Abstract
Cardiovascular (CV) disease fatality rates are higher for women compared with men with diabetes despite lower rates of obstructive coronary artery disease (CAD). Impaired coronary flow reserve (CFR), the ratio of adenosine-stimulated to rest myocardial blood flow (MBF), is an indicator of coronary microvascular dysfunction and predicts major adverse CV events. We performed a post hoc analysis to determine whether there was a sex disparity in coronary microvascular dysfunction among 46 men and 27 women with well-controlled type 2 diabetes and without clinical evidence of obstructive CAD. We found that women had a higher rest MBF, lower CFR, and worse diastolic function compared with men. In addition, rest MBF was positively correlated with worse diastolic function in women. We previously showed that mineralocorticoid blockade improved CFR in men and women with type 2 diabetes, implicating aldosterone in the pathophysiology of coronary microvascular dysfunction. We therefore examined aldosterone levels and found that women had larger increases in aldosterone in response to an angiotensin-II infusion than did men. In conclusion, among individuals with type 2 diabetes and good cardiometabolic control, women had worse myocardial perfusion and diastolic function compared with men. The greater aldosterone responsivity in women may be a mechanism for this sex effect.
Introduction
Although women experience fewer cardiovascular (CV) events than men of the same age, this advantage erodes among individuals with type 2 diabetes (1–3). Type 2 diabetes confers greater risk for coronary heart disease (CHD), myocardial infarction, and CHD-related death in women compared with men (4,5). Yet, obstructive coronary artery disease (CAD) is less prevalent among women than men (6).
Coronary microvascular dysfunction is increasingly recognized as an important risk factor for CV events (7). A well-established indicator of the presence of coronary microvascular dysfunction is impaired coronary flow reserve (CFR). CFR is the ratio of maximal adenosine-stimulated (stress) to rest myocardial blood flow (MBF) and is assessed on positron emission tomography (PET). Impaired CFR is independently associated with major adverse CV outcomes (8). Murthy et al. (9) showed that regardless of sex, an increase in CFR of 10% was associated with a 20% reduction in CV events.
Coronary microvascular dysfunction is more prevalent in individuals with diabetes and confers increased CV risk (10,11). A study among individuals with type 2 diabetes found that rates of cardiac death were comparable for individuals with obstructive CAD and for individuals without obstructive CAD but with impaired coronary vascular function (12).
In the current study, we investigated whether there was a sex disparity in coronary microvascular function among individuals with well-controlled type 2 diabetes and without clinical evidence of obstructive CAD. We hypothesized that women with type 2 diabetes would have lower CFR than men with type 2 diabetes given the worse CV outcomes in women despite lower rates of obstructive CAD.
Research Design and Methods
We performed a post hoc analysis of baseline data from a previously published double-blind, randomized, controlled study among individuals with type 2 diabetes without clinical evidence of CAD. Our prior study demonstrated improvement in CFR after 6 months of mineralocorticoid receptor (MR) blockade compared with treatment with placebo or hydrochlorothiazide (ClinicalTrials.gov, NCT00865124) (13). Participants were aged 18–70 years and had well-controlled type 2 diabetes. Exclusion criteria included any evidence of coronary, cerebrovascular, peripheral vascular, or renal disease based on history, physical examination, electrocardiogram, and screening blood tests. The full list of inclusion and exclusion criteria was previously published (13). The Partners HealthCare Institutional Review Board approved the protocol, and all participants provided written informed consent.
Subjects completed a 3-month run-in phase where they were started on enalapril (20 mg daily) and tapered off other antihypertensives. Amlodipine was added if systolic blood pressure (SBP) remained >140 mmHg. Simvastatin (20 mg) was added if LDL was >100 mg/dL and the participant was not already on a statin. Antidiabetic medications were adjusted to optimize glucose control.
After the 3-month run-in, participants were admitted to the Brigham and Women’s Hospital Center for Clinical Investigation and completed a baseline assessment, which included echocardiography, cardiac PET scan, cardiac MRI scan, and hormonal measurements using techniques previously described (14). All studies were performed with subjects in balance on a liberal sodium (250 mEq) diet. Stress (hyperemic) MBF and rest MBF from the left ventricle were obtained from the cardiac PET scan and used to calculate CFR as previously described (14). Echocardiography was used to determine E/e′. E/e′ is the ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (e′) and is a measure of diastolic function. Normal E/e′ is a value <8 with >15 being abnormal.
After subjects had been supine for 30 min, blood pressure was assessed every 5 min over a 30-min period using an automated blood pressure device (Dinamap). Morning blood was collected with subjects supine and fasting from midnight the previous night. To assess aldosterone response to a controlled stimulus, supine subjects were infused with angiotensin-II at 3 ng/kg/min for 60 min with measurement of serum aldosterone before and at the end of the infusion. The infusion rate was reduced to 1 ng/kg/min if SBP increased to >180 mmHg or diastolic blood pressure increased to >100 mmHg. Assays were performed as previously described (14); the lower limit of the aldosterone assay is 2.5 ng/dL. Any participant with imaging evidence of cardiac ischemia or prior myocardial infarction on late gadolinium-enhancement cardiac MRI or cardiac PET scan was excluded. This study used data obtained during this baseline, prerandomization assessment.
Statistical Analysis
The Shapiro-Wilk test was used to determine deviations from normality. CFR and stress MBF were normally distributed, whereas rest MBF (Shapiro-Wilk test P < 0.001) and diastolic function E/e′ (Shapiro-Wilk test P = 0.01) were not normally distributed. On log transformation, rest MBF and E/e′ both had a log-normal distribution (Shapiro-Wilk test P = 0.06 and P = 0.51, respectively). Consequently, natural log transformation was applied to rest MBF and E/e′ before analysis, and the coefficients were exponentiated and interpreted as percentage differences.
Multivariate regression was used to evaluate the primary outcome of sex differences in CFR. Covariates were chosen a priori to be the same as those used in our prior study and had been selected from among those associated with vascular function: hemoglobin A1c (HbA1c), BMI, race, SBP, statin use, and age (13). The same multivariate model was used to assess sex differences in rest MBF, stress MBF, and diastolic function. Partial correlation was used to assess the relationship between rest MBF and diastolic function, retaining only those covariates contributing significantly to the model (race, HbA1c, and SBP). Other factors (HDL, fasting blood glucose, and thiazolidinedione use) that differed between men and women at baseline did not influence our outcomes. Significance was considered for a two-sided P value of ≤0.05. All statistical analysis was performed using Stata 15.1 software (StataCorp LLC, College Station, TX).
Results
The analysis included 73 study participants (63% men) (see Table 1 for characteristics of the study population). As specified by the study protocol, all study participants were on the ACE inhibitor enalapril and had excellent glycemic, blood pressure, and lipid management, according to the 2012 American Diabetes Association guidelines (15).
Table 1.
Characteristics of study population (n = 73)
| Men n = 46 | Women n = 27 | P value | |
|---|---|---|---|
| Age (years) | 55 ± 7 | 52 ± 8 | 0.11 |
| Race | 0.19 | ||
| Caucasian | 31 (67) | 14 (52) | |
| African American | 12 (26) | 10 (37) | |
| Other | 3 (7) | 3 (11) | |
| BMI (kg/m2) | 32.0 ± 4.6 | 32.0 ± 4.8 | 0.99 |
| Blood pressure (mmHg) | |||
| Systolic | 125 ± 12 | 126 ± 15 | 0.79 |
| Diastolic | 76 ± 9 | 74 ± 10 | 0.37 |
| Statin use | 36 (78) | 21 (78) | 0.96 |
| Fasting laboratory data | |||
| HbA1c (%) | 6.8 ± 0.8 | 7.0 ± 0.7 | 0.26 |
| Blood glucose (mg/dL) | 110 ± 20 | 121 ± 28 | 0.08 |
| Total cholesterol (mg/dL) | 148 ± 29 | 154 ± 34 | 0.42 |
| LDL (mg/dL) | 83 ± 22 | 77 ± 27 | 0.31 |
| HDL (mg/dL) | 41 ± 11 | 49 ± 12 | 0.01* |
| Triglycerides (mg/dL) | 124 ± 69 | 124 ± 60 | 0.95 |
| Creatinine clearance rate (mL/min) | 74.8 ± 17.9 | 79.3 ± 20.6 | 0.32 |
| Duration of diabetes (years) | 7.9 ± 6.3 | 7.8 ± 6.5 | 0.93 |
| Diabetes medications | |||
| Metformin | 38 (78) | 23 (79) | 0.77 |
| Insulin | 6 (12) | 4 (14) | 0.74 |
| Sulfonylurea | 17 (35) | 9 (32) | 0.51 |
| Thiazolidinedione | 0 (0) | 3 (11) | 0.05 |
| Glucagon-like peptide 1 analog | 2 (4) | 3 (11) | 0.35 |
| Dipeptidyl peptidase 4 inhibitor | 1 (2) | 0 (0) | 0.64 |
Data are expressed as mean ± SD for continuous variables and as absolute numbers (%) for categorical variables. Comparisons across sexes were performed using the unpaired Student t test, Fisher exact test, and χ2 test for continuous, binary, and categorical variables, respectively.
*P < 0.05 between men and women.
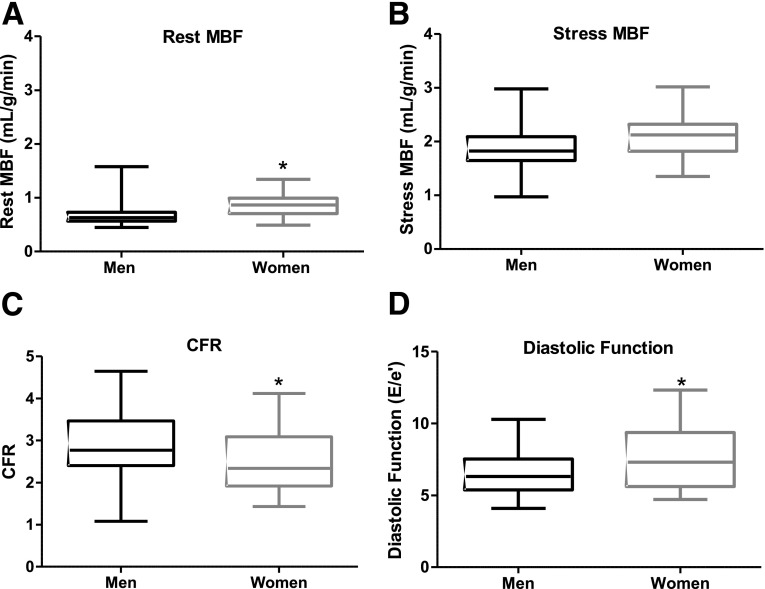
Median rest MBF was 0.63 mL/g/min in men (interquartile range [IQR] 0.57–0.73) and 0.86 mL/g/min in women (IQR 0.71–0.95) (Fig. 1A). On multivariate regression, rest MBF was 35% higher in women compared with men (P < 0.0010). In the model, increased age and SBP contributed to higher rest MBF. Rest MBF was 20% higher in those >60 years old compared with those <50 years old. For every increase of 10 mmHg in SBP, there was a 6% increase in rest MBF.
Figure 1.
Sex differences in rest MBF, stress MBF, CFR, and diastolic function. A: Median rest MBF was 0.63 mL/g/min in men (IQR 0.57–0.73) and 0.86 mL/g/min in women (IQR 0.71–0.95). *P < 0.001. B: Median stress MBF was 1.83 mL/g/min in men (IQR 1.65–2.07) and 2.17 mL/g/min in women (IQR 1.83–2.33, P = 0.08). C: Median CFR was 2.85 in men (IQR 2.41–3.46) and 2.38 in women (IQR 1.92–3.09). *P = 0.008. D: Median diastolic function (E/e′) was 6.3 in men (IQR 5.4–7.5) and 7.3 in women (IQR 5.6–9.4). *P = 0.008. Higher E/e′ values indicate worse diastolic function. All P values were adjusted for HbA1c, BMI, race, SBP, statin use, and age.
The median stress MBF was 1.83 mL/g/min in men (IQR 1.65–2.07) and 2.17 mL/g/min in women (IQR 1.83–2.33) (Fig. 1B). After adjusting for covariates on multivariate regression, stress MBF was not significantly different between the sexes (P = 0.08).
Because CFR is the ratio of stress MBF to rest MBF, the disproportionately higher rest MBF compared with stress MBF in women versus men led to a lower CFR in women with type 2 diabetes. The median CFR was 2.85 in men (IQR 2.41–3.46) compared with 2.38 in women (IQR 1.92–3.09), representing a 16% lower CFR in women (P = 0.008) (Fig. 1C).
Diastolic function was assessed with E/e′. Median E/e′ was 6.3 in men (IQR 5.4–7.5) and 7.3 in women (IQR 5.6–9.4; P = 0.008) (Fig. 1D). E/e′ was 18% higher (worse) in women compared with men. E/e′ was also 6% higher for every 10 mmHg higher in an individual’s SBP.
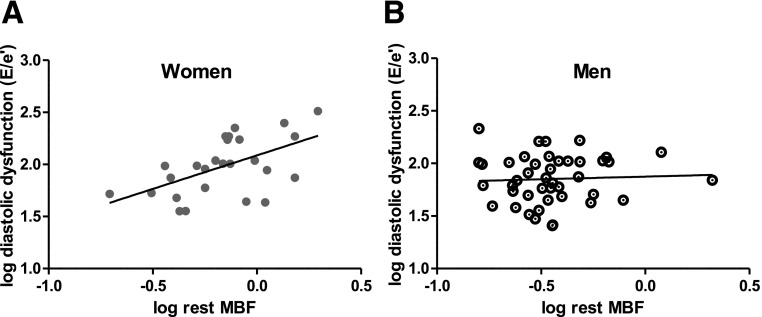
We then determined whether there was a correlation between rest MBF and diastolic function. Although no significant correlation was found between rest MBF and diastolic function in the overall study population (P value 0.08), there was a significant relationship among women. In women, higher rest MBF correlated with higher (worse) E/e′, controlling for race, HbA1c, and SBP (P = 0.05 and correlation coefficient of 0.43) (Fig. 2). There was no relationship between CFR and E/e′ in women, men, or the overall study population.
Figure 2.
A: Partial correlation of log-transformed rest MBF and log-transformed diastolic function (E/e′) among women (partial coefficient = 0.43 and P = 0.05). Higher E/e′ values correspond with worse diastolic function. B: There was no significant partial correlation of log-transformed rest MBF and log-transformed diastolic function (E/e′) among men (partial coefficient = 0.0054 and P = 0.97).
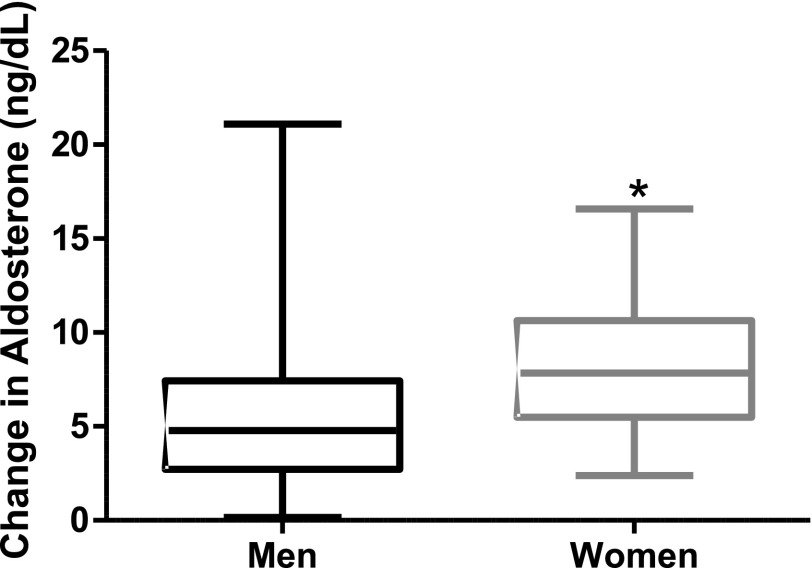
Because abnormal activation of the renin-angiotensin-aldosterone system leads to myocardial damage and because MR blockade has been shown to improve CFR in men and women with type 2 diabetes, we next examined aldosterone levels (13). There was no difference in baseline aldosterone levels between men (2.50 ng/dL [IQR 2.50–4.00]) and women (2.50 ng/dL [IQR 2.50–3.16], P = 0.644); however, peak angiotensin-II–stimulated aldosterone levels were lower in men (7.66 ng/dL [IQR 5.68–11.22]) than in women (11.23 ng/dL [IQR 8.27–14.83], P = 0.032). The aldosterone response to angiotensin-II (peak minus baseline) was smaller in men (median ∆4.77 ng/dL [IQR 2.72–7.35]) than in women (median ∆7.85 ng/dL [IQR 5.76–10.28], P= 0.027) (Fig. 3). Statin use was associated with a smaller increase in aldosterone in response to angiotensin-II, consistent with our previous report that statin use reduced aldosterone production (16).
Figure 3.
Sex differences in aldosterone response to angiotensin-II. Men had smaller increases in aldosterone in response to angiotensin-II infusion (median ∆4.77 ng/dL [IQR 2.72–7.35]) than women (median ∆7.85 ng/dL [IQR 5.76–10.28]). *P = 0.027.
Discussion
This study demonstrated that among individuals with type 2 diabetes with good cardiometabolic control and without clinical or imaging evidence of obstructive CAD, CFR is lower in women compared with men. To our knowledge, this is the first study to examine the effect of sex on MBF and coronary microvascular function in individuals with diabetes but without evidence of heart disease by symptoms and without evidence of obstructive cardiac disease by cardiac imaging (PET and MRI). Our observed difference of almost 0.5 in median CFR between women (2.38) and men (2.85) may be clinically relevant. In a large study of 1,218 men and women referred for rest/stress cardiac PET because of concerns for suspected CAD (mean CFR 1.9), an increase in CFR by 0.2 was associated with a 10% decrease in major adverse CV events (9). However, large-scale studies are needed to determine the specific relationship between CFR and clinical outcomes in individuals without CAD symptoms and with diabetes.
Lower coronary microvascular function predicts CV mortality in men and women with diabetes (8). Our finding that coronary microvascular function is worse in women compared with men may contribute to the excess CV mortality that has been observed in women with diabetes compared with men with diabetes (5). Taqueti et al. (17) showed that among patients with low CFR, women showed a higher frequency of nonobstructive CAD, whereas men showed a higher frequency of severely obstructive CAD. Despite manifesting a lower burden of obstructive angiographic CAD, women had an elevated risk of CVD events (17).
Our data indicate that the reduction in CFR in women is partly driven by higher rest MBF. This increase in rest MBF does not appear benign, because increases in rest MBF were associated with worse diastolic function. Further, a recent study demonstrated that increased rest MBF was an independent predictor of cardiac mortality (18). A key function of the coronary microvasculature is to adjust local blood flow to match tissue metabolic demands (19). Although the cause for higher rest MBF in women is not known, it may be that rest MBF is increased in women due to an increase in rest cardiac metabolic demand. Intriguingly, aldosterone has been shown to cause mitochondrial dysfunction (20). Other studies have demonstrated this phenomenon of higher rest MBF in women (9,21), but these studies enrolled individuals with angina referred for cardiac stress testing.
Impaired CFR may involve remodeling of the microvasculature, altered endothelial function, and smooth muscle dysfunction; however, the specific mechanisms involved in diabetes are not yet fully defined. Insulin-resistant, obese rodents develop impaired coronary endothelium-dependent vasodilation and diastolic dysfunction, both of which are improved with blockade of the MR (22). In male diabetic rodents, coronary vasoconstriction is enhanced and coronary vasodilation impaired, and MR blockade improves endothelium-dependent coronary vasodilatory function in addition to altering expression of genes involved in the regulation of nitric oxide production and inflammation (22,23). These preclinical studies suggest that excess MR activity mediates impairments in endothelium-dependent coronary vasodilation in diabetes. Further, in nondiabetic, hypertensive rodents, aldosterone damages the intramural coronary arterioles, induces perivascular inflammation and fibrinoid necrosis, causes cardiac inflammation, myocardial necrosis, and fibrosis, and impairs mitochondrial function of cardiac fibroblasts (24,25). Thus, it may be that excess MR activation alters coronary microvascular function through effects on inflammation, fibrosis, mitochondrial function, vasoconstriction, and vasodilation.
In an animal model of CV injury induced by low nitric oxide and angiotensin-II administration, MR blockade markedly reduced coronary microvascular and myocardial injury to similar levels in male and female rodents. However, this model caused more aldosterone-mediated myocardial injury in female versus male rodents, and this was due to angiotensin-II raising aldosterone to a higher level in female versus male rodents (26). Human females also show increased sensitivity to angiotensin-II; we showed increased angiotensin-II stimulated aldosterone in women versus men with hypertension (26). In humans, blockade of the receptor for aldosterone improved CFR in individuals with diabetes (13), suggesting that excess MR activation is a key contributor to microvascular dysfunction in men and women with diabetes. In this study, men and women both showed improvements with MR blockade, but there was inadequate sample size (17 men and 6 women) to assess a sex effect. Our current finding that women with diabetes had increased angiotensin-II–stimulated aldosterone versus men raises the possibility that although activation of the MR is an important contributor to impaired coronary microvascular function in both men and women with diabetes, the excess exposure to aldosterone in women may help explain the excess CVD incidence in women with diabetes.
Advantages of this study are that we carefully controlled cardiometabolic factors (blood pressure, lipids, and glucose) for the 3 months before the study. Fingerstick blood glucose before PET imaging was not elevated, which is important because elevated blood glucose at the time of cardiac PET reduces coronary microvascular function. In addition, this was a study in individuals without clinical or imaging evidence of obstructive CAD or scarring, so we were presumably examining early changes in diabetic CV disease.
Our study has several limitations. It is a relatively small, cross-sectional, post hoc analysis, and the original study was not designed to assess sex effects. However, our ability to detect significant sex differences suggests there is a strong signal. We cannot state that the observed decrease in coronary microvascular function in women will translate into increased CV morbidity and mortality. However, the association of increased rest MBF with reductions in diastolic function suggest the higher rest MBF is not benign. Further prospective randomized studies are warranted to identify additional mechanisms contributing to these sex disparities.
Conclusions
These findings suggest that among individuals with type 2 diabetes and good cardiometabolic control, women have more impairment in myocardial perfusion and diastolic function compared with men. There may be unique pathophysiologic drivers in females leading to more extensive coronary microvascular dysfunction. One potential mechanism for this sex effect is dysregulated aldosterone production.
Article Information
Funding. This work was supported by National Institutes of Health grants UL-1TR-000170 (Harvard Catalyst, Harvard Clinical and Translational Science Center), T32-HL-007609 (A.V.H.), K24-HL-103845 (G.K.A.), and R01-HL-087060 (G.K.A.)
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.V.H. and B.A.R. performed the statistical analysis. A.V.H., M.F.D.C., and G.K.A. were involved in developing the research question. A.V.H. and G.K.A. wrote the manuscript. R.Y.K. directed imaging. R.Y.K., A.D.R., R.G., and M.F.D.C. reviewed and edited the manuscript. A.D.R. and R.G. recruited subjects. A.D.R., R.G., and G.K.A. helped with clinical management of study participants and conducted the study. M.F.D.C. directed PET imaging and analysis. G.K.A. procured funding. G.K.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 100th Endocrine Society Annual Meeting, Chicago, IL, 17–20 March 2018.
Footnotes
Clinical trial reg. no. NCT00865124, clinicaltrials.gov
See accompanying article, p. 474.
References
- 1.Tunstall-Pedoe H. Myth and paradox of coronary risk and the menopause. Lancet 1998;351:1425–1427 [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Wilson PW. Risk factors that attenuate the female coronary disease advantage. Arch Intern Med 1995;155:57–61 [PubMed] [Google Scholar]
- 3.Hu G, Jousilahti P, Qiao Q, Katoh S, Tuomilehto J. Sex differences in cardiovascular and total mortality among diabetic and non-diabetic individuals with or without history of myocardial infarction. Diabetologia 2005;48:856–861 [DOI] [PubMed] [Google Scholar]
- 4.Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA 1991;265:627–631 [PubMed] [Google Scholar]
- 5.Regensteiner JG, Golden S, Huebschmann AG, et al.; American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Epidemiology and Prevention, Council on Functional Genomics and Translational Biology, and Council on Hypertension . Sex differences in the cardiovascular consequences of diabetes mellitus: a scientific statement from the American heart association. Circulation 2015;132:2424–2447 [DOI] [PubMed] [Google Scholar]
- 6.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol 2009;54:1561–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herzog BA, Husmann L, Valenta I, et al. . Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol 2009;54:150–156 [DOI] [PubMed] [Google Scholar]
- 8.Cortigiani L, Rigo F, Gherardi S, et al. . Prognostic effect of coronary flow reserve in women versus men with chest pain syndrome and normal dipyridamole stress echocardiography. Am J Cardiol 2010;106:1703–1708 [DOI] [PubMed] [Google Scholar]
- 9.Murthy VL, Naya M, Taqueti VR, et al. . Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Carli MF, Janisse J, Grunberger G, Ager J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol 2003;41:1387–1393 [DOI] [PubMed] [Google Scholar]
- 11.Pepine CJ, Ferdinand KC, Shaw LJ, et al.; ACC CVD in Women Committee . Emergence of nonobstructive coronary artery disease: a woman’s problem and need for change in definition on angiography. J Am Coll Cardiol 2015;66:1918–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murthy VL, Naya M, Foster CR, et al. . Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012;126:1858–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg R, Rao AD, Baimas-George M, et al. . Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes 2015;64:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao AD, Shah RV, Garg R, et al. . Aldosterone and myocardial extracellular matrix expansion in type 2 diabetes mellitus. Am J Cardiol 2013;112:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association Standards of medical care in diabetes--2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baudrand R, Pojoga LH, Vaidya A, et al. . Statin use and adrenal aldosterone production in hypertensive and diabetic subjects. Circulation 2015;132:1825–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taqueti VR, Shaw LJ, Cook NR, et al. . Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation 2017;135:566–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta A, Taqueti VR, van de Hoef TP, et al. . Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation 2017;136:2325–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pries AR, Badimon L, Bugiardini R, et al. . Coronary vascular regulation, remodelling, and collateralization: mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur Heart J 2015;36:3134–3146 [DOI] [PubMed] [Google Scholar]
- 20.Ibarrola J, Sadaba R, Martinez-Martinez E, et al. . Aldosterone impairs mitochondrial function in human cardiac fibroblasts via A-kinase anchor protein 12. Sci Rep 2018;8:6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufmann PA, Camici PG. Myocardial blood flow measurement by PET: technical aspects and clinical applications. J Nucl Med 2005;46:75–88 [PubMed] [Google Scholar]
- 22.Bender SB, DeMarco VG, Padilla J, et al. . Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension 2015;65:1082–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown SM, Meuth AI, Davis JW, Rector RS, Bender SB. Mineralocorticoid receptor antagonism reverses diabetes-related coronary vasodilator dysfunction: a unique vascular transcriptomic signature. Pharmacol Res 2018;134:100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocha R, Stier CT Jr, Kifor I, et al. . Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology 2000;141:3871–3878 [DOI] [PubMed] [Google Scholar]
- 25.Ibarrola J, Sadaba R, Martinez-Martinez E, et al. . Aldosterone impairs mitochondrial function in human cardiac fibroblasts via A-kinase anchor protein 12. Sci Rep 2018;8:6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shukri MZ, Tan JW, Manosroi W, et al. . Biological sex modulates the adrenal and blood pressure responses to angiotensin II. Hypertension 2018;71:1083–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]