Abstract
Background
Thinning of the fibrous cap of atherosclerotic plaque is a major component of plaque vulnerability. The high resolution of optical coherence tomography (OCT) provides an accurate measurement of fibrous-cap thickness. Endothelial dysfunction is associated with inflammation and enhanced local expression of matrix metalloproteinases. We investigated the association between endothelial dysfunction and OCT-derived thin-cap fibroatheroma (TCFA) in patients with acute coronary syndromes (ACS).
Methods
Seventy-four patients with ACS, who underwent both OCT examinations of the culprit lesion before percutaneous coronary intervention and peripheral endothelial function assessment as assessed by logarithmic value of reactive hyperemia index (Ln_RHI), were enrolled. Age-, sex-, hypertension-, and diabetes-matched non-coronary artery disease (non-CAD) patients were also enrolled (n=15).
Results
Ln_RHI levels were significantly lower in ACS patients compared with non-CAD patients (0.56±0.26 vs 0.74±0.22, P=0.01). Furthermore, the Ln_RHIs of ACS patients with TCFA (n=44) were significantly lower than those of ACS patients without TCFA (n=30) (0.50±0.24 vs 0.65±0.26, P=0.01). There was a weak but significant positive correlation between Ln_RHI and fibrous-cap thickness (Spearman’s ρ=0.25, P=0.03). Multivariate logistic regression analysis identified lower Ln_RHI as an independent factor associated with TCFA in ACS patients (OR per 0.1 increase in Ln_RHI: 0.78 [95% CI: 0.62–0.98], P=0.03).
Conclusion
Advanced endothelial dysfunction significantly correlates with a thin fibrous cap of coronary plaques in patients with ACS.
Keywords: peripheral endothelial function, optical coherence tomography, thin-cap fibroatheroma, plaque vulnerability
Introduction
Endothelial dysfunction is a key component in plaque formation, progression, and disruption.1,2 Past studies have reported that impaired endothelial function was associated with an increased inflammatory response and enhanced local expression of matrix metalloproteinases.1,3 Reactive hyperemia-peripheral arterial tonometry (RH-PAT) is a noninvasive, automatic, reproducible, and quantitative clinical test that can be used to evaluate peripheral endothelial function,4 and has been shown to be associated with cardiovascular risk factors,5 coronary endothelial dysfunction,6 and cardiovascular events.7
The depletion of matrix components from the fibrous cap, caused by an imbalance between synthesis and breakdown, leads to cap thinning.8 This predisposes the fibrous cap to rupture, either spontaneously or in response to hemodynamic or other triggers. Optical coherence tomography (OCT) is a recently developed intracoronary imaging technique with a high resolution of 10–20 µm, enabling detailed visualization of plaque microstructure.9
It is unclear whether thin-cap fibroatheromas (TCFAs) as assessed by OCT are accompanied by endothelial dysfunction. In this study, we examined whether endothelial dysfunction as assessed by RH-PAT is related to OCT-derived TCFA on coronary plaque in patients with acute coronary syndromes (ACS).
Methods
Subjects
Seventy four patients with angiographically proven ACS (luminal stenosis of at least 50%) who underwent both OCT examinations before balloon dilatation or coronary stent implantation and RH-PAT examinations before discharge were enrolled in this study from July 2010 to April 2012. Participants were given diagnosis of unstable angina pectoris (UA), non-ST-segment elevation acute myocardial infarction (NSTEMI), or ST-segment elevation acute myocardial infarction (STEMI). UA was defined as typical angina at rest, accelerated angina or new onset angina according to Braunwald’s class I–III, with no evidence of myocardial necrosis. Myocardial infarction was diagnosed in accordance with the “Universal Definition of Myocardial Infarction”.10 Myocardial necrosis was defined as elevated levels of troponin T or I, or creatine kinase-MB fraction, according to hospital’s reference ranges. ST-segment elevation was defined as an elevation of ≥0.2 mV in ≥2 contiguous leads on electrocardiography. The OCT procedure could not be performed in patients with cardiogenic shock, clinically significant left main trunk disease, severe tortuous or calcified coronary artery, or renal insufficiency with a serum creatinine level of >2.0 mg/dL.
During the same period, patients matched for age, sex, and the occurrence of hypertension and diabetes mellitus, who were hospitalized to undergo surgery for osteoarthritis and had never received diagnosis of coronary artery disease (CAD) were enrolled as non-CAD patients (n=15). We used the group matching method to select non-CAD patients. Only noninvasive assessments were performed in non-CAD patients, including coronary risk factors and RH-PAT.
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Yokohama City University Institutional Review Board and was conducted in accordance with the guidelines of the ethics committee of our institution. Written informed consent was obtained from each patient before enrollment. This study is registered at ClinicalTrials.gov protocol registration system (identification # NCT01578616).
Diagnostic coronary angiography
Coronary angiography was performed using a 4–6 French (Fr) Judkins catheter via a radial or femoral artery approach. CAD was defined as the presence of >50% stenosis in at least one major coronary artery. The culprit lesion for each patient was identified on the basis of coronary angiography, electrocardiography, transthoracic echocardiography, and OCT by at least two experienced cardiologists.
OCT image acquisition
OCT studies were performed using a 6-Fr guiding catheter to observe the culprit lesion before percutaneous coronary intervention (PCI), and the technique was described previously.11 Briefly, a 0.016-inch OCT image wire (ImageWire, LightLab Imaging, Westford, MA, USA) was positioned at the distal end of the culprit lesion through a 3-Fr occlusion balloon catheter (Helios, Goodman, Nagoya, Japan). The occlusion balloon catheter was inflated to 0.4–0.6 atmospheres at a proximal site of the culprit lesion, and lactated Ringer’s solution was infused into the coronary artery from the distal tip of the occlusion balloon catheter. The solution was infused at a rate of 2.5–4.5 mL/s with the use of an injector pump. To observe the proximal lesion, we used a non-occlusive technique.12
OCT image analysis
Acquired images were analyzed by two experienced investigators who were blinded to all clinical information. In case of disagreement, the image was reviewed by a third investigator. Plaque disruption, calcification, and thrombus were noted. Calcification was defined as a signal-poor or heterogeneous region with sharply delineated borders. Intracoronary thrombus was identified as a mass protruding into the lumen from the vessel wall. The OCT images were analyzed using previously validated criteria for plaque characterization, and fibrous-cap thickness was determined as previously described.13 Intra- and inter-observer differences for the measurements of fibrous-cap thickness were low (6±20 and 11±22 µm), and the correlation coefficients were high for repeated measurements by the same observer (R=0.97, P<0.001), as well as for measurements by two different observers (R=0.90, P<0.001). TCFA was defined as the presence of a thin fibrous cap (<70 µm) overlying a lipid-rich plaque (a lipid arc >90°).
RH-PAT examinations
RH-PAT studies were performed in the fasting state early in the morning before receiving any medications. Therefore, endothelial function was not assessed before coronary plaque assessment, but after. Reactive hyperemic changes in finger pulse volume were recorded using an Endo-PAT2000 device (Itamar Medical, Caesarea, Israel) before discharge. The principle of PAT has been described previously.14 Briefly, the PAT signals were recorded by tonometry probes placed on one finger of each hand. The cuff was inflated to 60 mmHg above the systolic pressure or 200 mmHg for 5 minutes. After a 5-minute equilibration period, the cuff was deflated to induce RH.
The RH-PAT data were automatically analyzed online in an operator-independent manner (Endo-PAT2000 software, version 3.0.4). Logarithmic transformation of reactive hyperemia index (Ln_RHI) was used in analyses, as previously reported.5,15
Blood tests
Venous blood samples were obtained at admission for measurement of hemoglobin A1c, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, creatinine, and high-sensitivity C-reactive protein levels. The estimated glomerular filtration rate was determined using the prediction equation proposed by the Japanese Society of Nephrology and was based on that described in the Modification of Diet in Renal Disease Study.16
Statistical analyses
All analyses were performed using PASW 18 software for Windows (SPSS Inc., Tokyo, Japan). Baseline characteristics were compared after stratification according to TCFA. Patients with Ln_RHI ≤0.510 (median) were classified as the low Ln_RHI group. This cut-off point is equal to the historical threshold, which was reported by Bonetti et al, and we defined endothelial dysfunction as Ln_RHI ≤0.510.6 Continuous variables are expressed as means ± SD or medians (IQR). Categorical variables are presented as frequencies and percentages. Student’s t-test or the Mann–Whitney test was used to compare continuous variables, as appropriate. Comparisons between categorical variables were evaluated using the chi-squared test or Fisher’s exact test. Spearman correlation was used to evaluate possible associations between Ln_RHI and fibrous-cap thickness.
Multivariate logistic regression analysis was used to evaluate the independent association between endothelial function and TCFA, and the Hosmer–Lemeshow goodness-of-fit statistic was calculated. A two-tailed P-value of <0.05 was considered to indicate statistical significance.
Results
Baseline characteristics
Baseline characteristics of the study population are presented in Table 1. Mean age was 67.0±10.6 years, and 75.7% of ACS patients was male. Sixteen patients presented with UA, 29 NSTEMI, and 29 STEMI. TCFA was observed in 44 patients. There were no significant differences in baseline characteristics between ACS patients with and without TCFA (Table 1). RHI value was not affected by time between onset and RH-PAT examination or time between OCT measurement and RH-PAT examination (data not shown).
Table 1.
Baseline clinical characteristics
| Characteristics | Non-CAD patients | All ACS | P-value* | ACS patients | P-value^ | |
|---|---|---|---|---|---|---|
| Thin-cap fibroatheroma | ||||||
| No | Yes | |||||
| n=15 | n=74 | n=30 | n=44 | |||
| Age, years | 68.3±12.0 | 67.0±10.6 | 0.68 | 64.9±10.4 | 68.5±10.5 | 0.16 |
| Male | 10 (66.7%) | 56 (75.7%) | 0.52 | 25 (83.3%) | 31 (70.5%) | 0.27 |
| Body mass index, kg/m2 | 25.8±6.3 | 23.8±3.2 | 0.07 | 24.6±2.7 | 23.3±3.5 | 0.09 |
| Current smoking | 3 (20.0%) | 33 (44.6%) | 0.09 | 14 (46.7%) | 19 (43.2%) | 0.82 |
| Hypertension | 10 (66.7%) | 51 (68.9%) | >0.99 | 23 (76.7%) | 28 (63.6%) | 0.31 |
| Diabetes mellitus | 5 (33.3%) | 25 (33.8%) | >0.99 | 10 (33.3%) | 15 (34.1%) | >0.99 |
| Dyslipidemia | 8 (53.3%) | 57 (78.6%) | 0.10 | 22 (73.3%) | 35 (79.5%) | 0.58 |
| Clinical diagnosis | 0.26 | |||||
| Unstable angina pectoris | 16 (21.6%) | 9 (30.0%) | 7 (15.9%) | |||
| NSTEMI | 29 (39.2%) | 12 (40.0%) | 17 (38.6%) | |||
| STEMI | 29 (39.2%) | 9 (30.0%) | 20 (45.5%) | |||
| Culprit coronary artery | 0.24 | |||||
| Left anterior descending | 41 (55.4%) | 20 (66.7%) | 21 (47.7%) | |||
| Left circumflex | 14 (18.9%) | 5 (16.7%) | 9 (20.5%) | |||
| Right | 19 (25.7%) | 5 (16.7%) | 14 (31.8%) | |||
| Stent | 0.82 | |||||
| Any drug-eluting stent | 43 (58.1%) | 18 (60.0%) | 25 (56.8%) | |||
| Only bare-metal stent | 31 (41.9%) | 12 (40.0%) | 19 (43.2%) | |||
| Left ventricular ejection fraction, % | 59.7±10.4 | 57.3±13.4 | 60.7±7.3 | 0.18 | ||
| eGFR, mL/min/1.73 m2 | 70.6±18.7 | 68.7±21.1 | 0.74 | 72.9±28.9 | 65.8±13.0 | 0.16 |
| HMG-CoA RIs before admission | 16 (21.6%) | 7 (23.3%) | 9 (20.5%) | 0.78 | ||
| Medication at Endo-PAT examination | ||||||
| HMG-CoA RIs | 4 (26.7%) | 73 (98.6%) | <0.001 | 29 (96.7%) | 44 (100%) | 0.41 |
| Beta blocker | 3 (20.0%) | 48 (64.9%) | 0.003 | 19 (63.3%) | 29 (65.9%) | >0.99 |
| ACE-I or ARB | 9 (60.0%) | 56 (75.7%) | 0.22 | 24 (80.0%) | 32 (72.7%) | 0.59 |
| LDL cholesterol, mg/dL | 103.1±32.0 | 129.0±38.2 | 0.02 | 121.8±28.4 | 133.9±43.3 | 0.18 |
| HDL cholesterol, mg/dL | 57.5±14.3 | 52.7±16.4 | 0.29 | 52.7±19.2 | 52.7±14.4 | 0.99 |
| Triglycerides, mg/dL | 97 (79–158) | 127 (83–173) | 0.20 | 106 (69–187) | 134 (97–171) | 0.14 |
| Hemoglobin A1c, % | 6.0±1.0 | 6.2±0.7 | 0.19 | 6.3±0.8 | 6.2±0.6 | 0.54 |
| High-sensitivity CRP, mg/L | 1.11 (0.46–1.70) | 1.45 (0.70–2.90) | 0.30 | 1.13 (0.49–2.72) | 1.56 (0.73–3.02) | 0.34 |
Notes: Data are means ± SD, median values (25th–75th percentile range) or n (%).
P-value represent comparisons of ACS patients and control, and
P-value represent comparisons of ACS patients with TCFA vs ACS patients without TCFA and were calculated by the unpaired t-test, Mann–Whitney U test, or Fisher’s exact test.
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitors; ACS, acute coronary syndromes; ARB, angiotensin II receptor blockers; CAD, coronary artery disease; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HMG-CoA RIs, hydroxymethylglutaryl-CoA reductase inhibitors; LDL, low-density lipoprotein; NSTEMI, non-ST-segment elevation acute myocardial infarction; STEMI, ST-segment elevation acute myocardial infarction; TCFA, thin-cap fibroatheroma.
OCT findings in high and low Ln_RHI groups
The high and low Ln_RHI groups did not differ with respect to the percentages of patients with thrombus, plaque rupture, and calcification (Table 2). The frequency of STEMI was numerically higher in the low Ln_RHI group compared with the high Ln_RHI group, but did not reach statistical significance. Importantly, the fibrous-cap thickness in patients with low Ln_RHI was significantly lower than those with high Ln_RHI (63.0 [57.0–68.5], 70.0 [55.0–103.5], P=0.04). In addition, the frequency of TCFA was about 1.8-fold higher in the low Ln_RHI group (P=0.009).
Table 2.
Results of optical coherence tomography
| Endothelial function | P-value | ||
|---|---|---|---|
| High | Low | ||
| n=37 | n=37 | ||
| STEMI | 12 (41.4%) | 17 (58.6%) | 0.34 |
| Plaque rupture | 17 (45.9%) | 13 (35.1%) | 0.48 |
| Thrombus | 21 (56.8%) | 24 (64.9%) | 0.63 |
| Calcification | 8 (21.6%) | 9 (24.3%) | >0.99 |
| Fibrous-cap thickness, µm | 70.0 (55.0–103.5) | 63.0 (57.0–68.5) | 0.036 |
| Thin-cap fibroatheroma | 16 (43.2%) | 28 (75.7%) | 0.009 |
Notes: Data are median values (25th–75th percentile range) or n (%). Significance was assessed by the unpaired t-test, the Mann–Whitney U test or Fisher’s exact test. Patients were divided by clinical diagnosis and median value of Ln_RHI (0.510).
Abbreviations: Ln_RHI, logarithmic value of reactive hyperemia index; STEMI, ST-segment elevation acute myocardial infarction.
Association between Ln_RHI level and fibrous-cap thickness
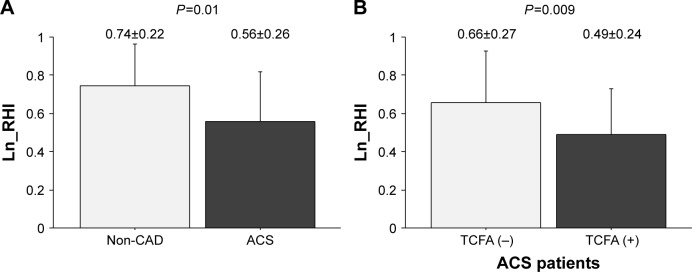
Ln_RHI was significantly lower in ACS patients than in non-CAD patients (0.56±0.26, 0.74±0.22, P=0.01) (Figure 1A). Ln_RHI levels in ACS patients without TCFA were intermediate between those of non-CAD patients and ACS patients with TCFA, and the difference in Ln_RHI levels between ACS patients with TCFA and without TCFA was statistically significant (0.50±0.24, 0.65±0.26, P=0.01) (Figure 1B). Although the number of patients in each group was very small, the relation of Ln_RHI with the presence of TCFA seemed to be similar to that with UA, NSTEMI, and STEMI (UA without TCFA 0.70±0.30, UA with TCFA 0.49±0.22, NSTEMI without TCFA 0.60±0.27, NSTEMI with TCFA 0.50±0.26, STEMI without TCFA 0.67±0.22, and STEMI with TCFA 0.50±0.25).
Figure 1.
Clinical presentation, plaque vulnerability, and Ln_RHI.
Notes: The bars represent mean Ln_RHI in each group. The T-bars indicate standard deviation. Ln_RHI was significantly decreased in patients with ACS (A), especially in those with TCFA (B).
Abbreviations: ACS, acute coronary syndromes; CAD, coronary artery disease; Ln_RHI, logarithmic value of reactive hyperemia index; TCFA, thin-cap fibroatheroma.
Ln_RHI is an independent factor associated with TCFA
Simple logistic regression analysis demonstrated that Ln_RHI was significantly associated with the presence of TCFA (Table 3), and even after adjustment for traditional risk factors and clinical diagnosis of STEMI, Ln_RHI was independently related to TCFA (OR per 0.1 increase in Ln_RHI: 0.78 [95% CI: 0.62–0.98], P=0.03) (Table 3).
Table 3.
Logistic regression analysis of factors related to thin-cap fibroatheroma in patients with acute coronary syndromes
| OR | 95% CI | P-value | |
|---|---|---|---|
| Univariate | |||
| Ln_RHI (per 0.1) | 0.79 | 0.65–0.96 | 0.017 |
| Multivariate model with age, gender, hypertension, diabetes, LDL and HDL cholesterol, smoking, and STEMI | |||
| Ln_RHI (per 0.1) | 0.78 | 0.62–0.98 | 0.029 |
Notes: Hosmer–Lemeshow test goodness-of-fit; chi-squared and P are 10.4 and 0.24, respectively.
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; Ln_RHI, logarithmic value of reactive hyperemia index; STEMI, ST-segment elevation acute myocardial infarction.
Discussion
Our study demonstrated that endothelial function as evaluated by Ln_RHI was significantly impaired in ACS patients, especially in those with TCFA. Ln_RHI positively correlated with fibrous-cap thickness and was significantly associated with the presence of vulnerable plaques with thin fibrous caps in ACS patients. This is the first study to evaluate the association between fibrous-cap thickness as measured by OCT and endothelial function assessed by RH-PAT in patients with ACS. These findings suggest that RH-PAT might be a clinically useful means in identifying high-risk patients with advanced endothelial dysfunction and TCFA.
An atherosclerotic plaque prone to thrombosis and which can cause complications leading to coronary occlusion is termed a vulnerable plaque.17 For the purpose of predicting plaques which are at risk for precipitating thrombosis, the identification of vulnerable plaque has received considerable attention. In the last few years, OCT imaging has become available as a high-resolution, light-based imaging technique that can identify microstructures in atherosclerotic plaques.13 Using OCT, we can accurately measure fibrous-cap thickness in vivo. OCT provides a more precise delineation of thin fibrous caps than gray-scale intravascular ultrasound (IVUS).18
The pathological features of the most common type of vulnerable plaque include a large lipid pool inside the plaque, a thin fibrous cap, and increased macrophage infiltration within the cap, which results in the expression of proteolytic enzymes that weaken the fibrous cap and ultimately promote plaque disruption.19–21 The composition and vulnerability of plaque rather than the severity of associated stenosis are considered the most important determinants of thrombus-mediated ACS.22 Although the identification of vulnerable coronary plaques by integrated backscatter IVUS or virtual histology IVUS is clinically useful for predicting future cardiovascular events,23–25 a previous three-vessel IVUS study reported that the rate of complications, including coronary dissection and perforation was 1.6%.23 Therefore, clinical techniques that can noninvasively evaluate plaque vulnerability are desired.
The vascular endothelium plays a critical role in vascular homeostasis. Several studies have reported that endothelial dysfunction is a significant risk factor for future cardiovascular events.7,26,27 Endothelial cells regulate vascular tone, platelet activity, coagulation factors, vascular inflammation, and cell migration and proliferation.1 Impaired endothelial function is associated with an increased inflammatory response,1 thrombogenicity,28 and enhanced local expression of matrix metalloproteinases.3 Thus, it is suggested that endothelial function is an integrated parameter of all atherogenic and atheroprotective factors.
Digital RH-PAT is a simple noninvasive method for the assessment of peripheral endothelial function,4,5,14,29 and can be used to predict coronary endothelial function.6 The Framingham Heart Study reported that RHI levels are related to various cardiovascular risk factors, particularly diabetes mellitus, body mass index, hypercholesterolemia, and smoking.5 Rubinshtein et al demonstrated that assess ment of peripheral endothelial function on the basis of RHI in addition to the Framingham risk score more accurately predicts cardiovascular events than the Framingham risk score alone.30 In the present study, we demonstrated that Ln_RHI was significantly associated with vulnerable coronary plaques possessing a thin fibrous cap independently of coronary risk factors. Although OCT has several advantages, including high resolution of the order of few microns (10–15 µm), it cannot be widely applied due to the invasive nature. On the other hand, Endo-PAT is a noninvasive method to evaluate peripheral endothelial function, and thus, it can be clinically useful in the risk classification. Our results support the independent prognostic utility of RHI for cardiovascular events.
Our study had several limitations. First, this was a preliminary study of a small group of subjects, reducing the power of the statistical analyses. Our results could be derived from the type I error. Although we used the cut-off value of Ln_RHI 0.510, the relevant cut-off value for “endothelial dysfunction” may be different in another setting. Second, only culprit lesions were evaluated by OCT, not all three coronary arteries. Third, thrombus may affect the analysis of the plaque behind it, in particular making it difficult to detect the ruptured site. In the current study, patients with low Ln_RHI had high frequency of thrombus and low frequency of ruptured plaque. Plaque rupture might be underestimated by thrombus. Fourth, RH-PAT examinations in this study were performed after PCI. Further studies are needed to determine the predictive value of RHI for TCFA detected on three-vessel OCT. Fifth, the long-term prognostic values of RHI and TCFA were not assessed. Sixth, in this study, medications, including statins, beta-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers were more frequently prescribed in ACS patients than in the control group. In general, these drugs are effective to improve endothelial function. Seventh, in this study, we enrolled matched patients without CAD who underwent surgery for osteoarthritis as non-CAD patients. As we previously reported,15 even if subjects who undergo coronary angiography do not have epicardial CAD, they frequently have non-obstructive CAD. Endothelial function is similarly attenuated in those with non-obstructive CAD compared with those with obstructive CADs. If we routinely perform detailed and complicated examinations during coronary angiography to evaluate coronary spasm and coronary microvascular function in all subjects, we could choose a relevant control group, which consists of subjects without any CADs. As we recently reported,31 RHI 2.10 (Ln_RHI 0.742), which is equal to the mean level of Ln_RHI in the non-CAD patients of the current study, is considered as the cut-off value for normal endothelial function and thus, our control group could be relevant. Eighth, all patients who were enrolled in this study had normal or almost normal renal function. Renal dysfunction can decrease endothelial function, thus, in patients with advanced renal dysfunction, results might be different.
Conclusion
Our results showed that advanced endothelial dysfunction, which was assessed after OCT examination, was significantly associated with the presence of TCFA in ACS patients. However, further prospective studies with large-scale subjects are needed to establish the predictive value of RHI for vulnerable plaques with TCFA.
Footnotes
Data sharing statement
The data sets used and analyzed during this study are available from the corresponding author on request.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Widlansky ME, Gokce N, Keaney JF, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42(7):1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 3.Rajavashisth TB, Liao JK, Galis ZS, et al. Inflammatory cytokines and oxidized low density lipoproteins increase endothelial cell expression of membrane type 1-matrix metalloproteinase. J Biol Chem. 1999;274(17):11924–11929. doi: 10.1074/jbc.274.17.11924. [DOI] [PubMed] [Google Scholar]
- 4.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146(1):168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 5.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham heart study. Circulation. 2008;117(19):2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44(11):2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzawa Y, Sugiyama S, Sumida H, et al. Peripheral endothelial function and cardiovascular events in high-risk patients. J Am Heart Assoc. 2013;2(6):e000426. doi: 10.1161/JAHA.113.000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92(3):657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 9.Yabushita H, Bouma BE, Houser SL, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106(13):1640–1645. doi: 10.1161/01.cir.0000029927.92825.f6. [DOI] [PubMed] [Google Scholar]
- 10.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 11.Kubo T, Imanishi T, Takarada S, et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50(10):933–939. doi: 10.1016/j.jacc.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 12.Kitabata H, Tanaka A, Kubo T, et al. Relation of microchannel structure identified by optical coherence tomography to plaque vulnerability in patients with coronary artery disease. Am J Cardiol. 2010;105(12):1673–1678. doi: 10.1016/j.amjcard.2010.01.346. [DOI] [PubMed] [Google Scholar]
- 13.Jang IK, Tearney GJ, MacNeill B, et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111(12):1551–1555. doi: 10.1161/01.CIR.0000159354.43778.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonetti PO, Barsness GW, Keelan PC, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41(10):1761–1768. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 15.Matsuzawa Y, Sugiyama S, Sugamura K, et al. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55(16):1688–1696. doi: 10.1016/j.jacc.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 16.Imai E, Matsuo S, Makino H, et al. Chronic kidney disease Japan cohort (CKD-JAC) study: design and methods. Hypertens Res. 2008;31(6):1101–1107. doi: 10.1291/hypres.31.1101. [DOI] [PubMed] [Google Scholar]
- 17.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108(14):1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki M, Bouma BE, Bressner J, et al. Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. J Am Coll Cardiol. 2006;48(1):81–88. doi: 10.1016/j.jacc.2006.02.062. [DOI] [PubMed] [Google Scholar]
- 19.Farb A, Burke AP, Tang AL, et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93(7):1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 20.Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349(24):2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 21.Kolodgie FD, Burke AP, Farb A, et al. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol. 2001;16(5):285–292. doi: 10.1097/00001573-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. 2003;41(4 Suppl S):15S–22S. doi: 10.1016/s0735-1097(02)02834-6. [DOI] [PubMed] [Google Scholar]
- 23.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 24.Sano K, Kawasaki M, Ishihara Y, et al. Assessment of vulnerable plaques causing acute coronary syndrome using integrated backscatter intravascular ultrasound. J Am Coll Cardiol. 2006;47(4):734–741. doi: 10.1016/j.jacc.2005.09.061. [DOI] [PubMed] [Google Scholar]
- 25.Amano T, Matsubara T, Uetani T, et al. Lipid-rich plaques predict non-target-lesion ischemic events in patients undergoing percutaneous coronary intervention. Circ J. 2011;75(1):157–166. doi: 10.1253/circj.cj-10-0612. [DOI] [PubMed] [Google Scholar]
- 26.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 27.Shimbo D, Grahame-Clarke C, Miyake Y, et al. The association between endothelial dysfunction and cardiovascular outcomes in a population-based multi-ethnic cohort. Atherosclerosis. 2007;192(1):197–203. doi: 10.1016/j.atherosclerosis.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Diodati JG, Dakak N, Gilligan DM, Quyyumi AA. Effect of atherosclerosis on endothelium-dependent inhibition of platelet activation in humans. Circulation. 1998;98(1):17–24. doi: 10.1161/01.cir.98.1.17. [DOI] [PubMed] [Google Scholar]
- 29.Matsuzawa Y, Sugiyama S, Sugamura K, et al. Successful diet and exercise therapy as evaluated on self-assessment score significantly improves endothelial function in metabolic syndrome patients. Circ J. 2013;77(11):2807–2815. doi: 10.1253/circj.cj-13-0549. [DOI] [PubMed] [Google Scholar]
- 30.Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31(9):1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka A, Tomiyama H, Maruhashi T, et al. Physiological diagnostic criteria for vascular failure. Hypertension. 2018;72:1060–1071. doi: 10.1161/HYPERTENSIONAHA.118.11554. [DOI] [PubMed] [Google Scholar]