Abstract
Visceral obesity is associated with insulin resistance and higher risk of type 2 diabetes and metabolic diseases. A limited ability of adipose tissues to remodel through the recruitment and differentiation of adipose stem cells (ASCs) is associated with adipose tissue inflammation and fibrosis and the metabolic syndrome. We show that the lower adipogenesis of omental (Om) compared with abdominal subcutaneous (Abdsc) ASCs was associated with greater secretion of TGFβ ligands that acted in an autocrine/paracrine loop to activate SMAD2 and suppress adipogenesis. Inhibition of TGFβ signaling rescued Om ASC differentiation. In Abdsc ASCs, low concentrations of dexamethasone suppressed TGFβ signaling and enhanced adipogenesis, at least in part by increasing TGFBR3 protein that can sequester TGFβ ligands. Om ASCs were resistant to these dexamethasone effects; recombinant TGFBR3 increased their differentiation. Pericellular fibrosis, a hallmark of dysfunctional adipose tissue, was greater in Om and correlated with higher level of tissue TGFβ signaling activity and lower ASC differentiation. We conclude that glucocorticoids restrain cell-autonomous TGFβ signaling in ASCs to facilitate adipogenesis and healthy remodeling in Abdsc and these processes are impaired in Om. Therapies directed at overcoming glucocorticoid resistance in visceral adipose tissue may improve remodeling and help prevent metabolic complications of visceral obesity.
Introduction
Fat distribution and, as a consequence, body shape vary widely among individuals. Humans store only ∼5–20% of their body fat in intraabdominal visceral adipose depots, but they have a disproportionate influence on health (1). Visceral adipose tissues are highly susceptible to chronic inflammation and pericellular fibrosis, which are associated with tissue and systemic metabolic dysfunction in obesity (2–4). To maintain optimal function, adipose tissues must emodel. The inability to recruit and differentiate adipose stem cells (ASCs) leads to excess adipocyte hypertrophy and dysfunction and macrophage infiltration that further drives tissue inflammation (5). Mechanisms that limit this homeostatic remodeling in the omental (Om) depot, one of the two major visceral depots, remain unclear.
Primary cultures of human visceral Om ASCs differentiate less well than those from the abdominal subcutaneous (Abdsc) depots (6,7). A lower level of adipogenesis is maintained in cloned Om ASCs, suggesting cell-autonomous differences (7). In the tissue context, local factors including inflammatory cytokines and members of the tumor growth factor β (TGFβ) superfamily inhibit adipogenesis (8–10). Hormonal factors such as glucocorticoids also regulate adipogenesis (11,12). We and others have observed depot differences in glucocorticoid actions between human Om and Abdsc adipose tissues (13–15). Therefore, we tested whether differential sensitivity to a glucocorticoid receptor agonist, dexamethasone (Dex), contributes to depot differences in adipogenesis.
Most of the existing literature focuses on the increased number of macrophages in Om adipose tissue (16,17) as the source of antiadipogenic and profibrotic factors (18,19). Macrophage-conditioned media (CM) (20) or coculture with activated or adipose tissue macrophages (21) inhibits differentiation in Abdsc ASCs, but these treatments do not further suppress the already low level of adipogenesis of Om ASCs (21). ASCs also secrete antiadipogenic factors including proinflammatory cytokines (8,10,22) and TGFβ ligands, activin A (encoded by INHBA) and TGFβ1 (9,22,23). The potential roles of depot-dependent intrinsic properties of ASCs as autocrine/paracrine regulators of human adipose expansion and remodeling have received far less attention. In addition to inhibiting adipogenesis, TGFβ ligands induce myofibroblast-like phenotypes in ASCs with high production of collagens and matricellular proteins (19,24) and, hence, may drive adipose tissue fibrosis. We therefore assessed the importance of TGFβ signaling in depot differences in adipogenesis and the relationship of TGFβ signaling activity at the tissue level with pericellular fibrosis and differentiation capacity of ASCs derived from the same donors. Our previous study noted that TGFβ receptor 3 (TGFBR3) mRNA levels were higher and more robustly induced by low concentrations of Dex in Abdsc than Om adipose tissue (15). The ectodomain of TGFBR3 can be shed, releasing soluble TGFBR3 into the extracellular space where it sequesters TGFβ ligands, inhibiting their activity (25). Dex dampens TGFβ signaling via SMAD2/3 through induction of TGFBR3 in lung fibroblasts (26). Thus, we explored potential depot differences in the effects of Dex on TGFBR3 and their importance in the regulation of adipogenesis.
We demonstrated that Om ASCs produced higher levels of TGFβ ligands that acted in an autocrine/paracrine loop to inhibit differentiation and may promote tissue fibrosis. Om ASCs were less sensitive to the effects of Dex to suppress TGFβ signaling and enhance adipogenesis. Further, we uncovered potential mechanisms through which glucocorticoids regulate TGFβ signaling and adipogenesis depot dependently.
Research Design and Methods
Tissue Collection and ASC Culture
Institutional review boards at the Boston Medical Center and the Mount Sinai School of Medicine approved all protocols, and all subjects signed informed consent. Exclusion criteria were systemic inflammatory conditions, current cancer, and active smoking. Adipose tissues were sampled during elective surgeries from 51 donors; 4 had diabetes (HbA1c >6.5 [48 mmol/mol]), and inclusion of their data did not affect results. Subject characteristics from medical records are provided in Supplementary Table 1. Adipose tissues were processed as previously described (15); aliquots were frozen in liquid nitrogen immediately after excision, fixed in Z-fix solution (27), or transferred to the laboratory. ASCs were isolated with collagenase digestion, cultured, and differentiated as previously described (28). Late markers of adipogenesis or triacylglycerol (TAG) content were used to assess the degree of differentiation.
Dex Modulation of TGFβ Signaling, TGFBR3 Expression, and Adipogenesis
Paired Om and Abdsc ASCs at confluence were treated with varying concentrations of Dex overnight, and SMAD2 phosphorylation (ratio of phosphorylated to total SMAD2 [SMAD2P]) and TGFBR3 protein were measured. Dex regulation of adipogenesis was assessed by differentiating paired Om and Abdsc ASCs with varying concentrations of Dex.
CM Preparation and Testing
CM was collected from confluent pairs of Om and Abdsc ASCs that were cultured with DMEM/F12 + 1% FBS with or without Dex (10 nmol/L) for 20 h. Effects of paired Om and Abdsc CM from different donors were tested on well-differentiating Abdsc ASCs from another donor. The differentiation media were prepared to contain CM (25% v/v) and standard concentrations of all other components. For assessment of whether Om CM affected differentiation through activation of TGFβ signaling, Abdsc ASCs were differentiated with or without CM (25% v/v) and/or the TGFBR1 kinase inhibitor SB431542 (SB) (5 μmol/L). The acute effects of CM on SMAD2P were tested by treating Abdsc ASCs with CM (or control media) after a 15-min preincubation with or without SB.
Adipose tissue CM samples were obtained by culturing intact fragments of paired Om and Abdsc adipose tissues from eight donors. Immediately after excision and transport to the laboratory, adipose tissues were minced into 5- to 10-mg fragments and placed in organ cultures for 24 h (15). CM was collected and added during differentiation of ASCs (50% v/v).
Secreted Factors and Adipogenesis
IL-6 and activin A levels in CM were measured with ELISA (R&D Systems, Minneapolis, MN) or quantitative immunoblotting, respectively. Total and active TGFβ levels were determined using a bioassay (29). Abdsc ASCs were differentiated with addition of recombinant human (rh) IL-6, rhTGFβ1, or rhTNFα (PeproTech, Rocky Hill, NJ) during the 7-day induction period followed by maintenance under the standard condition (28). The effects of BMP4 on Om ASC adipogenesis were tested with addition of BMP4, added during days −2 to 2 of adipogenesis (30). The effects of TGFBR3 on TGFβ signaling activity were assessed by incubating confluent Om ASCs with rhTGFBR3 overnight (100 nmol/L) (R&D Systems) and measuring SMAD2P. For testing of the effects of TGFBR3 on adipogenesis, rhTGFBR3 (100 nmol/L) was added during differentiation of Om ASCs.
siRNA-Mediated Knockdown
ASCs were transfected with 40 nmol/L siRNA using HiPerFect (Qiagen, Germantown, MD) (31). Knockdown (KD) levels were confirmed at confluence, and their effects on TGFβ signaling activity or differentiation were assessed. With a high plating density (15,000 cells/cm2), cells reached confluence 2–3 days after transfection and no differences in growth rates were observed.
Isolation of Adipose Progenitors
Freshly isolated stromal vascular cells prepared from paired samples of Om and Abdsc adipose tissues were used for flow cytometry (27). Antibodies were purchased from eBioscience (FITC CD45, FITC CD14, FITC CD31, and Cy5 CD34) and BioLegend (phycoerythrin CD29, and brilliant blue CD90). Total stromal and sorted cells were used for RNA extraction and quantitative PCR measurement of cell markers or plated for testing adipogenesis.
Gene and Protein Expression
RNA was isolated using Trizol and quantitative RT-PCR was performed, and relative expression levels are presented (15). For Western blotting, lysates from adipose tissues or cells were prepared as previously described (12,27). Proteins were resolved in NuPAGE 10% Bis-Tris gels and transferred to polyvinylidene fluoride membranes (Thermo Fisher Scientific), followed by incubation with antibodies: activin A (Abcam, Cambridge, MA), SMA (Millipore, Burlington, MA), ADPN (R&D Systems), ATGL, PPARγ, FABP4, total SMAD2/3, phosphorylated SMAD2 (S465/467), SMAD4, TGFBR3 (Cell Signaling Technology), and HSP90 (Santa Cruz Biotech, Santa Cruz, CA). Chemiluminescence signals were detected and quantified.
Tissue Histology, Staining, and Measurements of Cell Size and Fibrosis
Fixed adipose tissue was embedded in paraffin, sliced into 5-µm-thick sections, and used for hematoxylin-eosin (27), picrosirius red (4), or Masson’s trichrome staining (Thermo Fisher Scientific). Digital images were acquired with a Nikon TE 200 microscope equipped with an Olympus DP72 camera and used for calculation of average adipocyte weight (μg lipid/cell) (27). An Olympus IX70 inverted microscope equipped with an Optronics camera was used for polarized light microscopy. Pericellular fibrosis was measured with ImageJ software in at least 10 random fields and presented as the percentage of red-stained area to total surface area (4).
Statistics
Data are presented as means ± SEM for the number of independent studies indicated in figure legends. One- or two-way repeated-measures (RM)-MANOVA was used. When main effects or interaction of Dex and depot were significant, post hoc comparisons were made by Dunnett or t tests as indicated in figure legends (GraphPad Prism or SAS JMP Pro 13). P values ≤0.05 were considered statistically significant.
Results
Lower Adipogenesis of Om ASCs Inversely Correlated With SMAD2P
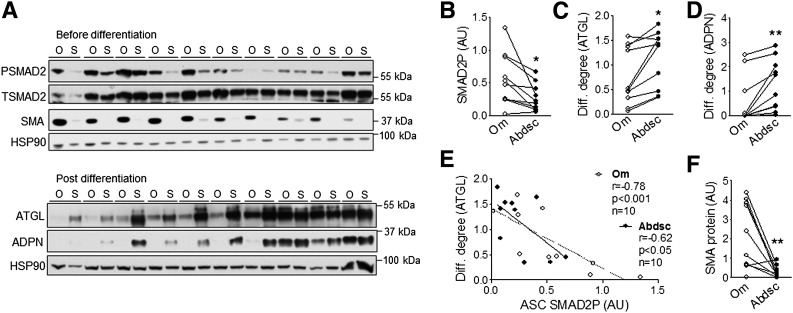
For assessment of the importance of autocrine/paracrine TGFβ signaling activity in the lower level of adipogenesis observed in Om ASCs (6,7), SMAD2P and differentiation degree were measured in paired Om and Abdsc ASCs derived from 10 different donors. Basal SMAD2P, measured prior to differentiation, was 2.4-fold higher in Om than Abdsc ASCs (Fig. 1A and B). Om ASCs did not differentiate as well as Abdsc ASCs, as indicated by the lower levels of adipose triglyceride lipase (ATGL) (Fig. 1A and C) and adiponectin (ADPN) (Fig. 1A and D). SMAD2P negatively correlated with expression of differentiation markers (Fig. 1E).
Figure 1.
Higher basal SMAD2P in Om ASCs negatively correlated with adipogenesis. A: Phosphorylated (PSMAD2) and total (TSMAD2) SMAD2, SMA, and HSP90 (a loading control) levels were measured in paired Om (O) and Abdsc (S) ASCs derived from 10 different donors at confluence before differentiation (top panels). ASCs derived from the same donors were differentiated, and markers of adipocytes, ATGL and ADPN, and HSP90 protein levels were measured on day 14 (bottom panels). B: Quantification of SMAD2P and differentiation (Diff.) degree as determined by ATGL (C) and ADPN (D) protein levels relative to HSP90. E: Negative correlations between basal SMAD2P and differentiation degree (ATGL). F: Quantification of SMA protein relative to HSP90 before differentiation. *P < 0.05, **P < 0.01 (paired t tests); n = 10. AU, arbitrary units.
Higher level of TGFβ signaling activity may induce a myofibroblast-like phenotype in Om ASCs (19,24). Consistent with this idea, α-smooth muscle actin (SMA) protein levels were 7.6-fold higher in Om than Abdsc ASCs (Fig. 1A and F). Further, SMA levels correlated positively with SMAD2P (r = 0.76, P = 0.02, n = 9) and negatively with differentiation (r = −0.88, P < 0.001, n = 10) in Om ASCs.
Higher Secretion of Antiadipogenic TGFβ Ligands From Om ASCs
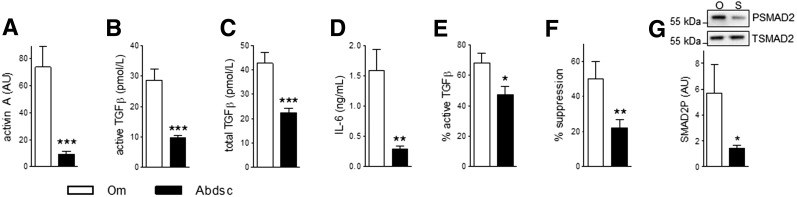
The observation that Om ASCs exhibited elevated SMAD2P led us to test the hypothesis that these cells generated more TGFβ ligands. Levels of activin A (8.2-fold), active TGFβ (3.1-fold), total TGFβ (3.1-fold), and IL-6 (8.9-fold) were higher in Om than Abdsc ASC CM (Fig. 2A–D). The ratio of active to total TGFβ was 1.8-fold higher in Om (Fig. 2E). Addition of Om versus Abdsc CM more potently inhibited differentiation (2.3-fold) (Fig. 2F). The percent suppression of adipogenesis by Om CM positively correlated with levels of activin A, active TGFβ, and IL-6 (Supplementary Fig. 1A–C). Levels of active TGFβ, activin A, and IL-6 in CM highly correlated with each other (Supplementary Fig. 1D–F). Although IL-6 levels negatively correlated with adipogenesis, addition of IL-6 (20 ng/mL) had no effect and a higher concentration (100 ng/mL) improved differentiation by ∼50% (Supplementary Fig. 2). As expected (8,32), both TGFβ1 (10 ng/mL) and TNFα (3 ng/mL) inhibited adipogenesis (Supplementary Fig. 2).
Figure 2.
Higher levels of activin A, TGFβ, and IL-6 in Om ASC CM. CM samples were obtained by culturing 14 pairs of Om and Abdsc ASCs and levels of activin A (A), active TGFβ (B), total TGFβ (C), IL-6 (D), and calculated percentage of active to total TGFβ (E) were determined as described in the Research Design and Methods. F: Abdsc ASCs were differentiated with addition of CM (25% v/v), and adipogenesis was measured on day 14 (TAG relative to DNA quantity). Data are presented as % suppression compared with cells differentiated with addition of control media. G: Abdsc ASCs were treated with CM from Om (O) or Abdsc (S) ASCs for 15 min and used for assessment of SMAD2P. Representative Western images and quantification of SMAD2P are presented. *P < 0.05, **P < 0.01, ***P < 0.001, paired t tests; n = 14 (A–F) or n = 5 (G). AU, arbitrary units.
The TGFβ family member BMP4 has been reported to overcome the low rate of adipose progenitors (APs) in mouse “visceral” epididymal adipose tissue (30), so we tested its effect in Om ASCs. Addition of BMP4 (3 nmol/L) during days −2 to +2 of adipogenesis (30) modestly improved adipogenesis in Om ASCs (31 ± 8% [P < 0.05, n = 6]).
Inhibition of TGFβ Signaling Enhanced Adipogenesis of Om ASCs to Levels Similar to Those of Abdsc ASCs
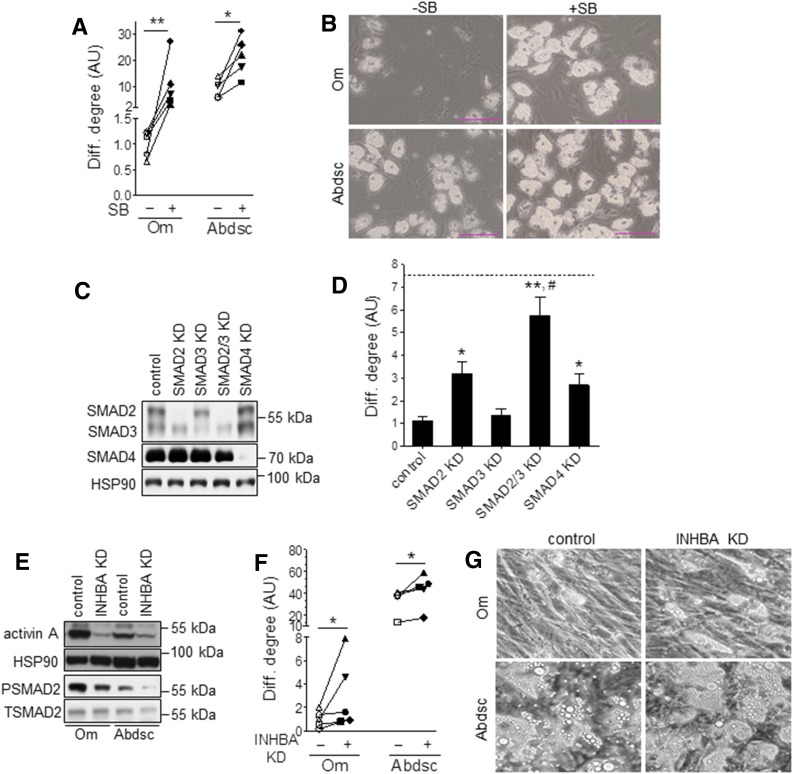
CM from Om compared with Abdsc ASCs more markedly stimulated SMAD2P (fourfold) (Fig. 2G). The TGFBR1 (ALK5) kinase inhibitor SB (5 μmol/L) completely blocked the Om ASC CM-mediated stimulation of SMAD2P as well as suppression of differentiation (Supplementary Fig. 3A and B), indicating that TGFβ ligands were the main antiadipogenic factors secreted by Om ASCs. Furthermore, blocking autocrine/paracrine TGFβ signaling with SB enhanced the differentiation of Om ASCs (10.5-fold) to levels similar to those of the control Abdsc ASCs (Fig. 3A and B). SB also enhanced Abdsc ASC differentiation (2.4-fold). These results suggest that TGFβ signaling tonically inhibited adipogenesis in a cell-autonomous manner in both depots.
Figure 3.
Blockade of TGFβ signaling pathway markedly improved adipogenesis in Om ASCs. A: Paired Om and Abdsc ASCs were differentiated in the absence (−) or presence (+) of SB (5 μmol/L), and differentiation (Diff.) degree (ATGL protein) was measured on day 14. Each symbol represents ASCs derived from a different donor. Scale bars, 100 μm. B: Representative images of differentiated cells. Effects of depot and SB on adipogenesis were determined by two-way ANOVA followed by paired t tests (*P < 0.05, **P < 0.01; n = 5). C: Om ASCs were transfected with control, SMAD2, SMAD3, both SMAD2 and 3 (SMAD2/3), or SMAD4 siRNA, and KD levels were confirmed at confluence with immunoblotting. D: Cells were differentiated and PPARγ mRNA levels were measured as an adipocyte marker; dotted line represents the average value for paired Abdsc ASCs, differentiated in the control condition. Data were first analyzed by RM-ANOVA followed by Dunnett test to compare experimental values with the control (*P < 0.05 and **P < 0.01) and a paired t test to compare SMAD2/3 KD with SMAD2 KD alone (#P < 0.05); n = 6. E: Paired Om and Abdsc ASCs were transfected with control or activin A (INHBA) siRNA, and expression levels of activin A, phosphorylated SMAD2 (PSMAD) and total SMAD2 (TSMAD2), and HSP90 were measured at confluence. F: Differentiation degree was evaluated by measuring ATGL protein levels on day 14. Each symbol represents ASCs derived from a different subject. G: Representative images of differentiated cells are presented. Depot and siRNA effects on adipogenesis were determined by two-way ANOVA followed by paired t tests (*P < 0.05; n = 5).
KD of SMAD2 (>90%) (Fig. 3C) increased adipogenesis by 3.2-fold, while silencing of SMAD3 alone (∼60%) had no effect (Fig. 3D). SMAD4 KD (>90%) also improved differentiation (2.7-fold). The combined KD of SMAD2 and SMAD3 greatly increased adipogenesis compared with the control (5.7-fold) and SMAD2 KD alone (1.8-fold). Moreover, silencing of both SMAD2 and -3 enhanced differentiation of Om ASCs to levels similar to those of Abdsc ASCs. Activin A KD (>95%) decreased SMAD2P and improved adipogenesis in both Om and Abdsc ASCs (Fig. 3E–G). However, although KD of activin A improved the degree of differentiation of Om ASCs, it remained lower than the control Abdsc ASCs.
Dex Attenuated Expression of Antiadipogenic TGFβ Ligands
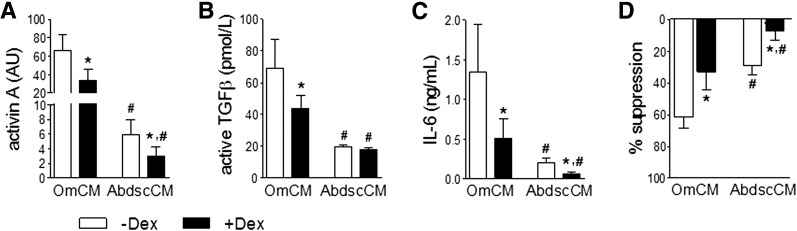
Because glucocorticoids regulate the mRNA expression of multiple members of the TGFβ superfamily and inflammatory factors (9,13,15), we tested whether Dex affected their protein levels. Dex decreased the amount of activin A, active TGFβ, and IL-6 in Om CM (Fig. 4A–C). In accordance, Om CM collected with Dex was twofold less effective in suppressing adipogenesis (Fig. 4D). Dex also decreased the lower levels of activin A and IL-6 in Abdsc CM, and CM collected in the presence of Dex was fourfold less antiadipogenic.
Figure 4.
Dex decreased levels of antiadipogenic TGFβ ligands in ASC CM. CM was collected from five pairs of Om and Abdsc ASCs in the absence (−) or presence (+) of Dex, and the amount of activin A (A), active TGFβ (B), and IL-6 (C) was measured with quantitative immunoblotting, bioassay, and ELISA, respectively. D: Abdsc ASCs were differentiated with or without addition of CM (25% v/v). Differentiation degree (TAG relative to DNA quantity) was determined on day 14 and presented as % suppression compared with the control, differentiated with addition of the control media. *Dex effects and #depot differences were determined by two-way ANOVA followed by post hoc paired t tests (P < 0.05; n = 5).
Om ASCs Were Resistant to Dex Suppression of SMAD2P
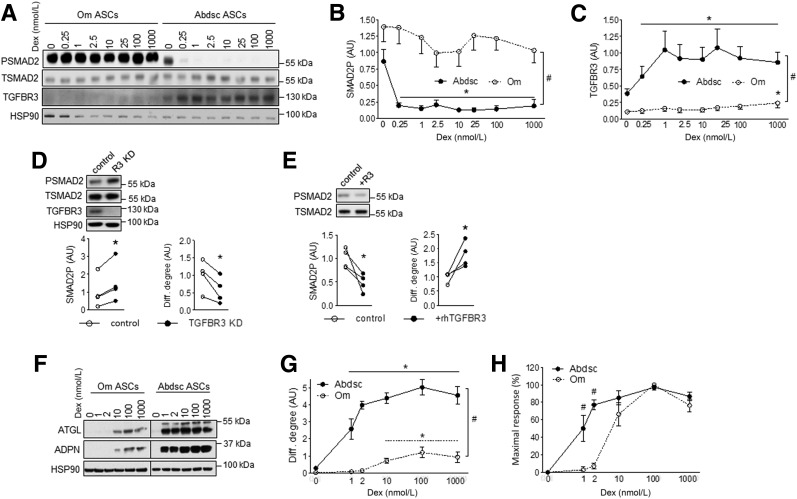
We previously showed that Om adipose tissue was less sensitive to Dex-mediated regulation of multiple pathways and transcripts (15). Thus, we hypothesized that Om versus Abdsc ASCs may also be less sensitive to Dex. In Abdsc ASCs, very low concentrations of Dex (0.25–1 nmol/L) markedly suppressed SMAD2P, while even very high concentrations of Dex (100–1,000 nmol/L) were ineffective in Om ASCs (Fig. 5A and B), indicating that Om ASCs were resistant to the Dex-mediated suppression of TGFβ signaling.
Figure 5.
Resistance to Dex-mediated induction of TGFBR3 contributed to higher level of TGFβ signaling activity and lower level of adipogenesis in Om ASCs. Confluent Om and Abdsc ASCs were incubated with Dex (0, 0.25, 1, 2.5, 10, 25, 100, and 1,000 nmol/L) overnight, and phosphorylated (PSMAD2) and total (PSMAD2) SMAD2, TGFBR3, and HSP90 protein levels were measured: representative Western blot (A), densitometry of SMAD2P (B), and relative expression levels of TGFBR3 to HSP90 (C) in samples from six independent donors are presented. Depot differences in dose response curves were significant by two-way ANOVA (depot × Dex interaction, P < 0.05; n = 6). #Depot differences were significant at all Dex concentrations (P < 0.05 [paired t tests]). *Dex effects, P < 0.05 (Dunnett test). D: Abdsc ASCs were transfected with control or TGFBR3 siRNA, and phosphorylated and total SMAD2, TGFBR3 and HSP90 protein levels were measured at confluence (left). Cells were differentiated and adipogenesis (ATGL protein) was determined on day 14 (right). *P < 0.05 (paired t tests), n = 4. E: Confluent Om ASCs were treated with rhTGFBR3 (100 nmol/L) overnight, and phosphorylated and total SMAD2 levels were measured (left). Om ASCs were differentiated with or without rhTGFBR3 (100 nmol/L), and differentiation (Diff.) degree (ATGL protein) was determined on day 14 (right). *P < 0.05 (paired t test), n = 4. Paired Om and Abdsc ASCs from four independent donors were differentiated with varying concentrations of Dex (0, 1, 2, 10, 100, and 1,000 nmol/L): representative immunoblots of ATGL, ADPN, and HSP90 on day 14 (F); quantification of ATGL protein levels relative to HSP90 (G); and ATGL levels calculated as a % of maximal response (H). Dex and depot interactions were significant for both differentiation degree and % maximal response by RM-ANOVA (P < 0.05). #Depot differences (P < 0.05, paired t tests) and *Dex effects (P < 0.05, Dunnett test); n = 4. AU, arbitrary units.
Proadipogenic Role of Dex Induction of TGFBR3 (Betaglycan)
Motivated by our previous observation that Dex more potently increases TGFBR3 mRNA in human Abdsc than Om depot (15) and findings that TGFBR3 can be released to modulate TGFβ signaling (25,26), we compared Dex effects on TGFBR3 in Om and Abdsc ASCs. Total cell-associated TGFBR3 protein levels were much higher (13-fold) in Abdsc than Om ASCs. Low concentrations of Dex (0.25–1 nmol/L) increased TGFBR3 protein in Abdsc ASCs (Fig. 5A and C). The dose response curves were markedly right shifted in Om ASCs. Only a very high concentration of Dex (1,000 nmol/L) increased TGFBR3 protein levels in Om ASCs but to levels far lower than in Abdsc ASCs. TGFBR3 KD (>90%) in Abdsc ASCs increased SMAD2P by ∼80% and decreased adipogenesis by ∼45% (Fig. 5D). Conversely, treatment of Om ASCs with rhTGFBR3 (100 nmol/L) suppressed SMAD2P by ∼50% and improved differentiation by ∼85% (Fig. 5E).
Om ASCs Were Resistant to the Proadipogenic Effects of Dex
Because Om ASCs were less sensitive to the effects of Dex on SMAD2P and TGFBR3, we hypothesized that they were also less sensitive to the glucocorticoid-mediated promotion of adipogenesis (11,12). Low concentrations of Dex (1–2 nmol/L) markedly enhanced adipogenesis in Abdsc but not Om ASCs (Fig. 5F and G). Higher concentrations of Dex increased adipogenesis in Om ASCs, but the magnitude of the response was much smaller and the dose response curves (% maximal response) were right shifted (Fig. 5H), indicating that Om ASCs were less responsive as well as sensitive to Dex.
CM From Om Adipose Tissue Fragments Was More Antiadipogenic
To assess depot differences in the production of antiadipogenic factors in the more physiologically relevant context of intact tissue, we compared the ability of CM from paired samples of human Om and Abdsc adipose tissues to affect adipogenesis. CM from Om inhibited differentiation more than Abdsc adipose tissues (% suppression: 70 ± 4% vs. 35 ± 7% [P < 0.01, n = 8]).
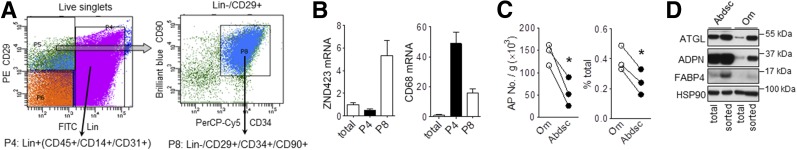
Accumulation of Poorly Differentiating APs in Om
To gain insight into mechanisms that limited Om ASC differentiation in vivo, we used flow cytometry to sort APs, i.e., cells more committed to an adipogenic fate from freshly isolated stromal cells (Fig. 6A) (27). Compared with the total cell population, the sorted Om APs (CD45−/CD14−/CD31−/CD29+/CD34+/CD90+ [P8]) were depleted of macrophages (CD45+/CD14+/CD31+ [P4]) and enriched in ZNF423, a transcription factor expressed early in adipogenesis (Fig. 6B). More APs were present in Om per gram of tissue (2.5-fold) or as a percentage of the total stromal cell number (1.6-fold) (Fig. 6C). Although the selected population of Om APs exhibited higher levels of adipogenesis than mass cultures, their differentiation remained lower than for Abdsc APs, as indicated by protein levels of differentiation markers (Fig. 6D).
Figure 6.
Accumulation of poorly differentiating APs in human Om adipose tissue. A: FACS strategy: freshly isolated stromal vascular cells from Om and Abdsc adipose tissues were first sorted to remove hematopoietic and endothelial cell (Lin+, CD45+/CD14+/CD31+, P4) population. Lin−/CD29+ were then further selected for CD34+/CD90+ cells (P8). B: mRNA expression levels of ZNF423 (marker for AP [left]) and CD68 (macrophage marker [right]) were measured in total stromal and sorted cells from Om. Data are from three donors. C: The number of APs (CD45−/CD14−/CD31−/CD29+/CD34+/CD90+) is presented as cells per gram of adipose tissue (left) and percent of the total number of stromal cells (right). *P < 0.05 (paired t tests). D: Paired samples of total stromal cells and sorted APs from Om and Abdsc were grown and differentiated. Protein expression levels of adipocyte markers, ATGL, ADPN, FABP4, and HSP90, were measured on day 14 of differentiation. Representative blots from two independent experiments are shown.
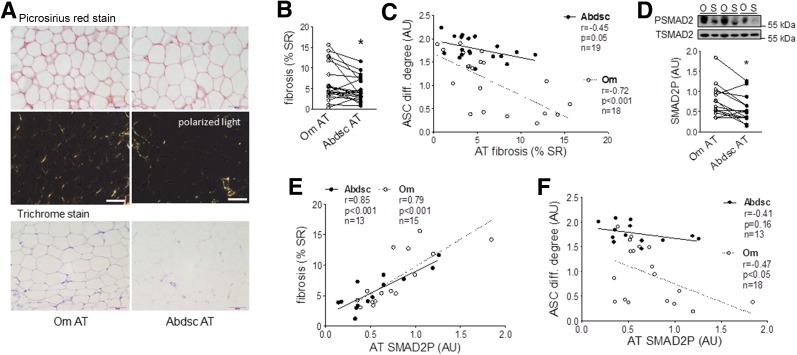
Pericellular Fibrosis Correlated Positively With Tissue SMAD2P and Negatively With ASC Differentiation
To assess the in vivo relevance of the lower level of adipogenesis of Om ASCs to tissue dysfunction, we measured pericellular fibrosis in adipose tissues from the same donors. Pericellular fibrosis was 1.5-fold higher in Om than Abdsc as indicated by picrosirius red and trichrome staining (Fig. 7A and B), and this remained significant after statistical adjustment for BMI (F = 5.32, P = 0.03, df = 18) or adipocyte volume (F = 8.4, P = 0.009, df = 18). Further, pericellular fibrosis negatively correlated with the differentiation of ASCs isolated from the same tissues (Fig. 7C).
Figure 7.
Higher pericellular fibrosis in Om was correlated positively with tissue TGFβ signaling activity and negatively with ASC differentiation (diff.). A: Adipose tissue (AT) samples were used for picrosirius red and trichrome staining of collagen, and representative images from a donor are presented. Scale bars, 100 µm. B: Levels of pericellular fibrosis were quantified in picrosirius red–stained slides and presented as percent of stained to total area (% SR). *P < 0.05 (paired t test), n = 20 (mean ± SEM BMI 42.7 ± 2.1 kg/m2 [range 23–63] and age 38.7 ± 2.6 years [20–56], with 14 females and 6 males). C: Inverse correlations between pericellular fibrosis and differentiation degree of ASCs derived from the same donors. D: SMAD2P in Om and Abdsc adipose tissues was measured with immunoblotting. *P < 0.05 (paired t test; n = 14). E: Positive associations between tissue SMAD2P and fibrosis (percent of stained to total area). F: Negative associations between tissue SMAD2P and differentiation degree of ASCs derived from the same donors. The numbers of subjects, Pearson correlation coefficients (r), P values, and number of samples are shown in each panel. AU, arbitrary units. O, Om; S, Abdsc.
We next assessed the importance of TGFβ signaling activity in adipose tissue in vivo. Basal SMAD2P measured in flash-frozen adipose samples was 1.8-fold higher in Om than Abdsc (Fig. 7D) and highly positively correlated with pericellular fibrosis in both depots (Fig. 7E). Furthermore, tissue SMAD2P negatively correlated with differentiation of Om ASCs from the same tissues (Fig. 7F). The lower tissue SMAD2P in Abdsc did not significantly correlate with variations in differentiation of ASCs.
Discussion
The impaired capacity of visceral compared with subcutaneous adipose tissues to remodel and expand through hyperplasia is thought to account for depot dysfunction and, hence, the association of visceral adiposity with insulin resistance and metabolic health. Here, we show that the lower rates of differentiation in primary human Om than Abdsc ASCs were attributable to higher autocrine/paracrine production of TGFβ ligands and resistance to Dex suppression of TGFβ signaling, as illustrated in Fig. 8. We also provide evidence for a potential mechanism through which Dex regulates TGFβ signaling depot dependently; in Abdsc but not in Om ASCs, Dex induced TGFBR3 that functioned to dampen TGFβ signaling and enhance adipogenesis. The in vivo relevance of TGFβ signaling for tissue dysfunction was supported by findings that higher tissue SMAD2P in Om, indicative of TGFβ activity, positively correlated with pericellular fibrosis, a hallmark of dysfunctional adipose tissue (5), and negatively correlated with lower levels of ASC differentiation. Collectively, these data suggest that insensitivity to glucocorticoid suppression of the TGFβ signaling pathway plays an essential role in limiting hyperplastic expansion and remodeling in visceral adipose tissue and thereby metabolic health.
Figure 8.
Incomplete suppression of TGFβ signaling by glucocorticoids (GCs) in Om ASCs inhibits adipogenesis and may promote fibrosis. This model summarizes our findings and emphasizes the role of ASCs as a source of TGFβ ligands and their regulation by glucocorticoids. Om ASCs secrete higher levels of TGFβ ligands, activin A, and TGFβ, which act in an autocrine/paracrine loop to inhibit adipogenesis. Although glucocorticoids, as modeled by Dex, inhibited the high production of TGFβ ligands in Om and Abdsc ASCs, Om levels remained higher than levels for Abdsc. Abdsc ASCs were more sensitive to glucocorticoid-mediated inhibition of TGFβ signaling through SMAD2P (not shown) and stimulation of adipogenesis. Depot-dependent TGFβ signaling activity and its suppression by glucocorticoids were at least partially mediated by TGFBR3, which was more abundantly expressed and potently induced by glucocorticoids in Abdsc ASCs. The higher level of TGFβ activity may induce a myofibroblast-like phenotype (gray-shaded cells) in some Om ASCs as indicated by the higher SMA expression of Om cultures. These cells may proliferate and accumulate in the depot, contributing to pericellular fibrosis in Om. The in vivo clinical relevance of our cell culture model was supported by the strong associations of interindividual variations in pericellular fibrosis with basal tissue TGFβ signaling activity and rates of adipogenesis of ASCs isolated from the same tissues. Bold and larger fonts and thicker lines or arrows indicate the quantities of factors or magnitude of effects, respectively.
We provide strong evidence that higher production of TGFβ ligands by Om than Abdsc ASCs limits their differentiation. Not only did a bioassay show higher TGFβ activity level but also Om CM more potently stimulated SMAD2P and inhibited adipogenesis, and these effects were blocked by inhibition of TGFBR1 signaling. Further, inhibition of cell-autonomous TGFBR1 kinase activity and its downstream signaling by KD of both SMAD2 and SMAD3 enabled Om ASCs to differentiate to the same level observed in Abdsc ASCs. Levels of activin A and TGFβ in CM covaried and strongly correlated with rates of differentiation and the antiadipogenic effects of ASC CM. Therefore, their individual effects are difficult to evaluate. KD experiments showed that activin A (INHBA) only moderately improved the limited differentiation capacity of Om ASCs, indicating that TGFβ (9,32–34) as well as other effectors such as aortic carboxypeptidase-like protein (34) is also important.
Culture with Dex inhibited TGFβ signaling to promote adipogenesis in both Abdsc and Om ASCs; yet, Om was far less sensitive, as we previously observed for many other pathways and transcripts in human adipose tissues (15). Our results suggest that one mechanism by which Dex regulated TGFβ signaling and adipogenesis in Abdsc was through induction of TGFBR3 protein as shown by loss-of-function studies, similar to the findings in lung fibroblasts (26). Further, addition of exogenous rhTGFBR3 reduced SMAD2P and improved differentiation in Om ASCs, suggesting that TGFBR3 functions extracellularly (25,35). Indeed, with mass spectrometry, we found that TGFBR3 was present in the secretome of confluent Abdsc ASCs (M.-J.L., S.K.F., unpublished observations). The ratio of active to total TGFβ was also higher in Om than Abdsc ASC CM, implicating depot-dependent regulation of TGFβ bioactivity through the release from its latent form (36). Mechanisms by which glucocorticoids regulate TGFβ activity in the tissue context therefore merit further study.
The depot differences in sensitivity to Dex suppression of SMAD2P and adipogenesis highlight the importance of understanding mechanisms regulating glucocorticoid receptor actions in ASCs and adipose tissues. Proinflammatory cytokines including TNFα are known to phosphorylate glucocorticoid receptor at S226 and decrease its activity (37,38), providing a potential link between high inflammation (22,39) and resistance to glucocorticoids in the visceral depot. The inability of glucocorticoids to fully suppress both inflammation and TGFβ signaling in Om to the levels observed in Abdsc adipose tissues (13,15) and ASCs, as shown with measurement of secreted IL-6 herein, may contribute to a feed-forward cascade of events that exacerbate the antiadipogenic and profibrotic microenvironment in this depot.
Cortisol is thought to drive hyperplastic expansion in the visceral fat by increasing adipogenesis (14,40). Local cortisol levels are higher in obese visceral adipose tissue (41), but levels may be insufficient to fully suppress TGFβ and inflammatory signaling in ASCs and other cell types to promote adipogenesis. Nevertheless, Om adipocytes are highly responsive to the ability of glucocorticoids plus insulin to upregulate adipocyte pathways that promote lipid storage (13,15,42,43), permitting the expansion of Om via hypertrophy with deleterious effects on adipose function.
The low adipogenic potential of Om ASCs may be related to a WT1-mediated activation of retinoic acid (RA) signaling (44). Takeda et al. (44) noted higher levels of endogenous retinol and RA in Om ASCs and found that exogenous RA suppressed adipogenesis at an early stage. Activation of WNT and TGFβ/SMAD signaling by retinoids may also contribute to the low adipogenic capacity of Om ASCs, as suggested by studies of human bone marrow mesenchymal stem cells (45) and mouse adipogenic cell lines (46). Multiple signaling pathways likely converge to limit the hyperplastic growth and remodeling of visceral fat.
TGFβ ligands can induce a myofibroblast-like phenotype in subcutaneous ASCs, i.e., greater proliferative but lesser adipogenic capacity and greater production of collagens and other matricellular proteins (9,19,24) that may increase adipose tissue fibrosis. Although macrophages produce TGFβ ligands (9,24), we show that ASCs are also an important source within adipose tissue. Further, our data suggest that high level of TGFβ signaling activity in Om is associated with the accumulation of APs with low adipogenic capacity. This population may include the PDGFRα+CD9high APs that have been shown to be associated with fibrosis in the Om of obesity (47).
Pericellular fibrosis around adipocytes was 1.5-fold higher in Om than Abdsc adipose tissues, independent of adipocyte size and BMI, and was strongly correlated with low adipogenic potential in Om ASCs in a very diverse group of subjects. Other investigators did not find a statistically significant depot difference in pericellular fibrosis (4,48). These somewhat discrepant results are likely to be related to variations in the sample populations as well as methodological differences. Studies that measured total collagen staining may be confounded by inclusion of fibrous septa that have a different metabolic impact (4,48,49).
Potential limitations of this study include a lack of body composition data to assess the relationships of adipogenesis and TGFβ signaling activity in ASCs with interindividual variations in the mass or cellularity of Om and Abdsc adipose tissues. We were not powered to assess the influence of degree of obesity or adipocyte size, age, race, or sex, and we did not have extensive characterization of metabolic phenotypes of subjects. It will be important for future studies to determine how the mechanisms that we identified here change as a function of visceral adiposity independent of total body fat, regional adipocyte size, BMI, race/ethnicity, sex, and age. In addition, we assessed adipogenic potential in standard cultures on plastic, a stiff matrix, that may have a differential effect in Om ASCs. However, the higher basal SMAD2P in Om adipose tissue nevertheless suggests the physiological relevance of the higher level of TGFβ signaling activity observed.
Summary and Conclusion
Insensitivity of Om ASCs to glucocorticoids leads to a higher level of TGFβ signaling activity that likely conspires with inflammatory factors to limit the ability of visceral adipose tissues to recruit new adipocytes and, therefore, their ability to homeostatically remodel or expand in a healthy fashion (Fig. 8). Therapeutic approaches to improve sensitivity to glucocorticoids and thereby suppress TGFβ signaling in ASCs may enhance remodeling and, if required, hyperplastic expansion in Om under the stress of obesity to maintain metabolic health.
Supplementary Material
Article Information
Acknowledgments. The authors thank the subjects who donated adipose tissue samples to the study and Drs. D.T. Hess, B.J. Carmine, P. Hendessi, and D.S. Wang of the Boston Medical Center (BMC) for providing surgical samples. The authors also thank Swati Bhattacharya (BMC) for clinical coordination and Ashley McCarthy and Dr. Caroline Apovian (BMC) for their support and assistance with gathering clinical information.
Funding. This work was supported by National Institutes of Health grants R01-DK-080448 and P30-DK-046200 (Adipocyte Biology and Metabolism Core) to S.K.F., P&F grants from Boston University Clinical and Translational Science Institute (UL1-TR-001430) and Einstein-Mount Sinai Diabetes Research Center (P30-DK-020541) to M.-J.L., a predoctoral fellowship to R.T.P. (T32-DK-007201), and funds from Icahn School of Medicine at Mount Sinai.
Duality of Interest. S.K.F. is currently a consultant for Talapo Therapeutics. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.-J.L., R.T.P., V.S., Y.W., and K.K. carried out the experiments. M.-J.L., R.T.P., and S.K.F. evaluated the data and wrote and edited the manuscript. M.-J.L. and S.K.F. conceived the study. M.-J.L. and S.K.F. designed all experiments. M.J. and M.D.L. suggested and assisted V.S. with polarized light microscopy. M.D.L. reviewed and suggested edits to the manuscript. M.-J.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the National Institutes of Health workshop The Adipose Niche: Role in Health and Disease, Bethesda, MD, 29–30 November 2016, and Nutrition 2018, the annual meeting of the American Society for Nutrition, Boston, MA, 9–12 June 2018.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-0955/-/DC1.
References
- 1.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med 2013;34:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blüher M, Bashan N, Shai I, et al. Activated Ask1-MKK4-p38MAPK/JNK stress signaling pathway in human omental fat tissue may link macrophage infiltration to whole-body insulin sensitivity. J Clin Endocrinol Metab 2009;94:2507–2515 [DOI] [PubMed] [Google Scholar]
- 3.Guglielmi V, Cardellini M, Cinti F, et al. Omental adipose tissue fibrosis and insulin resistance in severe obesity. Nutr Diabetes 2015;5:e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michaud A, Tordjman J, Pelletier M, et al. Relevance of omental pericellular adipose tissue collagen in the pathophysiology of human abdominal obesity and related cardiometabolic risk. Int J Obes 2016;40:1823–1831 [DOI] [PubMed] [Google Scholar]
- 5.Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest 2017;127:74–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Harmelen V, Röhrig K, Hauner H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism 2004;53:632–637 [DOI] [PubMed] [Google Scholar]
- 7.Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol 2002;282:R1286–R1296 [DOI] [PubMed] [Google Scholar]
- 8.Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes 2009;58:1550–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaragosi LE, Wdziekonski B, Villageois P, et al. Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes 2010;59:2513–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almuraikhy S, Kafienah W, Bashah M, et al. Interleukin-6 induces impairment in human subcutaneous adipogenesis in obesity-associated insulin resistance. Diabetologia 2016;59:2406–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauner H, Entenmann G, Wabitsch M, et al. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest 1989;84:1663–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MJ, Fried SK. The glucocorticoid receptor, not the mineralocorticoid receptor, plays the dominant role in adipogenesis and adipokine production in human adipocytes. Int J Obes 2014;38:1228–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MJ, Gong DW, Burkey BF, Fried SK. Pathways regulated by glucocorticoids in omental and subcutaneous human adipose tissues: a microarray study. Am J Physiol Endocrinol Metab 2011;300:E571–E580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindroos J, Husa J, Mitterer G, et al. Human but not mouse adipogenesis is critically dependent on LMO3. Cell Metab 2013;18:62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickering RT, Lee MJ, Karastergiou K, Gower A, Fried SK. Depot dependent effects of dexamethasone on gene expression in human omental and abdominal subcutaneous adipose tissues from obese women. PLoS One 2016;11:e0167337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005;46:2347–2355 [DOI] [PubMed] [Google Scholar]
- 17.Harman-Boehm I, Blüher M, Redel H, et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab 2007;92:2240–2247 [DOI] [PubMed] [Google Scholar]
- 18.Bourlier V, Zakaroff-Girard A, Miranville A, et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation 2008;117:806–815 [DOI] [PubMed] [Google Scholar]
- 19.Keophiphath M, Achard V, Henegar C, Rouault C, Clément K, Lacasa D. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol 2009;23:11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology 2007;148:868–877 [DOI] [PubMed] [Google Scholar]
- 21.Liu LF, Craig CM, Tolentino LL, et al. Adipose tissue macrophages impair preadipocyte differentiation in humans. PLoS One 2017;12:e0170728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Tchkonia T, Stout MB, et al. Inflammation and the depot-specific secretome of human preadipocytes. Obesity (Silver Spring) 2015;23:989–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klar AS, Biedermann T, Michalak K, et al. Human adipose mesenchymal cells inhibit melanocyte differentiation and the pigmentation of human skin via increased expression of TGF-β1. J Invest Dermatol 2017;137:2560–2569 [DOI] [PubMed] [Google Scholar]
- 24.Bourlier V, Sengenès C, Zakaroff-Girard A, et al. TGFbeta family members are key mediators in the induction of myofibroblast phenotype of human adipose tissue progenitor cells by macrophages. PLoS One 2012;7:e31274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elderbroom JL, Huang JJ, Gatza CE, et al. Ectodomain shedding of TβRIII is required for TβRIII-mediated suppression of TGF-β signaling and breast cancer migration and invasion. Mol Biol Cell 2014;25:2320–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartze JT, Becker S, Sakkas E, et al. Glucocorticoids recruit Tgfbr3 and Smad1 to shift transforming growth factor-β signaling from the Tgfbr1/Smad2/3 axis to the Acvrl1/Smad1 axis in lung fibroblasts. J Biol Chem 2014;289:3262–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y, Lee MJ, Ido Y, Fried SK. High-fat diet-induced obesity regulates MMP3 to modulate depot- and sex-dependent adipose expansion in C57BL/6J mice. Am J Physiol Endocrinol Metab 2017;312:E58–E71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee MJ, Fried SK. Optimal protocol for the differentiation and metabolic analysis of human adipose stromal cells. Methods Enzymol 2014;538:49–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 1994;216:276–284 [DOI] [PubMed] [Google Scholar]
- 30.Macotela Y, Emanuelli B, Mori MA, et al. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes 2012;61:1691–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MJ, Pickering RT, Puri V. Prolonged efficiency of siRNA-mediated gene silencing in primary cultures of human preadipocytes and adipocytes. Obesity (Silver Spring) 2014;22:1064–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petruschke T, Röhrig K, Hauner H. Transforming growth factor beta (TGF-beta) inhibits the differentiation of human adipocyte precursor cells in primary culture. Int J Obes Relat Metab Disord 1994;18:532–536 [PubMed] [Google Scholar]
- 33.Choy L, Skillington J, Derynck R. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J Cell Biol 2000;149:667–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jager M, Lee MJ, Li C, Farmer SR, Fried SK, Layne MD. Aortic carboxypeptidase-like protein enhances adipose tissue stromal progenitor differentiation into myofibroblasts and is upregulated in fibrotic white adipose tissue. PLoS One 2018;13:e0197777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkins LM, Horst B, Lancaster CL, Mythreye K. Dually modified transmembrane proteoglycans in development and disease. Cytokine Growth Factor Rev 2018;39:124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson IB, Rifkin DB Regulation of the bioavailability of TGF-β and TGF-β-related proteins. Cold Spring Harb Perspect Biol 2016;8:a021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ismaili N, Garabedian MJ. Modulation of glucocorticoid receptor function via phosphorylation. Ann N Y Acad Sci 2004;1024:86–101 [DOI] [PubMed] [Google Scholar]
- 38.Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol 2013;131:636–645 [DOI] [PubMed] [Google Scholar]
- 39.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 1998;83:847–850 [DOI] [PubMed] [Google Scholar]
- 40.Wong JC, Krueger KC, Costa MJ, et al. A glucocorticoid- and diet-responsive pathway toggles adipocyte precursor cell activity in vivo. Sci Signal 2016;9:ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veilleux A, Laberge PY, Morency J, Noël S, Luu-The V, Tchernof A. Expression of genes related to glucocorticoid action in human subcutaneous and omental adipose tissue. J Steroid Biochem Mol Biol 2010;122:28–34 [DOI] [PubMed] [Google Scholar]
- 42.Fried SK, Russell CD, Grauso NL, Brolin RE. Lipoprotein lipase regulation by insulin and glucocorticoid in subcutaneous and omental adipose tissues of obese women and men. J Clin Invest 1993;92:2191–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gathercole LL, Morgan SA, Bujalska IJ, Hauton D, Stewart PM, Tomlinson JW. Regulation of lipogenesis by glucocorticoids and insulin in human adipose tissue. PLoS One 2011;6:e26223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeda K, Sriram S, Chan XH, et al. Retinoic acid mediates visceral-specific adipogenic defects of human adipose-derived stem cells. Diabetes 2016;65:1164–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao J, Ma Y, Yao W, Zhang X, Wu D. Retinoids regulate adipogenesis involving the TGFβ/SMAD and Wnt/β-catenin pathways in human bone marrow mesenchymal stem cells. Int J Mol Sci 2017;18:E842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marchildon F, St-Louis C, Akter R, Roodman V, Wiper-Bergeron NL. Transcription factor Smad3 is required for the inhibition of adipogenesis by retinoic acid. J Biol Chem 2010;285:13274–13284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marcelin G, Ferreira A, Liu Y, et al. A PDGFRα-mediated switch toward CD9high adipocyte progenitors controls obesity-induced adipose tissue fibrosis. Cell Metab 2017;25:673–685 [DOI] [PubMed] [Google Scholar]
- 48.Divoux A, Tordjman J, Lacasa D, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010;59:2817–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wernstedt Asterholm I, Scherer PE. Fibrosis-streaks and splatters: some things are not always what they seem to be. Obesity (Silver Spring) 2016;24:552–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.