charlson comorbidity index was calculated using 5 years of data, starting with data from 2 years before diagnosis, and for the 0-to-5-, 0-to-6-, and 0-to-7-year early exposure periods, it was calculated using data from the first 5 years after diabetes diagnosis.
Finally, models adjusted for glycemic exposure after each of the seven early exposure periods. This variable was defined similarly to how glycemic exposure was defined during the early exposure periods. For each patient, the mean HbA1c value was calculated for the time period after each early exposure period and before incidence of the outcome under study for each of the 21 Cox proportional hazards models. These 21 mean values were then categorized as HbA1c <6.5% (<48 mmol/mol), 6.5% to <7.0% (48 to <53 mmol/mol), 7.0% to <8.0% (53 to <64 mmol/mol), 8.0% to <9.0% (64 to <75 mmol/mol), or ≥9.0% (≥75 mmol/mol).
Statistical Analysis
We summarized data for patient characteristics using means and SDs for continuous variables and counts and percentages for categorical variables stratified by the 0-to-1-year early exposure period. We used the χ2 test for bivariate statistics for categorical variables and the Mann-Whitney U test for continuous variables.
In total, 21 Cox proportional hazards models were used to examine associations between glycemic control for the seven successively longer early exposure periods and risk for subsequent microvascular or macrovascular events or death (Fig. 1). The measure of time was the number of years since diabetes diagnosis.
We adjusted for demographics (age at diagnosis, sex, race/ethnicity), diagnosis year, cardiovascular risk factors, HbA1c after the early exposure period, and comorbidity. Because we were interested in incident future events, for each of the seven early exposure periods, we excluded patients who had prevalent (preexisting) microvascular (or macrovascular) complications during the relevant early exposure period for models examining microvascular (or macrovascular) complications, as appropriate. For example, if a patient had a diagnosis of end-stage renal disease in year 2 after diabetes diagnosis, the patient was included in models that examined the association between the 0- and 1-year early glycemic exposure period and incident future microvascular complications. However, this patient would be excluded from models examining associations between the 0- and 2-year early glycemic exposure period and incident future microvascular complications.
For each of the 21 models, patient follow-up was censored after the first occurrence of the outcome of interest, dropout from KPNC (for advanced microvascular and macrovascular events), or end of follow-up (31 December 2013). Overall, 1,732 patients (5.0%) dropped out, and 32,930 (94.8%) were administratively censored at the end of follow-up. For each combination of seven early exposure periods and three outcomes, the time to event or censoring was the number of years from the beginning of the month following the end of the early exposure period to the beginning of the month of the incident outcome.
We conducted sensitivity analyses to assess how results changed with other modeling assumptions. Because the models with various early exposure periods had different follow-up periods, which may affect results, we conducted a sensitivity analysis for the microvascular and macrovascular outcomes in which we right censored patient follow-up at 5 years of follow-up. A two-sided P < 0.05 was considered statistically significant for all analyses. Analyses were completed using SAS 9.4 statistical software (SAS Institute, Cary, NC).
Results
Overall, 34,737 eligible patients were included in the study. Mean follow-up after diagnosis was 13.0 years (SD 1.9 years) (Table 1). The mean age at diagnosis was 56.8 years (SD 11.0 years). During the 1st year after diagnosis (0-to-1-year early glycemic exposure period), 41.1% (n = 14,286) of patients had an average HbA1c <6.5% (<48 mmol/mol), 16.9% (n = 5,877) had an average HbA1c 6.5% to <7.0% (48 to <53 mmol/mol), 13.6% (n = 4,730) had an average HbA1c 7.0% to <8.0% (53 to <64 mmol/mol), 4.1% (n = 1,418) had an average HbA1c 8.0% to <9.0% (64 to <75 mmol/mol), 3.7% (n = 1,290) had an average HbA1c ≥9.0% (≥75 mmol/mol), and 20.5% (n = 7,136) did not have a second HbA1c value. Patients who had a mean HbA1c <6.5% (<48 mmol/mol) in the 0-to-1-year early exposure period were older, more likely to be non-Hispanic white, and less likely to be current smokers and had a lower BMI and total cholesterol than those with higher mean HbA1c levels. In addition, they were less likely to be taking both metformin and sulfonylureas during their 1st year after diagnosis. During the first 7 years after diabetes diagnosis, only 4.9% of patients had the same HbA1c level for each of the 7 years; 50.4% of patients had >1 year with a higher HbA1c level, and 30.4% of patients had >1 year with a lower HbA1c level; 14.3% of patients had years with both higher and lower HbA1c levels. Overall, chronic kidney disease stage 4 or 5 was present in only 53 patients.
Table 1.
Characteristics of patients with newly diagnosed type 2 diabetes and 10 years of survival, stratified by mean HbA1c during the 0-to-1-year early glycemic exposure period
| HbA1c | ||||||||
|---|---|---|---|---|---|---|---|---|
| Overall | <6.5% (<48 mmol/mol) | 6.5% to <7.0% (48 to <53 mmol/mol) | 7% to <8.0% (53 to <64 mmol/mol) | 8.0% to <9.0% (64 to <75 mmol/mol) | ≥9.0% (75 mmol/mol) | Missing | P value | |
| Patients, n (%) | 34,737 (100) | 14,286 (41.1) | 5,877 (16.9) | 4,730 (13.6) | 1,418 (4.1) | 1,290 (3.7) | 7,136 (20.5) | |
| Follow-up time (year), mean (SD) | 13.0 (1.9) | 12.9 (1.9) | 12.6 (1.8) | 12.8 (1.8) | 13.1 (1.8) | 13.3 (1.9) | 13.3 (2.0) | <0.0001 |
| Age at diagnosis (year), mean (SD) | 56.8 (11.0) | 57.9 (10.9) | 57.9 (10.9) | 56.2 (11.0) | 53.4 (10.7) | 52.0 (10.0) | 55.7 (10.7) | <0.0001 |
| Female sex, n (%) | 16,216 (46.7) | 6,329 (44.3) | 3,015 (51.3) | 2,451 (51.8) | 706 (49.8) | 588 (45.6) | 3,127 (43.8) | <0.0001 |
| Race/ethnicity, n (%) | <0.0001 | |||||||
| Non-Hispanic white | 17,625 (50.7) | 7,996 (56.0) | 2,859 (48.7) | 2,177 (46.0) | 591 (41.7) | 506 (39.2) | 3,496 (49.0) | |
| Non-Hispanic black | 3,253 (9.4) | 1,154 (8.1) | 555 (9.4) | 494 (10.4) | 166 (11.7) | 201 (15.6) | 683 (9.6) | |
| Hispanic | 4,531 (13.0) | 1,831 (12.8) | 703 (12.0) | 596 (12.6) | 197 (13.9) | 218 (16.9) | 986 (13.8) | |
| Asian | 6,351 (18.3) | 2,148 (15.0) | 1,231 (21.0) | 1,046 (22.1) | 328 (23.1) | 231 (17.9) | 1,367 (19.2) | |
| Mixed | 2,158 (6.2) | 857 (6.0) | 377 (6.4) | 311 (6.6) | 101 (7.1) | 91 (7.1) | 421 (5.9) | |
| Other/missing | 819 (2.4) | 300 (2.1) | 152 (2.6) | 106 (2.2) | 35 (2.5) | 43 (3.3) | 183 (2.6) | |
| HbA1c (%), mean (SD) | 6.7 (1.2) | 6.0 (0.4) | 6.7 (0.1) | 7.4 (0.3) | 8.4 (0.3) | 10.5 (1.5) | — | <0.0001 |
| BMI (kg/m2), mean (SD) | 32.0 (6.4) | 31.7 (6.2) | 31.8 (6.4) | 32.1 (6.5) | 32.8 (6.6) | 32.8 (6.9) | 32.3 (6.5) | <0.0001 |
| Systolic blood pressure (mmHg), n (%) | <0.0001 | |||||||
| ≤120 | 7,613 (21.9) | 3,257 (22.8) | 1,339 (22.8) | 1,028 (21.7) | 293 (20.7) | 301 (23.3) | 1,395 (19.6) | |
| 121–129 | 5,119 (14.7) | 2,187 (15.3) | 899 (15.3) | 703 (14.9) | 204 (14.4) | 193 (15.0) | 933 (13.1) | |
| 130–139 | 8,847 (25.5) | 3,680 (25.8) | 1,536 (26.1) | 1,262 (26.7) | 370 (26.1) | 281 (21.8) | 1,718 (24.1) | |
| 140–159 | 9,492 (27.3) | 3,721 (26.1) | 1,554 (26.4) | 1,275 (27.0) | 387 (27.3) | 391 (30.3) | 2,164 (30.3) | |
| ≥160 | 3,666 (10.6) | 1,441 (10.1) | 549 (9.3) | 462 (9.8) | 164 (11.6) | 124 (9.6) | 926 (13.0) | |
| Diastolic blood pressure (mmHg), n (%) | <0.0001 | |||||||
| ≤80 | 22,090 (63.6) | 9,468 (66.3) | 3,806 (64.8) | 3,015 (63.7) | 857 (60.4) | 759 (58.8) | 4,185 (58.7) | |
| 81–84 | 4,357 (12.5) | 1,739 (12.2) | 747 (12.7) | 607 (12.8) | 182 (12.8) | 167 (13.0) | 915 (12.8) | |
| 85–89 | 3,452 (9.9) | 1,329 (9.3) | 597 (10.2) | 464 (9.8) | 153 (10.8) | 132 (10.2) | 777 (10.9) | |
| 90–99 | 3,787 (10.9) | 1,364 (9.6) | 576 (9.8) | 506 (10.7) | 178 (12.6) | 187 (14.5) | 976 (13.7) | |
| ≥100 | 1,051 (3.0) | 386 (2.7) | 151 (2.6) | 138 (2.9) | 48 (3.4) | 45 (3.5) | 283 (4.0) | |
| TC (mg/dL), mean (SD) | 205.7 (45.0) | 199.0 (39.5) | 204.8 (42.6) | 208.2 (42.2) | 212.7 (58.9) | 222.6 (62.5) | 213.8 (49.2) | <0.0001 |
| HDL-C (mg/dL), mean (SD) | 45.3 (11.4) | 45.4 (11.6) | 46.3 (11.5) | 45.2 (11.2) | 44.7 (11.1) | 44.0 (11.3) | 44.5 (11.3) | <0.0001 |
| Smoking status, n (%) | <0.0001 | |||||||
| Never | 21,440 (61.7) | 8,844 (61.9) | 3,769 (64.1) | 2,938 (62.1) | 842 (59.4) | 753 (58.4) | 4,294 (60.2) | |
| Current | 2,071 (6.0) | 792 (5.5) | 345 (5.9) | 340 (7.2) | 100 (7.1) | 120 (9.3) | 374 (5.2) | |
| Unknown | 10,973 (31.6) | 4,535 (31.7) | 1,723 (29.3) | 1,415 (29.9) | 464 (32.7) | 409 (31.7) | 2,427 (34.0) | |
| Charlson comorbidity index, mean (SD) | 1.6 (1.0) | 1.6 (1.1) | 1.6 (1.0) | 1.6 (1.0) | 1.6 (1.0) | 1.6 (0.9) | 1.5 (0.9) | <0.0001 |
| Oral diabetes medication, n (%) | <0.0001 | |||||||
| Biguanide only | 5,383 (10) | 2,024 (11) | 947 (13) | 935 (15) | 257 (13) | 215 (11) | 1,005 (5) | |
| Sulfonylurea only | 17,218 (31) | 6,163 (32) | 1,898 (26) | 1,960 (32) | 779 (40) | 812 (42) | 5,606 (31) | |
| Biguanide and sulfonylurea only | 2,363 (4) | 628 (3) | 331 (4) | 471 (8) | 252 (13) | 303 (16) | 378 (2) | |
| None | 29,850 (54) | 10,340 (54) | 4,189 (57) | 2,717 (45) | 667 (34) | 584 (31) | 11,353 (62) | |
| Insulin, n (%) | 1,344 (2) | 512 (3) | 109 (1) | 167 (3) | 68 (3) | 107 (6) | 381 (2) | <0.0001 |
| Statin, n (%) | 12,578 (23) | 5,257 (27) | 2,471 (34) | 1,727 (28) | 464 (24) | 314 (16) | 2,345 (13) | <0.0001 |
| Microvascular events, n (%) | 3,185 (9.2) | 1,070 (7.5) | 475 (8.1) | 459 (9.7) | 182 (12.8) | 263 (20.4) | 736 (10.3) | <0.0001 |
| Macrovascular events, n (%) | 10,864 (31.3) | 4,499 (31.5) | 1,822 (31.0) | 1,473 (31.1) | 450 (31.7) | 452 (35.0) | 2,168 (30.4) | <0.0001 |
| Death, n (%) | 1,807 (5.2) | 744 (5.2) | 268 (4.6) | 224 (4.7) | 68 (4.8) | 66 (5.1) | 437 (6.1) | <0.0001 |
Percentages may not total 100% as a result of rounding. To convert TC and HDL-C to millimoles per liter, multiply by 0.259. HDL-C, HDL cholesterol; TC, total cholesterol.
Microvascular and Macrovascular Events
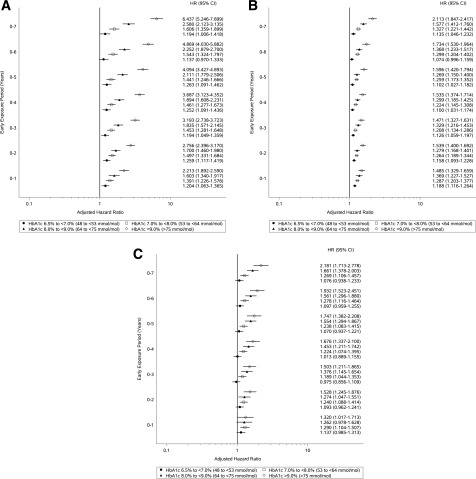
Figure 2A and B depicts HRs comparing microvascular and macrovascular event rates for the various HbA1c early exposure periods and levels, compared with an HbA1c <6.5% (<48 mmol/mol) for the same early exposure periods. With regard to our hypothesis that the legacy effect begins the 1st year after diagnosis, we found that patients with HbA1c levels ≥6.5% (≥48 mmol/mol) for the 0-to-1-year early exposure period had a higher risk for microvascular and macrovascular events than patients with HbA1c levels <6.5% (<48 mmol/mol) for the same period (e.g., HbA1c 6.5% to <7.0% [48 to <53 mmol/mol]: HR 1.204 [95% CI 1.063–1.365]) (Table 2).
Figure 2.
A: Microvascular events (vs. HbA1c <6.5% [<48 mmol/mol]). B: Macrovascular events (vs. HbA1c <6.5% [<48 mmol/mol]). C: Mortality (vs. HbA1c <6.5% [<48 mmol/mol]). HRs adjusted for year of diagnosis, age at diagnosis, sex, race/ethnicity, BMI, systolic and diastolic blood pressure, total cholesterol, HDL cholesterol, smoking status, HbA1c after each early exposure period, and comorbidity.
Table 2.
Associations among various early HbA1c exposure periods and subsequent outcomes
| Microvascular events |
Macrovascular events |
Death |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Early period and mean glycemic control | n/total n | Adjusted HR (95% CI) | P value | n/total n | Adjusted HR (95% CI) | P value | n/total n | Adjusted HR (95% CI) | P value |
| 0–1 year | |||||||||
| HbA1c | |||||||||
| <6.5% (<48 mmol/mol) | 864/14,080 | Reference | 3,668/13,455 | Reference | 744/14,286 | Reference | |||
| 6.5% to <7.0% (48 to <53 mmol/mol) | 372/5,774 | 1.204 (1.063–1.365) | 0.004 | 1,497/5,552 | 1.188 (1.116–1.264) | <0.0001 | 268/5,877 | 1.137 (0.985–1.313) | 0.079 |
| 7.0% to <8.0% (53 to <64 mmol/mol) | 385/4,656 | 1.391 (1.226–1.578) | <0.0001 | 1,244/4,501 | 1.287 (1.203–1.377) | <0.0001 | 224/4,730 | 1.290 (1.104–1.507) | 0.001 |
| 8.0% to <9.0% (64 to <75 mmol/mol) | 154/1,390 | 1.603 (1.340–1.917) | <0.0001 | 383/1,351 | 1.369 (1.227–1.527) | <0.0001 | 68/1,418 | 1.262 (0.978–1.628) | 0.073 |
| ≥9.0% (≥75 mmol/mol) | 232/1,259 | 2.213 (1.892–2.590) | <0.0001 | 382/1,220 | 1.485 (1.329–1.659) | <0.0001 | 66/1,290 | 1.320 (1.017–1.713) | 0.037 |
| Missing | 647/7,047 | 1.354 (1.218–1.505) | <0.0001 | 1,899/6,867 | 1.112 (1.050–1.177) | 0.0003 | 437/7,136 | 1.235 (1.094–1.394) | 0.001 |
| 0–2 years | |||||||||
| HbA1c | |||||||||
| <6.5% (<48 mmol/mol) | 809/14,673 | Reference | 3,612/13,860 | Reference | 817/14,940 | Reference | |||
| 6.5% to <7.0% (48 to <53 mmol/mol) | 451/7,517 | 1.259 (1.117–1.419) | 0.0002 | 1,866/7,143 | 1.158 (1.093–1.228) | <0.0001 | 368/7,675 | 1.093 (0.962–1.241) | 0.172 |
| 7.0% to <8.0% (53 to <64 mmol/mol) | 544/7,144 | 1.497 (1.331–1.684) | <0.0001 | 1,788/6,842 | 1.264 (1.189–1.344) | <0.0001 | 368/7,295 | 1.240 (1.088–1.414) | 0.001 |
| 8.0% to <9.0% (64 to <75 mmol/mol) | 258/2,529 | 1.700 (1.460–1.980) | <0.0001 | 620/2,426 | 1.279 (1.168–1.401) | <0.0001 | 128/2,592 | 1.274 (1.047–1.551) | 0.016 |
| ≥9.0% (≥75 mmol/mol) | 408/2,159 | 2.756 (2.396–3.170) | <0.0001 | 624/2,112 | 1.539 (1.400–1.692) | <0.0001 | 126/2,235 | 1.528 (1.245–1.876) | <0.0001 |
| 0–3 years | |||||||||
| HbA1c | |||||||||
| <6.5% (<48 mmol/mol) | 619/12,475 | Reference | 2,931/11,635 | Reference | 717/12,769 | Reference | |||
| 6.5% to <7.0% (48 to <53 mmol/mol) | 412/7,883 | 1.194 (1.049–1.359) | 0.007 | 1,818/7,347 | 1.126 (1.059–1.197) | 0.0001 | 375/8,057 | 0.975 (0.856–1.109) | 0.696 |
| 7.0% to <8.0% (53 to <64 mmol/mol) | 551/8,310 | 1.453 (1.281–1.648) | <0.0001 | 1,931/7,873 | 1.208 (1.134–1.286) | <0.0001 | 433/8,541 | 1.189 (1.044–1.353) | 0.009 |
| 8.0% to <9.0% (64 to <75 mmol/mol) | 293/3,081 | 1.835 (1.571–2.145) | <0.0001 | 732/2,952 | 1.329 (1.216–1.453) | <0.0001 | 162/3,188 | 1.376 (1.145–1.654) | 0.001 |
| ≥9.0% (≥75 mmol/mol) | 406/2,084 | 3.193 (2.738–3.723) | <0.0001 | 563/2,041 | 1.471 (1.327–1.631) | <0.0001 | 120/2,182 | 1.503 (1.211–1.865) | 0.0002 |
| 0–4 years | |||||||||
| HbA1c | |||||||||
| <6.5% (<48 mmol/mol) | 493/10,627 | Reference | 2,322/9,692 | Reference | 621/10,898 | Reference | |||
| 6.5% to <7.0% (48 to <53 mmol/mol) | 401/8,097 | 1.252 (1.091–1.436) | 0.001 | 1,719/7,410 | 1.100 (1.031–1.174) | 0.004 | 399/8,302 | 1.013 (0.889–1.155) | 0.842 |
| 7.0% to <8.0% (53 to <64 mmol/mol) | 542/9,306 | 1.461 (1.277–1.673) | <0.0001 | 2,019/8,710 | 1.224 (1.145–1.308) | <0.0001 | 482/9,608 | 1.224 (1.074–1.395) | 0.002 |
| 8.0% to <9.0% (64 to <75 mmol/mol) | 312/3,567 | 1.894 (1.608–2.231) | <0.0001 | 770/3,352 | 1.299 (1.185–1.425) | <0.0001 | 187/3,697 | 1.453 (1.211–1.742) | <0.0001 |
| ≥9.0% (≥75 mmol/mol) | 412/2,115 | 3.687 (3.123–4.352) | <0.0001 | 534/2,073 | 1.535 (1.374–1.714) | <0.0001 | 118/2,232 | 1.676 (1.337–2.100) | <0.0001 |
| 0–5 years | |||||||||
| HbA1c | |||||||||
| <6.5% (<48 mmol/mol) | 399/9,209 | Reference | 1,862/8,226 | Reference | 552/9,475 | Reference | |||
| 6.5% to <7.0% (48 to <53 mmol/mol) | 377/8,244 | 1.263 (1.091–1.462) | 0.002 | 1,596/7,389 | 1.102 (1.027–1.182) | 0.007 | 423/8,465 | 1.070 (0.937–1.221) | 0.318 |
| 7.0% to <8.0% (53 to <64 mmol/mol) | 519/10,118 | 1.441 (1.246–1.666) | <0.0001 | 2,038/9,315 | 1.259 (1.173–1.352) | <0.0001 | 518/10,500 | 1.238 (1.083–1.415) | 0.002 |
| 8.0% to <9.0% (64 to <75 mmol/mol) | 331/3,841 | 2.111 (1.779–2.506) | <0.0001 | 727/3,556 | 1.269 (1.150–1.400) | <0.0001 | 200/3,984 | 1.554 (1.294–1.867) | <0.0001 |
| ≥9.0% (≥75 mmol/mol) | 418/2,184 | 4.094 (3.427–4.893) | <0.0001 | 524/2,134 | 1.596 (1.420–1.794) | <0.0001 | 114/2,313 | 1.747 (1.382–2.208) | <0.0001 |
| 0–6 years | |||||||||
| HbA1c | |||||||||
| <6.5% (<48 mmol/mol) | 343/8,192 | Reference | 1,524/7,122 | Reference | 505/8,463 | Reference | |||
| 6.5% to <7.0% (48 to <53 mmol/mol) | 319/8,328 | 1.137 (0.970–1.333) | 0.112 | 1,433/7,362 | 1.074 (0.996–1.159) | 0.064 | 432/8,580 | 1.097 (0.959–1.255) | 0.177 |
| 7.0% to <8.0% (53 to <64 mmol/mol) | 523/10,696 | 1.543 (1.324–1.797) | <0.0001 | 1,967/9,671 | 1.299 (1.204–1.402) | <0.0001 | 547/11,120 | 1.278 (1.116–1.464) | 0.0004 |
| 8.0% to <9.0% (64 to <75 mmol/mol) | 322/4,034 | 2.252 (1.879–2.700) | <0.0001 | 713/3,683 | 1.368 (1.233–1.517) | <0.0001 | 207/4,199 | 1.561 (1.296–1.880) | <0.0001 |
| ≥9.0% (≥75 mmol/mol) | 424/2,233 | 4.869 (4.030–5.882) | <0.0001 | 486/2,158 | 1.734 (1.530–1.964) | <0.0001 | 116/2,375 | 1.932 (1.523–2.451) | <0.0001 |
| 0–7 years | |||||||||
| HbA1c | |||||||||
| <6.5% (<48 mmol/mol) | 272/7,372 | Reference | 1,222/6,245 | Reference | 476/7,660 | Reference | |||
| 6.5% to <7.0% (48 to <53 mmol/mol) | 295/8,371 | 1.194 (1.006–1.418) | 0.043 | 1,320/7,226 | 1.135 (1.046–1.232) | 0.002 | 441/8,654 | 1.076 (0.938–1.233) | 0.295 |
| 7.0% to <8.0% (53 to <64 mmol/mol) | 481/11,178 | 1.606 (1.359–1.899) | <0.0001 | 1,783/9,959 | 1.327 (1.221–1.442) | <0.0001 | 562/11,672 | 1.269 (1.106–1.457) | 0.001 |
| 8.0% to <9.0% (64 to <75 mmol/mol) | 310/4,202 | 2.580 (2.123–3.135) | <0.0001 | 689/3,797 | 1.577 (1.412–1.760) | <0.0001 | 213/4,389 | 1.661 (1.378–2.003) | <0.0001 |
| ≥9.0% (≥75 mmol/mol) | 414/2,201 | 6.437 (5.246–7.899) | <0.0001 | 447/2,107 | 2.113 (1.847–2.417) | <0.0001 | 115/2,362 | 2.181 (1.713–2.778) | <0.0001 |
All models adjusted for year of diagnosis, age at diagnosis, sex, race/ethnicity, BMI, systolic and diastolic blood pressure, total cholesterol, HDL cholesterol, smoking status, HbA1c after each early period, and comorbidity.
With regard to our hypothesis that the legacy effect depends on the level of glycemic exposure, we found that longer periods of early glycemic exposure at HbA1c levels 6.5% to <8.0% (48 to <64 mmol/mol) did not increase the risk of microvascular or macrovascular events during follow-up. However, longer periods of exposure to HbA1c levels ≥8.0% (≥64 mmol/mol) were associated with an increasing risk of microvascular events. HbA1c levels ≥9.0% (≥75 mmol/mol) for early exposure periods >0–4 years were associated with an increasing risk of macrovascular events.
Mortality
Figure 2C presents the risks of mortality for various durations and levels of early glycemic exposure compared with an HbA1c <6.5% (<48 mmol/mol) for the same durations of exposure. Compared with an HbA1c <6.5% (<48 mmol/mol) for the 0-to-1-year early exposure period, HbA1c levels of 7.0% to <8.0% (53 to <64 mmol/mol) and ≥9.0% (≥75 mmol/mol) were associated with a higher risk of mortality (HR 1.290 [95% CI 1.104–1.507] and 1.320 [1.017–1.713], respectively). HbA1c 8.0% to <9.0% (64 to <75 mmol/mol) for the 0-to-2-year early exposure period was associated with a greater risk of mortality (1.240 [1.088–1.414]) compared with an HbA1c <6.5% (<48 mmol/mol) for the same early exposure period. For all early exposure periods, HbA1c 6.5% to <7.0% (48 to <53 mmol/mol) was not associated with a higher risk of mortality during follow-up.
Longer early exposure periods with HbA1c levels <8.0% (<53 mmol/mol) were not associated with increases in mortality risk. However, longer early exposure periods with HbA1c levels ≥8.0% (≥53 mmol/mol) were associated with increasing mortality risk.
Early Missing HbA1c
During the 1st year after diagnosis (0-to-1-year early exposure period), patients without an HbA1c value after the first 3 months after diagnosis had a higher risk of microvascular (HR 1.354 [95% CI 1.218–1.505]) and macrovascular (1.112 [1.050–1.177]) events and mortality (1.235 [1.094–1.394]) (Table 2).
Sensitivity Analyses
When follow-up time was right censored at 5 years, relationships between the duration and intensity of early glycemic exposure and microvascular and macrovascular events did not change (Supplementary Fig. 2).
Conclusions
In this large cohort study of patients with newly diagnosed diabetes and at least 10 years of survival after diagnosis, we found that diabetes control during the 1st year after diagnosis was strongly associated with future risk for diabetic complications and mortality, even after adjusting for glycemic control after the 1st year. Overall, the duration and intensity of early glycemic control were both closely aligned with outcomes. Compared with an HbA1c <6.5% (<48 mmol/mol) for the 1st year after diagnosis, higher HbA1c levels were associated with a higher risk for microvascular and macrovascular events, and HbA1c levels ≥7.0% (≥58 mmol/mol) were associated with a higher risk for mortality. The risk of complications did not increase significantly when the early period was characterized by longer periods of HbA1c levels of 6.5% to <8.0% (48 to <64 mmol/mol) (rather than just the 1st year after diabetes diagnosis). However, longer exposure to HbA1c levels ≥8.0% (≥64 mmol/mol) was associated with an increased risk for microvascular events and mortality.
The current study suggests that failure to achieve an HbA1c <6.5% (<48 mmol/mol) within the 1st year after diabetes diagnosis is sufficient to establish an irremediable long-term future risk of microvascular and macrovascular complications. In addition, failure to achieve an HbA1c <7.0% (<53 mmol/mol) within the 1st year after diabetes diagnosis may lead to an irreversible increased risk of mortality. These findings are supported by previous cohort studies that showed that failure to intensify diabetes treatments in patients with elevated HbA1c values is associated with increased retinopathy and cardiovascular events (18,19).
Longer periods of early exposure to a mean HbA1c <8.0% (<64 mmol/mol) in the current study were not associated with an increased risk of complications; however, lower periods of early exposure to an HbA1c ≥8.0% (≥64 mmol/mol) were associated with an increased future risk of microvascular events and mortality. The interaction between the duration and intensity of glycemic exposure and outcomes aligns with the pathophysiologic understanding of the glycemic legacy effect and patient outcomes from the DCCT and UKPDS. In cellular and animal models, persistent harm associated with historical hyperglycemia has been attributed to mitochondrial superoxide overproduction, leading to increased advanced glycation end products, activation of protein kinase C, fructose production, and increased flux through the hexosamine pathway (20,21). In the DCCT, patients with elevated levels of advanced glycation end products had higher rates of microvascular complications (22). Furthermore, patients randomized to early intensive glycemic control in the UKPDS and DCCT had a legacy of benefits that lasted 10 and 25 additional years, respectively (1,4).
The current results suggest that the patient’s lifetime history of glycemic control may be necessary to understand why patients with longstanding diabetes develop complications despite excellent control later in the course of their disease. The association between the first years of glycemic control and outcomes also should be considered when formulating public health and health care policy regarding the intensity of efforts to control hyperglycemia to improve diabetes outcomes for patients with longstanding diabetes. Young (ages 18–39 years) and middle-aged adults (ages 40–64 years), however, have been found to have substantially higher glycemic levels than older adults (23), which is a missed opportunity; treating patients with diabetes earlier and more intensively has the potential to confer substantial, long-term improvements in public health. Our finding of a relationship between missing data in the 1st year being predictive of future events also suggests the value for aggressive follow-up or educational efforts for patients who do not use offered services. Thus, public health and health care policy should put a greater emphasis on achieving early glycemic control as an avenue for improving lifetime outcomes for patients with diabetes.
This study has several strengths and limitations. Because we studied only patients with newly diagnosed diabetes and at least 10 years of survival, the results are not generalizable to patients with established diabetes or to those with a high risk of mortality after diabetes diagnosis. It is possible that patients with established diabetes may benefit from HbA1c <6.5% (<48 mmol/mol) control, but the benefits may take decades to become evident, and patients may die as a result of nondiabetes-related causes in the meantime. For example, in the Veterans Affairs Diabetes Trial (VADT), which randomized patients with established diabetes (mean 11.5 years) to intensive glycemic control or standard therapy (median HbA1c 6.9% [52 mmol/mol] vs. 8.4% [68 mmol/mol]) for 5.6 years, reductions in major cardiovascular events were detected only after 10 years of total follow-up (24). A long-term follow-up cohort study in a nonclinical trial population will be necessary to explore the effects of early versus later glycemic exposure in patients with established diabetes. In addition, the observed patterns between glycemic exposure and clinical outcomes may differ if we used other thresholds for defining glycemic exposure. The current study questions required a long follow-up, during which important secular changes in diabetes screening, (25) intensity of glycemic control (26), and outcomes (27) may have affected our findings. Although we adjusted for year of diagnosis to address issues with secular trends, residual confounding may still be present.
The study population was a managed care population from northern California, and this population is notable for its location, ethnic diversity, and sociodemographics. However, of note, 90% of patients with diabetes have health insurance (28), and KPNC cares for patients with a full range of insurance. In addition, because patients in KPNC are all offered uniform access to care within an integrated health care delivery system, confounding as a result of differential access to care is less of an issue by using KPNC data. Because this epidemiologic study includes all patients in KPNC with diabetes who survived 10 years after diagnosis, the predictor-outcome relationships that we identified should be widely generalizable, even if the distributions of HbA1c exposure observed in this population may not apply to other settings. Furthermore, previous studies have demonstrated that quality of care at KPNC is comparable to other large health care delivery systems across the U.S. (29).
A major limitation is the inability to comment on causality. It is possible that HbA1c at the time of diagnosis reflects inherent differences in baseline disease severity or patient characteristics that determine long-term outcomes. Furthermore, we relied on historical administrative databases to gather outcome data rather than a clinical adjudication of end points. Although there can be misclassification, we do not anticipate bias as a result of coding of events because that misclassification would unlikely differ systematically by timing, duration, and degree of glycemic exposure. We were unable to study the effect of aspirin, behavioral factors, or cause of death because these are not well documented in medical records, which may have led to an overestimation of the relationships between early HbA1c exposure and outcomes. We also did not analyze the effects of prescription medications because in the relationship between glycemic exposure and diabetic complications and mortality, medications can be time-dependent confounders, and using alternative modeling methods that address time-dependent confounding would preclude isolating the effects of specific early periods. Subsequent studies are necessary to explore how medications may moderate the legacy effect over time.
In conclusion, among patients with 10 years of survival after diabetes diagnosis, we found that HbA1c levels ≥6.5% (≥48 mmol/mol) for the 1st year after diagnosis was associated with a greater risk of future diabetic complications compared with an HbA1c <6.5% for the 1st year after diagnosis. HbA1c levels ≥7.0% (≥53 mmol/mol) for the 1st year after diagnosis were associated with an increased risk of future mortality. Increasing periods of exposure to HbA1c levels ≥8.0% (≥64 mmol/mol) were associated with an increased risk of microvascular events and mortality. This study suggests that the legacy effect exists outside of trial populations, begins as early as the 1st year after diagnosis, and depends on the level of glycemic exposure. These findings underscore the urgency of early diagnosis of diabetes and the future consequences of failing to achieve near-normal glycemia soon after patients are diagnosed with diabetes.
Supplementary Material
Article Information
Funding. N.L. was supported by a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant K23-DK-092783, the American Diabetes Association (1-18-JDF-037), and The University of Chicago Bucksbaum Institute for Clinical Excellence. E.S.H. is supported by NIDDK grant K24-DK-105340. N.L. and E.S.H. are members of the NIDDK Chicago Center for Diabetes Translation Research at The University of Chicago (P30-DK-092949). H.H.M., J.Y.L., and A.J.K. are members of the NIDDK Center for Diabetes Translational Research at Kaiser Permanente (P30-DK-092924). This study also was supported by the NIDDK-funded Diabetes & Aging Study (R01-DK-081796 and R56-AG-051683).
The funders had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of data; or the preparation, review, or approval of the manuscript.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. N.L. designed the study, analyzed and interpreted data, drafted the manuscript, and obtained funding. S.A.H. analyzed and interpreted data and reviewed/edited the manuscript. Y.G. contributed to the study design, analyzed and interpreted data, and reviewed/edited the manuscript. H.H.M. contributed to the study design and data interpretation, provided administrative support, and reviewed/edited the manuscript. J.Y.L. contributed to the data analysis and interpretation and reviewed/edited the manuscript. E.S.H. and A.J.K. contributed to the study design and data interpretation, reviewed/edited the manuscript, and obtained funding. N.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1144/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
See accompanying article, p. 349.
References
- 1.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 2.de Boer IH, Rue TC, Cleary PA, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group . Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med 2011;171:412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care 2016;39:686–69326861924 [Google Scholar]
- 5.Chalmers J, Cooper ME. UKPDS and the legacy effect. N Engl J Med 2008;359:1618–1620 [DOI] [PubMed] [Google Scholar]
- 6.Laiteerapong N, Karter AJ, Moffet HH, et al. . Ten-year hemoglobin A1c trajectories and outcomes in type 2 diabetes mellitus: The Diabetes & Aging Study. J Diabetes Complications 2017;31:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karter AJ, Parker MM, Moffet HH, et al. . Missed appointments and poor glycemic control: an opportunity to identify high-risk diabetic patients. Med Care 2004;42:110–115 [DOI] [PubMed] [Google Scholar]
- 8.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA 2002;287:2519–2527 [DOI] [PubMed] [Google Scholar]
- 9.Karter AJ, Schillinger D, Adams AS, et al. . Elevated rates of diabetes in Pacific Islanders and Asian subgroups: The Diabetes Study of Northern California (DISTANCE). Diabetes Care 2013;36:574–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: The Diabetes and Aging Study. Diabetes Care 2011;34:1329–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus: The Diabetes and Aging Study. JAMA Intern Med 2014;174:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karter AJ, Nundy S, Parker MM, Moffet HH, Huang ES. Incidence of remission in adults with type 2 diabetes: The Diabetes & Aging Study. Diabetes Care 2014;37:3188–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipska KJ, Warton EM, Huang ES, et al. . HbA 1c and risk of severe hypoglycemia in type 2 diabetes: The Diabetes and Aging Study. Diabetes Care 2013;36:3535–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care 2011;34:518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacks DB, John WG. Interpretation of hemoglobin A1c values. JAMA 2014;311:2271–2272 [DOI] [PubMed] [Google Scholar]
- 16.NGSP Factors that interfere with HbA1c test results [Internet], 2017. Available from http://www.ngsp.org/factors.asp. Accessed 2 January 2018
- 17.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–1251 [DOI] [PubMed] [Google Scholar]
- 18.Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osataphan S, Chalermchai T, Ngaosuwan K. Clinical inertia causing new or progression of diabetic retinopathy in type 2 diabetes: a retrospective cohort study. J Diabetes 2017;9:267–274 [DOI] [PubMed] [Google Scholar]
- 20.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–820 [DOI] [PubMed] [Google Scholar]
- 21.Ceriello A, Ihnat MA, Thorpe JE. Clinical review 2: the “metabolic memory”: is more than just tight glucose control necessary to prevent diabetic complications? J Clin Endocrinol Metab 2009;94:410–415 [DOI] [PubMed] [Google Scholar]
- 22.Genuth S, Sun W, Cleary P, et al.; DCCT Skin Collagen Ancillary Study Group . Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications participants with type 1 diabetes. Diabetes 2005;54:3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali MK, McKeever Bullard K, Imperatore G, Barker L, Gregg EW; Centers for Disease Control and Prevention (CDC) . Characteristics associated with poor glycemic control among adults with self-reported diagnosed diabetes--National Health and Nutrition Examination Survey, United States, 2007-2010. MMWR Suppl 2012;61(Suppl.):32–37 [PubMed] [Google Scholar]
- 24.Hayward RA, Reaven PD, Wiitala WL, et al.; VADT Investigators . Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–2206 [DOI] [PubMed] [Google Scholar]
- 25.Olson DE, Rhee MK, Herrick K, Ziemer DC, Twombly JG, Phillips LS. Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes Care 2010;33:2184–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumenthal KJ, Larkin ME, Winning G, Nathan DM, Grant RW. Changes in glycemic control from 1996 to 2006 among adults with type 2 diabetes: a longitudinal cohort study. BMC Health Serv Res 2010;10:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregg EW, Li Y, Wang J, et al. . Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 2014;370:1514–1523 [DOI] [PubMed] [Google Scholar]
- 28.Harris MI, Cowie CC, Eastman R. Health-insurance coverage for adults with diabetes in the U.S. population. Diabetes Care 1994;17:585–591 [DOI] [PubMed] [Google Scholar]
- 29.McCarthy D, Mueller K, Wrenn J. Kaiser Permanente: Bridging the Quality Divide with Integrated Practice, Group Accountability, and Health Information Technology. New York, NY, The Commonwealth Fund, 2009 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.