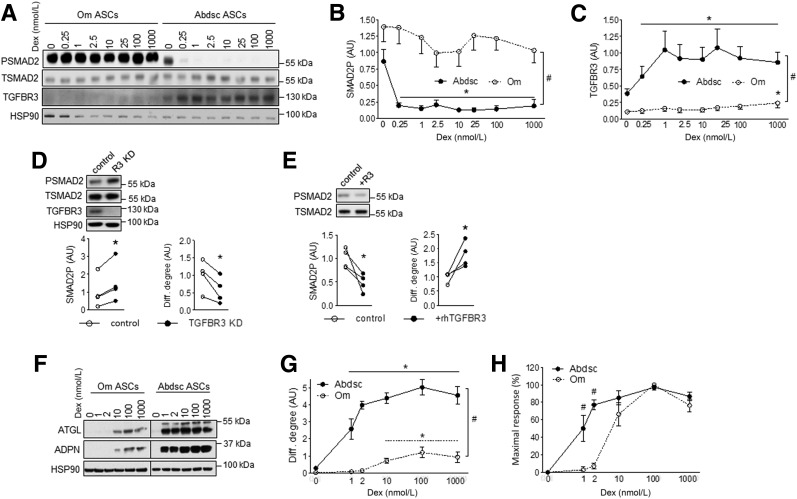
Figure 5.
Resistance to Dex-mediated induction of TGFBR3 contributed to higher level of TGFβ signaling activity and lower level of adipogenesis in Om ASCs. Confluent Om and Abdsc ASCs were incubated with Dex (0, 0.25, 1, 2.5, 10, 25, 100, and 1,000 nmol/L) overnight, and phosphorylated (PSMAD2) and total (PSMAD2) SMAD2, TGFBR3, and HSP90 protein levels were measured: representative Western blot (A), densitometry of SMAD2P (B), and relative expression levels of TGFBR3 to HSP90 (C) in samples from six independent donors are presented. Depot differences in dose response curves were significant by two-way ANOVA (depot × Dex interaction, P < 0.05; n = 6). #Depot differences were significant at all Dex concentrations (P < 0.05 [paired t tests]). *Dex effects, P < 0.05 (Dunnett test). D: Abdsc ASCs were transfected with control or TGFBR3 siRNA, and phosphorylated and total SMAD2, TGFBR3 and HSP90 protein levels were measured at confluence (left). Cells were differentiated and adipogenesis (ATGL protein) was determined on day 14 (right). *P < 0.05 (paired t tests), n = 4. E: Confluent Om ASCs were treated with rhTGFBR3 (100 nmol/L) overnight, and phosphorylated and total SMAD2 levels were measured (left). Om ASCs were differentiated with or without rhTGFBR3 (100 nmol/L), and differentiation (Diff.) degree (ATGL protein) was determined on day 14 (right). *P < 0.05 (paired t test), n = 4. Paired Om and Abdsc ASCs from four independent donors were differentiated with varying concentrations of Dex (0, 1, 2, 10, 100, and 1,000 nmol/L): representative immunoblots of ATGL, ADPN, and HSP90 on day 14 (F); quantification of ATGL protein levels relative to HSP90 (G); and ATGL levels calculated as a % of maximal response (H). Dex and depot interactions were significant for both differentiation degree and % maximal response by RM-ANOVA (P < 0.05). #Depot differences (P < 0.05, paired t tests) and *Dex effects (P < 0.05, Dunnett test); n = 4. AU, arbitrary units.