Abstract
Ubiquitin is covalently attached to substrate proteins in the form of a single ubiquitin moiety or polyubiquitin chains and has been generally linked to protein degradation, however, distinct types of ubiquitin linkages are also used to control other critical cellular processes like cell signaling. Over forty mammalian G protein-coupled receptors (GPCRs) have been reported to be ubiquitinated, but despite the diverse and rich complexity of GPCR signaling, ubiquitin has been largely ascribed to receptor degradation. Indeed, GPCR ubiquitination targets the receptors for degradation by lysosome, which is mediated by the ESCRT machinery, and the proteasome. This has led to the view that ubiquitin and ESCRTs primarily function as the signal to target GPCRs for destruction. Contrary to this conventional view, studies indicate that ubiquitination of certain GPCRs and canonical ubiquitin-binding ESCRTs are not required for receptor degradation and revealed that diverse and complex pathways exist to regulate endo-lysosomal sorting of GPCRs. In other studies, GPCR ubiquitination has been shown to drive signaling and not receptor degradation and further revealed novel insight into the mechanisms by which GPCRs trigger the activity of the ubiquitination machinery. Here, we discuss the diverse pathways by which ubiquitin controls GPCR endo-lysosomal sorting and beyond.
Keywords: ALIX, ARRDC3, β-arrestins, ESCRT, Exosomes, E3 ligases, G protein, HRS, NEDD4, PAR1
Graphical Abstract
Ubiquitination of GPCRs and endocytic adaptor proteins facilitates GPCR trafficking to lysosomes and. In addition, ubiquitination of GPCRs and GPCR effectors can mediate the activation of specific signaling pathways. GPCR ubiquitination is also critical for targeting some GPCRs for degradation by proteasomes. This review highlights the multiple pathways by which ubiquitin controls GPCR endo-lysosomal sorting and signaling, which expand the function of ubiquitin in regulating GPCR biology.
1 |. Introduction
Ubiquitination governs a multitude of cellular process and is one of the most common forms of posttranslational protein modifications. Studies predict that all proteins are ubiquitinated at least once during their life-times [1,2]. While our knowledge of the role of phosphorylation in regulating GPCR biology is extensive, there is a limited understanding of the diverse pathways by which ubiquitination controls GPCR function including trafficking, subcellular localization, abundance and signaling. GPCRs are the largest family of cell surface receptors expressed in mammals and include over 800 members that control a multitude of physiological functions. GPCRs respond to a diverse array of extracellular stimuli and signal primarily via heterotrimeric G proteins to elicit cellular responses. Given the abundance and pleiotropic functions of GPCRs, it is not surprising that dysregulation of GPCR signaling has been implicated in numerous diseases and has made this receptor class the largest target for FDA-approved drugs [3].
The first characterized role for ubiquitin as a GPCR endolysosomal sorting signal was discovered in the yeast Saccharomyces cerevisiae. The yeast Ste2 pheromone receptor requires direct ubiquitination for internalization from the plasma membrane [4,5]. Furthermore, discoveries in S. cerevisiae identified the role for ubiquitin in the sorting of Ste3 at the limiting membrane of the vacuole [6]. These discoveries paralleled investigations into the ubiquitin-dependent vacuolar trafficking of other transmembrane proteins, resulting in the discovery and classification of the ESCRT complexes [7–9] as well as the diversity of ubiquitin-binding adaptor proteins involved in early and late endocytosis [10–12]. In addition, ubiquitin promotes endocytic adaptor scaffolding during the internalization of GPCRs [13,14]. These studies demonstrated the critical role of ubiquitin in GPCR regulation, and stimulated the study of ubiquitin in the endocytic sorting and lysosomal degradation of human GPCRs as discussed below.
Ubiquitination likely offers novel and diverse mechanisms for regulation of GPCR biology. Therefore, a thorough understanding of the mechanisms by which key regulators and mediators of ubiquitination regulate GPCR signaling and trafficking is needed for defining dysregulated mechanisms in disease and identifying new targets for drug development for the treatment of human diseases. While ubiquitination of GPCRs has been largely ascribed to lysosomal or proteasomal degradation, emerging studies provide new insight into the multi-faceted and diverse functions of ubiquitin in regulating GPCR endo-lysosomal sorting and signaling. Here, we discuss the various GPCR endo-lysosomal sorting pathways that are conveyed through direct and indirect GPCR ubiquitination as well as the role of ubiquitination in regulating key endocytic adaptor proteins that facilitate GPCR endo-lysosomal sorting and new functions of ubiquitin in regulating GPCR biology.
2 |. Direct ubiquitination of GPCRs and endo-lysosomal sorting
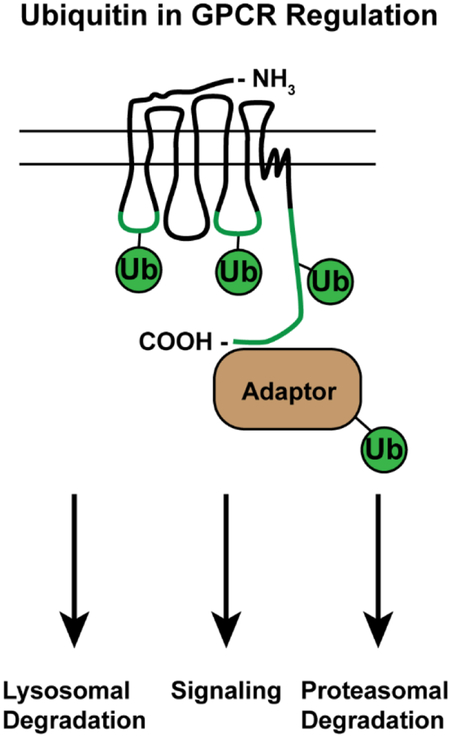
One of the most well-studied signals that direct GPCRs through the endo-lysosomal system is the covalent attachment of ubiquitin. Ubiquitin, a 76 amino acid protein, is covalently attached to substrate proteins by the sequential actions of E1, E2 and E3 ubiquitin ligases, where the E3 ligase confers substrate specificity and controls the type and length of specific ubiquitin chains. Ubiquitin is removed by ubiquitin-specific proteases (USPs) or deubiquitinating enzymes (DUBs). GPCRs can be ubiquitinated both on intracellular loops and on the C-terminal tail, and the ubiquitin modification can take the form of multiple mono-ubiquitinated lysine residues or as chains of polyubiquitin uniquely configured, a natural variation and key feature of protein ubiquitination. GPCR ubiquitination is a reversible posttranslational mediated by specific DUBs. Once ubiquitinated and trafficked to the early endosome, GPCRs can interact with the Endosomal Sorting Complexes Required for Transport (ESCRTs) machinery, which convey ubiquitinated GPCRs into intraluminal vesicles of multivesicular bodies (MVBs). The ESCRT-0, -I and -II complexes contain well-characterized binding proteins that sequentially directly interact with ubiquitinated cargo [15]. Thus, the ubiquitination state of the GPCR generally determines whether or not it is committed to a degradation pathway, whereas deubiquitinating enzymes may play a key role in maintaining populations of GPCRs within the specific subcellular compartments to promote other cellular functions. In contrast, there are also examples of receptors that traffic to lysosomes independently of receptor ubiquitination. Endo-lysosomal sorting of GPCRs such as the protease-activated receptor-1 (PAR1) and P2Y1 receptor is directed by a short peptide tyrosine-based motif (YPX3L) that is bound by the adaptor protein ALIX [16,17]. ALIX couples directly to the ESCRT-III complex, allowing these receptors to bypass the ubiquitin-binding ESCRTs and sort into intraluminal vesicles of MVBs. GPCR-associated sorting protein 1 (GASP-1)-mediated lysosomal sorting of the δ-opioid receptor represents another ubiquitin-independent pathway for GPCR degradation [18]. Interestingly, the recovery of ubiquitin-independent GPCRs like the δ-opioid receptor from the limiting membrane of the late endosome to the plasma membrane has also been observed [19], suggesting that these lysosomal pathways are also dynamically regulated. This section will discuss the most recent findings regarding the factors that control GPCR sorting at the limiting membrane of late endosomes and lysosomal degradation.
Ubiquitin is covalently attached to cytoplasmic lysine residues of target substrates, and the ubiquitin signal that directs GPCRs to the lysosome occurs on distinct cytoplasmic regions (Figure 1). GPCRs contain three intracellular loops (ICLs) and a carboxyl-terminal tail that are exposed to the cytoplasm and are accessible by E3 ubiquitin ligases. Ubiquitination of the C-terminal tail is sufficient to mediate lysosomal sorting of the chemokine C-X-C motif receptor type 4 (CXCR4) [20], and the κ-opioid receptor [21]. More recently, agonist-induced ubiquitination of the γ-aminobutyric acid receptor (GABAB) B1 subunit C-terminus has been demonstrated in live cells using BRET [22]. In contrast, the V2 vasopressin receptor is ubiquitinated primarily in in ICL3 [23]. The prototypical ubiquitin-dependent cargo, the β2-adrenergic receptor (β2AR), is specifically ubiquitinated on lysine residues within both its ICL3 and C-terminal tail prior to lysosomal degradation [24]. Furthermore, ubiquitination of the μ-opioid receptor ICL1 is a rate-limiting step in the packaging of the receptor into intraluminal vesicles prior to uptake by MVBs [25]. Ubiquitin can be attached as a single ubiquitin moiety or first conjugated onto itself to form chains of polyubiquitin at seven lysine residues (K6, K11, K27, K29, K33, K48 and K63) and via direct attachment to its N-terminus. The mechanism of GPCR ubiquitination can be assessed using ubiquitin mutants that harbor point mutations to individual or multiple lysine residues. GPCRs like the Frizzled receptor FZ4R [26], melanocortin receptor MC2R [27] and CXCR4 [20] are monoubiquitinated, likely on multiple lysine residues thus giving the appearance of polyubiquitination. In contrast, PAR1 [28], κ-opioid receptor [21], and β2-adrenergic receptor [29] are modified specifically by K63-linked polyubiquitin. Interestingly, the dopamine D4 receptor is modified by ubiquitin on lysine residues via the canonical isopeptide bond as well as on serine and threonine residues through an ester linkage, a process that regulates proteasomal degradation of the receptor [30]. These studies highlight the diversity of receptor ubiquitination sites and the flexibility within the ESCRT complexes to mediate receptor lysosomal degradation.
Figure 1:
The location of GPCR endo-lysosomal sorting signals. Sites of ubiquitination required for lysosomal degradation sorting have been mapped to intracellular loop (ICL) 1 and 3, as well as the C-terminal tail of the GPCRs listed (green). In contrast, YPX3L motifs required for lysosomal sorting have been discovered in ICL-2 of the GPCRs listed (orange).
The timing and dynamics of GPCR ubiquitination are controlled by E3 ubiquitin ligases as well as deubiquitinating enzymes or DUBs (Figure 2). Association of GPCRs with DUBs has multiple functions, including rescue of ubiquitinated GPCRs from lysosomal degradation. This mechanism allows cells under prolonged ligand stimulation to restore GPCR surface expression, or to maintain populations of GPCRs in endosomal compartments for continued signaling. Notably, ubiquitin-specific protease-33 (USP33) and USP20 mediate cellular resensitization under long-term stimulation by direct the deubiquitination of β2AR [31] (Figure 2A). Furthermore, deubiquitination by USP8 prevents lysosomal degradation of the human Frizzled receptor FZR4, facilitating persistent Wnt signaling [26]. In contrast, DUBs can also facilitate the lysosomal sorting of GPCRs through the regulation of receptor ubiquitination state at late endosomes. Inhibition of the DUBs AMSH and UBPY inhibit the early-to-late endosomal sorting of protease-activated receptor 2 (PAR2) [32]. Similarly, the deubiquitinating enzyme USP14 is required for deubiquitination and lysosomal sorting of CXCR4 [33] (Figure 2B) and the GABAB(B1) subunit [22]. Here, USP14 appears to function as an adaptor protein that facilitates packaging of receptors into budding intraluminal vesicles of MVBs. These results demonstrate that the dynamic cycle of GPCR ubiquitination, rather than the ubiquitination state of the receptor, is also critical for endo-lysosomal receptor trafficking.
Figure 2:
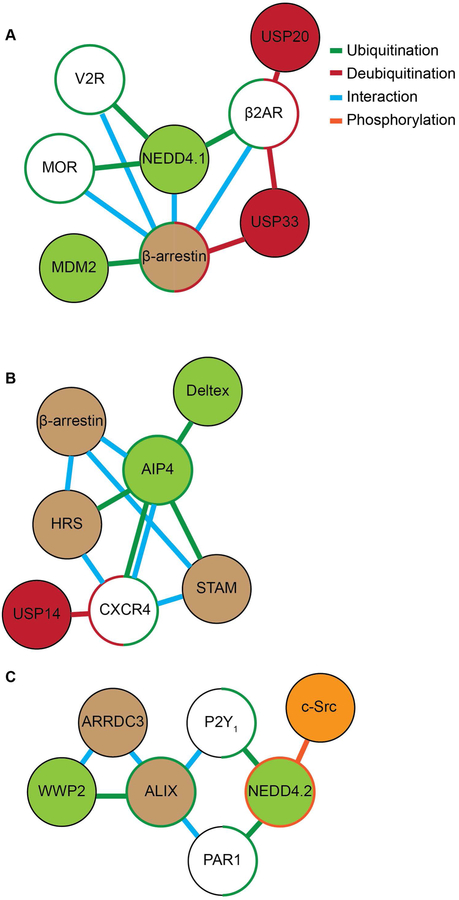
A map of known protein interactions and post-translational modifications involved in the regulation of GPCR lysosomal sorting and ubiquitin-dependent signaling. Direct ubiquitination is represented by green lines, red lines indicate deubiquitination, blue lines indicate direct interaction and orange lines indicate phosphorylation. A) The E3 ubiquitin ligase NEDD4.1 is recruited by β-arrestin to directly ubiquitinate β2-AR, MOR and V2R. β-arrestin is ubiquitinated by MDM2 and deubiquitinated by USP33. USP33 and USP20 deubiquitinate β2-AR. B) AIP4 directly interacts and ubiquitinates CXCR4, while USP14 mediates receptor deubiquitination. AIP4 is recruited by β-arrestin to ubiquitinate HRS and STAM during CXCR4 lysosomal trafficking. AIP4 is ubiquitinated and regulated by Deltex. C). The α-arrestin ARRDC3 recruits the NEDD4-family E3 ligase WWP2 to ubiquitinate and activate ALIX during the lysosomal sorting of PAR1 and P2Y1. c-Src phosphorylates and activates NEDD4.2 which ubiquitinates PAR1 and P2Y1, a process required for receptor signaling to the MAPK p38.
Ubiquitin is not the only endo-lysosomal sorting signal, and multiple GPCRs have been shown to harbor other sorting sequences that facilitate binding to late endosomal sorting adaptors, this is best characterized for PAR1. Although PAR1 is ubiquitinated upon agonist stimulation [28,34], receptor ubiquitination is not required for endo-lysosomal sorting. Instead, PAR1 sorting from early endosomes to late endosomes requires a YXXφ (where X is any amino acid and φ can be any bulky hydrophobic amino acid) within its C-terminal tail which is bound by the heterotetrameric adaptor protein complex-3 (AP-3), a clathrin adaptor protein complex [35]. Once at the limiting membrane of the late endosome, PAR1 interacts with the adaptor protein ALG-2-interacting protein X (ALIX) via a highly conserved YPX3L motif localized within ICL2 [16] (Figure 2C). The ICL2 of purinergic receptor P2Y1 also harbors a conserved YPX3L motif that directs receptor sorted to lysosomes in an ALIX-dependent manner [17] (Figure 1). ALIX directs receptors into budding intraluminal vesicles and couples YPX3L-motif GPCRs to the ESCRT-III complex. Similarly, lysosomal sorting of the δ-opioid receptor [36] and the D2 dopamine receptor [37] is mediated by the adaptor protein GASP-1, which couples these proteins to the ESCRT-0 complex independent of receptor ubiquitin. Recent findings have demonstrated that the δ-opioid receptor can recycle from the limiting membrane of the late endosome [19]. Interestingly, ALIX and the TIP47/Rab9 retrieval complex facilitate sorting of δ-opioid receptor to the trans-golgi network [19], where it can be re-sorted to the plasma membrane. These findings demonstrate that ALIX, GASP-1 and potentially other late endosomal adaptor proteins can mediate the endo-lysosomal trafficking, in some cases independent of ubiquitin and certain canonical ESCRT components, expanding the diversity of lysosomal sorting mechanisms for GPCRs beyond the direct ubiquitination and canonical ESCRTs.
3 |. Indirect ubiquitin-mediated GPCR endo-lysosomal sorting via modulation of endocytic adaptor proteins
In addition to direct ubiquitination of GPCRs, targeting of receptors to the endo-lysosomal pathway is controlled by ubiquitination of various endocytic adaptor proteins. β-arrestins are multi-functional adaptor proteins that directly regulate GPCR uncoupling from heterotrimeric G proteins, internalization from the plasma membrane and can facilitate a second wave of signaling in various subcellular compartments. β-arrestins are comprised of two major N- and C-domains, which consist primarily of β-sheets, an embedded polar core and an extended cytoplasmic tail that contains recognition motifs for clathrin and the AP-2 subunit β-adaptin and is rapidly recruited to agonist-activated GPCRs at the plasma membrane. β-arrestins engage activated GPCRs through interaction with a phosphate sensor mediated by the polar core and activation sensor conferred most notably by the finger loop that is exposed upon interaction with agonist-bound receptor [38,39]. While the precise conformation of the finger loop bound to the GPCR is not known, arrestin interacts with a highly conserved binding crevice of the GPCR that is similar to the region bound by the Gα subunit C-terminus [40]. Similar to GPCRs, posttranslational modifications of β-arrestins with ubiquitin is critical for regulating their function in controlling GPCR trafficking.
Ubiquitination of β-arrestin was shown to occur in response to β2AR stimulation and is mediated by the E3 ligase mouse double minute-2 (MDM2), and important for stabilizing the β-arrestin/β2AR complex (Figure 2A). The increased stability of the β-arrestin/β2AR interaction facilitates β2AR internalization by increasing interaction with components of the endocytic machinery [41,42]. Ubiquitination of β-arrestins has also been implicated in facilitating GPCR-induced mitogen-activated protein kinase (MAPK) signaling [41]. While MDM2 is important for stabilizing β2-arrestin/β2AR interaction and requires β-arrestin-2 for ubiquitination, MDM2 is selective in mediating β-arrestin ubiquitination and does not ubiquitinate β2AR. However, β-arrestin-2 is required for NEDD4–1 recruitment and ubiquitination of activated β2AR in response to stimulation by its full agonist isoproterenol, which is critical for β2AR endo-lysosomal sorting [43,44] (Figure 2A). Deubiquitination of β-arrestins is also regulated by agonist-activation of the β2AR and other GPCRs and is mediated by USP20 and USP33 and is important for β-arrestin-β2AR complex disassembly, lysosomal sorting and signaling [31,42] (Figure 2A). USP33 also mediates V2 vasopressin receptor-induced β-arrestin deubiquitination, endocytic trafficking and signaling using a similar process [42]. These studies indicate that ubiquitination of β-arrestins influences may aspects of GPCR function including endo-lysosomal sorting by enhancing E3 ligase recruitment and subsequent ubiquitination of the receptor.
In addition to ubiquitination of β-arrestins, agonist-activation of CXCR4 stimulates ubiquitination of ESCRT-0 components, which control CXCR4 endo-lysosomal sorting. In this case, the E3 ligase AIP4 (also known as ITCH), mediates ubiquitination of CXCR4 as well as the ESCRT-0 components, HRS and STAM, which have opposing functions in regulating CXCR4 endo-lysosomal sorting [45,46] (Figure 2B). Ubiquitination of HRS and STAM are regulated through a complex interplay mediated by β-arrestin-1 and AIP4 and is further controlled by the E3 ligase Deltex, which appears to regulate the activity of AIP4 [47,48] (Figure 2B). Besides CXCR4, surprising few other GPCRs have been shown to require both direct ubiquitination and components of the canonical ESCRT machinery for endo-lysosomal sorting and they include the δ-opioid receptor (DOR) [49] and PAR2 [50], but whether these GPCRs modify the ubiquitination status of ESCRT components to regulate their fate in the endo-lysosomal sorting pathway is not known.
As discussed above, a subset of GPCRs including PAR1 and P2Y1 are targeted to the endo-lysosomal sorting pathway independent of ubiquitination. While ubiquitination of these GPCRs is dispensable degradation, ubiquitination of the adaptor protein ALIX is important for shuttling the receptors through the endo-lysosomal sorting pathway. We found that ALIX activity is regulated by ubiquitination induced by agonist-activation of PAR1 [51]. Ubiquitination of ALIX is mediated by the E3 ligase WWP2 and results in ALIX dimerization and enhanced activity at sorting PAR1 to MVBs/lysosomes [51]. Importantly, the α-arrestin domain-containing protein-3 (ARRDC3) is responsible for activated PAR1-stimulated recruitment of the WWP2 to ALIX and subsequent ubiquitination (Figure 2C). We further showed that ARRDC3 is required for agonist-induced PAR1 interaction with ALIX and lysosomal degradation [51]. The P2Y1 receptor is also trafficked through a ubiquitin-independent and ALIX-dependent endo-lysosomal sorting pathway like PAR1 [17], suggesting that this pathway is broadly applicable to other GPCRs. Indeed, besides PAR1, seven other mammalian GPCRs were found to possess conserved ALIX YPXnL binding motifs within their second intracellular loop, including the adrenoreceptor α1B, angiotensin receptor AT2, galanin receptor GAL2, histamine receptor H2, neuropeptide FF receptor NPFF2, neuropeptide S receptor NPS, and purinergic receptor P2Y1 [16]. Moreover, ALIX has also been shown to bind to several other GPCRs that lack YPXnL motifs, including the vasopressin V2R and D1-like and D3 dopamine receptors, and regulate receptor trafficking [52,53]. These studies indicate that ALIX is likely to interact with receptors using divergent types of YPXnL motifs, including directly with ubiquitin. ALIX harbors ubiquitin binding sites and has been shown to bind directly with K63-linked ubiquitin and may recognize cargo via this mode of interaction [54]. Together, these studies illustrate that GPCR signaling can modulate the activity of the endo-lysosomal sorting machinery through ubiquitination of critical endocytic adaptor proteins and thereby has the capacity to regulate their own endo-lysosomal sorting efficiency.
4 |. Ubiquitin E3 ligases function in direct and indirect regulation of GPCR endo-lysosomal sorting
There are three classes of E3 ligases present in mammals including: really interesting new gene (RING), homologous to E6-APcarboxy terminus (HECT) and ring between ring (RHR). Of the six hundred E3 ligases, the majority are classified as RING E3 ligases, whereas the HECT E3 ligases are a considerably smaller class and includes twenty-eight members. The NEDD4 (neural precursor cell expressed, developmentally down-regulated-4) HECT domain-containing E3 ligases subfamily is comprised of nine members and are best known to mediate ubiquitination and endo-lysosomal sorting of GPCRs following ligand stimulation [55]. PAR2 is an exception and ubiquitinated by c-Cbl, a RING E3 ligase, in an agonist-dependent manner and required for endo-lysosomal sorting [32,56]. In contrast to NEDD4 E3 ligase, the RING E3 ligases have been largely reported to mediate constitutive ubiquitination of several GPCRs and appears to regulate a diverse array of trafficking processes [55]. Despite the large number of studies describing ligand-induced GPCR ubiquitination, our knowledge of the mechanisms by which GPCRs regulate E3 ligase activity to facilitate receptor endo-lysosomal sorting is limited. However, new developing studies now provide important insight into how GPCRs signaling initiates activation of E3 ligases to regulate receptor function.
The NEDD4 E3 ligases share a similar domain architecture including an N-terminal C2-domain, two to four WW domains connected by linker peptides and a catalytic HECT domain [57]. The C2 domain binds Ca2+, phospholipids and regulates NEDD4 cellular localization, whereas the WW domains mediate substrate recognition via P/LPxY motifs and phosphorylated serine/threonine residues. The activity of NEDD4 E3 ligases is regulated through release of an auto-inhibited state, which occurs through poorly understood allosteric mechanisms mediated by interactions with the C2 and WW domains [58]. Although numerous studies have implicated NEDD4 E3 ligase function in controlling GPCR endo-lysosomal sorting [43,46], it is not known how NEDD4 E3 ligases are released from auto-inhibition to increase HECT ubiquitin ligase activity following GPCR stimulation.
One mechanism by which NEDD4 E3 ligase catalytic activity is directed towards GPCRs occurs through recruitment to the receptor. Several studies have provided insight regarding mechanisms of NEDD4 E3 ligase recruitment and indicate that recruitment of NEDD4 E3 ligases to activated and phosphorylated GPCRs can occur either directly via recognition of receptor serine/threonine phosphorylated residues or indirectly via interaction with endocytic adaptor proteins [43,59]. This was first illustrated for the β2AR and CXCR4. β2AR activation results in phosphorylation and binding of β-arrestins, which are both required for agonist-induced β2AR ubiquitination [60]. In this case, the β-arrestin-2 isoform functions as the critical adaptor for NEDD4–1 recruitment to activated and phosphorylated β2AR [43]. Similarly, agonist-induced ubiquitination of the V2 vasopressin and μ-opioid receptor also requires β-arrestins [61]. Like other NEDD4 family members, the WW domains of NEDD4–1 mediate interaction with substrate proteins through recognition of PPXY motifs. However, PPXY motifs are absent in β-arrestins, indicating that β-arrestin-mediated recruitment of NEDD4 E3 ligases is mediated via distinct mechanisms. Despite the lack of PPXY motifs, β-arrestin-1 interacts with AIP4 via WW domains [47], whereas for NEDD4 interaction with β-arrestin-2 occurs via a non-WW domain region [43]. Unlike β-arrestins, the α-arrestin ARRDC3 contains an extended C-terminal region that harbors PPXY motifs that bind to WW domains of several NEDD4 E3 ligases and has been implicated in NEDD4–1 recruitment to activated β2AR [44,60]. Intriguingly, however, depletion of ARRDC3 by RNAi failed to perturb β2AR ubiquitination and receptor trafficking, suggesting that ARRDC3 may function secondarily by controlling β2AR endosomal retention [62]. In contrast to β2AR and μ-opioid receptor, the AIP4 is directly recruited to activated CXCR4 via WW domain interaction with phosphorylated serine residues in the C-tail domain of the receptor and occurs independent of β-arrestins [59]. These studies clearly indicate that different GPCRs use distinct direct and indirect mechanisms to recruit NEDD4 E3 ligases, making it difficult to predict how the catalytic activity of NEDD4 E3 ligases is specifically targeted towards certain GPCRs. However, to our knowledge virtually no studies have examined whether recruitment of NEDD4 E3 ligases to GPCRs modulates the catalytic activity of the enzymes or addressed how GPCRs “turn-on” E3 ligase activity, which is exquisitely regulated.
In addition to β2AR and CXCR4, PAR1 ubiquitination and function are well characterized. However, in contrast to these classic GPCRs, ubiquitination of PAR1 mediates p38 MAPK inflammatory signaling and not endo-lysosomal sorting. We previously showed that phosphorylation of PAR1 is required for agonist-stimulated ubiquitination [34]. In recent work, we further demonstrated that NEDD4–2 E3 ligase is rapidly recruited to and ubiquitinates PAR1 following ligand stimulation [28,63] (Figure 2C). However, it is not known if phosphorylation of PAR1 mediates recruitment of “active” NEDD4–2 to the receptor or enables PAR1 internalization and localization to endocytic vesicles containing “active” NEDD4–2 to facilitate substrate-enzyme interaction and ubiquitination.
It is also possible that GPCR signaling triggers NEDD4–2 catalytic activity and was recently examined for PAR1 and the P2Y1 receptor. Unexpectedly, we discovered that PAR1 stimulates c-Src-mediated tyrosine phosphorylation and activation of NEDD4–2, which promotes p38 inflammatory signaling [63] (Figure 2C). Intriguingly, c-Src is activated by PAR1 through a Gq and G12/13-dependent pathway. In this study, we also identified a unique phosphorylated tyrosine (Y)-485 residue located within the 2,3-linker peptide between WW domain 2 and 3 of NEDD4–2 in PAR1 agonist stimulated cells using mass spectrometry. Moreover, NEDD4–2 wildtype displayed a robust increase in catalytic activity following PAR1 stimulation, however, the NEDD4–2 Y-485 mutant exhibited impaired activity. In siRNA-mediated knockdown-rescue studies, expression of NEDD4–2 wildtype restored activated PAR1-induced p38 signaling, whereas the NEDD4–2 Y-485 mutant did not. The P2Y1 receptor also required c-Src and NEDD4–2 tyrosine phosphorylation for p38 MAPK signaling. These studies reveal a new mechanism by which signaling by GPCRs trigger NEDD4–2 catalytic activity to promote a second wave of signaling important for induction of inflammatory responses. Whether other GPCRs use a similar mechanism to activate NEDD4 E3 ligases to facilitate endo-lysosomal sorting or other functions is not known.
5 |. Alternative destinations for GPCRs – Autophagy, Exosome Packaging and the Proteasome
Recent discoveries have demonstrated that GPCRs can be trafficked to unexpected destinations, highlighting novel functions for GPCRs in regulating cellular homeostasis. The proteasome has emerged as a critical regulator of GPCR expression, and multiple findings have supported specific mechanisms for targeting the removal of GPCRs from endo-membranes prior to proteasomal degradation in the cytoplasm. Similarly, the relationship between GPCRs and autophagosomes has become an important research topic, as GPCRs are trafficked to autophagosomes in response to cell stress, while autophagosomes can also play an important role in regulating the recycling of some receptors. In addition, GPCR signaling can directly regulate autophagosome biogenesis, enhancing chemotaxis and inhibiting apoptosis. Finally, GPCRs have been discovered as critical cargoes shed from cells in exosomes and microparticles. These extracellular vesicles carry GPCRs and their signaling effectors to new destinations, initiating morphological changes in target cells. These findings highlight the diversity of GPCR regulation within the cell and highlight novel discoveries in GPCR trafficking and signaling.
Studies that have described the role of the proteasome in GPCR regulation have led to the discovery of novel functions for GPCRs in regulating neuronal development, hypertension and neuronal signaling. The proteasome is the critical final destination in the clearance of misfolded transmembrane proteins from the endoplasmic reticulum in a process known as ER-associated Degradation (ERAD) (Figure 3). Following de-novo synthesis, GPCRs like the δ-opioid receptor have been shown to be sorted directly from ER populations to the proteasome for degradation [64]. The E3 ligase parkin mediates ERAD of the orphan GPCR GPR37, regulating ER stress in Parkinson’s Disease [65]. GPR37 functions as an ER chaperone for the Wnt co-receptor LRP6 in neuronal progenitor cells [66], underscoring the importance of regulating GPR37 at the ER. In addition, a growing body of evidence suggests that GPCRs expressed on the cell surface can be directed to the proteasome in response to cellular signaling. The regulation of blood pressure in response to blood sodium levels involves the counter-regulatory dopamine and angiotensin II hormone signaling in smooth muscle cells. Once activated, the D5 dopamine receptor initiates the ubiquitination and proteasomal sorting of AT1 angiotensin receptors at the plasma membrane [67]. Dopamine signaling also modulates neuronal signaling, and the concentration of D4R dopamine receptors at the plasma membrane is tightly regulated by ubiquitination and proteasomal degradation [30]. In addition to ubiquitination on lysine residues, D4R is ubiquitinated on serines and threonines through an atypical ester bond linkage [30], a process critical for proteasomal targeting of D4R. These results open the possibility that other GPCRs may be regulated by both lysine isopeptide ubiquitination as well as ubiquitination of serine and threonine residues. These studies have added a new dimension to the mechanisms cells use ubiquitin to regulate GPCR signaling.
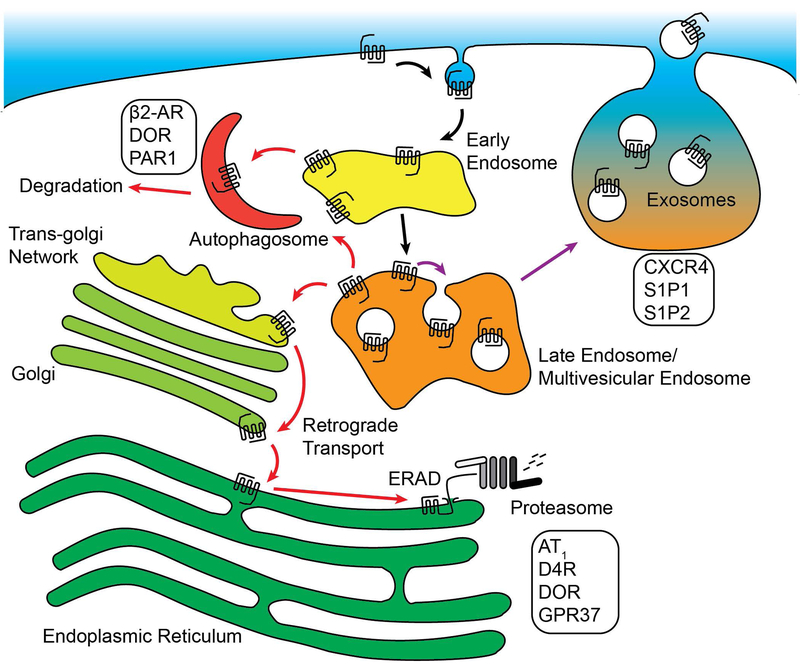
Figure 3:
Alternative trafficking pathways for GPCRs in response to ubiquitination. GPCRs can polyubiquitinated by K48-linked chains, targeting them for retrograde sorting to the ER prior to ERAD-mediated degradation by the proteasome. GPCRs can be sorted to autophagosomes from the limiting membrane of early or late endosomes, leading to rapid degradation that requires autophagosome adaptor proteins. Some receptors that are packaged into the intraluminal vesicles of multi-vesicular endosomes (MVEs) can be released from the cell in exosomes when MVEs fuse with the plasma membrane.
Autophagosomes are highly regulated membrane-bound organelles that degrade cellular components in response to cellular stress. One aspect of cellular response to stress is redistribution of specific GPCRs to autophagosomes (Figure 3). For example, the β2AR is sorted to autophagosomes under physiologically stressful conditions or persistent β2AR signaling [68]. This process requires the protein kinase A-dependent phosphorylation and deactivation of the deubiquitinating enzyme USP20, allowing for the ubiquitination of β2AR to persist and act as a sorting signal to autophagosomes [68]. In addition, autophagosome-specific adaptor proteins like Beclin 2 are involved in GPCR degradation. Beclin-2 interacts with the adaptor protein GASP-1, mediating the degradation of δ-opioid receptor [69]. Furthermore, host cell Beclin-2 regulates the lysosomal sorting and downregulation of constitutively active viral GPCRs found in Kaposi’s sarcoma-associated herpesvirus [70], protecting infected cells from pro-oncogenic signaling. Autophagosome-related adaptor proteins like ATG-5 have also been shown to regulate GPCR endo-lysosomal trafficking. PAR1 is constitutively internalized and recycled [71], and the recycling of PAR1 is mediated by the small GTPase Rab11B [72]. Interestingly, ATG-5 mediates the rapid degradation of PAR1 in the absence of Rab11B-dependent recycling [72], suggesting that the autophagosome or its machinery can function to regulate sorting of PAR1 under cellular stress conditions. Finally, GPCRs like the adenosine A2 receptor [73] regulate autophagosome biogenesis in order to inhibit neutrophil apoptosis. Similarly, the urotensin receptor UTS2R and CXCR4 [74] suppress the biogenesis of autophagosomes, a process critical for cell migration. These studies highlight the importance of autophagosomes in the regulation of GPCRs, and the connections between GPCR signaling and the control of autophagosome formation.
Extracellular vesicles (ECVs) have emerged as important biomarkers for disease and signaling factors that transport cargo such as microRNAs and proteins between cells. There are two subpopulations of ECVs that are defined by their mechanism of biogenesis: microparticles are shed by budding from the plasma membrane whereas exosomes are derived from the intraluminal vesicles of multivesicular bodies that have fused with the plasma membrane instead of lysosomes [75]. Once released, ECVs can fuse or be internalized by other cells, allowing for direct transfer of proteins and microRNAs that can alter the morphology of the target cell. Of particular interest are ECVs released from cancer cells, and their ability to transform cells near the tumor microenvironment. For example, the release of CXCR4 and its ligand SDF-1α (also known as CXCL12) in exosomes from highly metastatic hepatocarcinomas enhances the migration and invasion of non-metastatic hepatocarcinoma cells [76] (Figure 3). In addition, highly metastatic breast cancer cells release the GPCR S1P2 in exosomes that can initiate proliferation in nearby fibroblasts [77]. The S1P2 receptor isolated from MDA-MB-231 breast cancer ECVs is constitutively active due to cleavage of its N-terminus, and can signal to the ERK1/2 MAPK signaling pathway once transferred to fibroblasts [77] (Figure 3). Research into the exosomal sorting of the related S1P1 receptor has revealed a mechanism for how GPCRs are sorted away from degradative MVBs and into MVBs that contain exosomes. Continuous stimulation of the S1P1 receptor following internalization activates Rac1 and Cdc42 on MVBs [78], a process that requires the Gβγ subunits of the heterotrimeric Gi protein. Inhibition of Rho GTPase activity or the inhibition of Gβγ prevents the release of S1P1 in exosomes [78], suggesting that actin polymerization and GPCR signaling near the limiting membrane of MVBs is critical for exosome release. These findings have highlighted the importance of GPCRs in exosomal signaling, and provide critical mechanistic insight into how GPCRs are sorted into exosomes.
6 |. Conclusions and Perspectives
Our current understanding of the diverse mechanisms by which ubiquitin regulates GPCR function is limited and has been largely attributed to degradation. Of the forty GPCRs reported to be ubiquitinated, most functions have been linked to either lysosomal or proteasomal degradation. In some cases, endo-lysosomal sorting of ubiquitinated GPCRs is mediated by the canonical ubiquitin-binding ESCRT components and strictly requires receptor ubiquitination. However, several studies indicate that other GPCRs can enter intralumenal vesicles of MVBs/lysosomes independent of ubiquitin and the canonical ESCRTs, indicating that diverse and complex pathways exist to target the receptors for destruction. Ubiquitination of other key endocytic adaptors has also been shown to facilitate endo-lysosomal sorting of GPCRs to bypass the requirement for receptor ubiquitination and canonical ESCRTs. These studies indicate that ubiquitin controls GPCR trafficking via diverse mechanisms, however, the identification of the machinery that mediates ubiquitination of many of these GPCRs remains unknown. In addition, it is now becoming increasing clear that GPCR signaling controls the ubiquitination machinery and thereby can modulate ubiquitin-mediated functions like lysosomal sorting. New work on GPCRs and ubiquitin has also unveiled novel insight into how ubiquitination of GPCRs drives inflammatory signaling. This work also provides the first example of how activation of a GPCR “turns-on” an E3 ubiquitin ligase to trigger ubiquitin-driven signaling. Besides lysosomes, GPCRs can be targeted to alternative destinations within the cell including proteasomes, exosomes and the autophagic pathway, however the mechanisms and role of ubiquitin that regulated GPCR sorting to these pathways are even less clear. Given the prevalence of ubiquitination, it is likely that all GPCRs are ubiquitinated at least once during their life-times, the challenge now is to determine how and why GPCRs modified with ubiquitin.
Synopsis:
Ubiquitination of G protein-coupled receptors (GPCRs) is important for degradation by the lysosome and proteasome. While endo-lysosomal sorting of ubiquitinated GPCRs to the lysosome occurs via the canonical ESCRTs, not all GPCRs utilize ubiquitin or ESCRTs for receptor destruction. Here, we discuss the diverse pathways that exist to sort GPCRs to lysosomes independent of ubiquitination and canonical ubiquitin-binding ESCRTs. We also discuss ubiquitin-driven GPCR signaling and new mechanisms by which GPCR signaling controls the ubiquitination machinery to regulation cellular functions.
Acknowledgements:
This work was supported, in whole or in part by the National Institutes of Health (NIH)/NIGMS R35 GM127121 (J.T) and UC TRDRP 26IP-0050 (J.T.)
Footnotes
Conflict of interest: All authors on this work agree to its content and hereby declare no competing commercial interests relating to this submitted work.
References:
- 1.Clague MJ, Heride C, Urbé S. The demographics of the ubiquitin system. Trends Cell Biol 2015;25:417–426. [DOI] [PubMed] [Google Scholar]
- 2.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem 1998;67:425–479. [DOI] [PubMed] [Google Scholar]
- 3.Sriram K, Insel PA. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol Pharmacol 2018;93:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terrell J, Shih S, Dunn R, Hicke L. A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol Cell 1998;1:193–202. [DOI] [PubMed] [Google Scholar]
- 5.Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell 1996;84:277–287. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Davis NG. Ubiquitin-independent entry into the yeast recycling pathway. Traffic 2002;3:110–123. [DOI] [PubMed] [Google Scholar]
- 7.Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev Cell 2002;3:283–289. [DOI] [PubMed] [Google Scholar]
- 8.Bilodeau PS, Winistorfer SC, Kearney WR, Robertson AD, Piper RC. Vps27-Hse1 and ESCRT-I complexes cooperate to increase efficiency of sorting ubiquitinated proteins at the endosome. J Cell Biol 2003;163:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piper RC, Cooper AA, Yang H, Stevens TH. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J Cell Biol 1995;131:603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa R, Warren DT, Ayscough KR. Lsb5p interacts with actin regulators Sla1p and Las17p, ubiquitin and Arf3p to couple actin dynamics to membrane trafficking processes. Biochem J 2005;387:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hicke L, Schubert HL, Hill CP. Ubiquitin-binding domains. Nat Rev Mol Cell Biol 2005;6:610–621. [DOI] [PubMed] [Google Scholar]
- 12.Shih SC, Prag G, Francis SA, Sutanto MA, Hurley JH, Hicke L. A ubiquitin-binding motif required for intramolecular ubiquitylation, the CUE domain. EMBO J 2003;22:1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnell JD, Hicke L. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J Biol Chem 2003;278:35857–60. [DOI] [PubMed] [Google Scholar]
- 14.Dores MR, Schnell JD, Maldonado-Baez L, Wendland B, Hicke L. The Function of Yeast Epsin and Ede1 Ubiquitin-Binding Domains During Receptor Internalization. Traffic 2010;11:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurley JH, Emr SD. The ESCRT Complexes: Structure and Mechanism of a Membrane-Trafficking Network. Annu Rev Biophys Biomol Struct 2006;35:277–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dores MR, Chen B, Lin H, Soh UJK, Paing MM, Montagne WA, Meerloo T, Trejo J. ALIX binds a YPX3L motif of the GPCR PAR1 and mediates ubiquitin-independent ESCRT-III/MVB sorting. J Cell Biol 2012;197:407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dores MR, Grimsey NJ, Mendez F, Trejo J. ALIX Regulates the Ubiquitin-Independent Lysosomal Sorting of the P2Y1 Purinergic Receptor via a YPX3L Motif. PLoS One 2016;11:e0157587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marley A, von Zastrow M. Dysbindin promotes the post-endocytic sorting of G protein-coupled receptors to lysosomes. PloS one 2010;5:e9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charfi I, Abdallah K, Gendron L, Pineyro G. Delta opioid receptors recycle to the membrane after sorting to the degradation path. Cell Mol Life Sci 2018;75:2257–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem 2001;276:45509–45512. [DOI] [PubMed] [Google Scholar]
- 21.Li JG, Haines DS, Liu-Chen LY. Agonist-promoted Lys63-linked polyubiquitination of the human kappa-opioid receptor is involved in receptor down-regulation. Mol Pharmacol 2008;73:1319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahaie N, Kralikova M, Prézeau L, Blahos J, Bouvier M. Post-endocytotic Deubiquitination and Degradation of the Metabotropic γ-Aminobutyric Acid Receptor by the Ubiquitin-specific Protease 14. J Biol Chem 2016;291:7156–7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin NP, Lefkowitz RJ, Shenoy SK. Regulation of V2 vasopressin receptor degradation by agonist-promoted ubiquitination. J Biol Chem 2003;278:45954–9. [DOI] [PubMed] [Google Scholar]
- 24.Xiao K, Shenoy SK. Beta2-adrenergic receptor lysosomal trafficking is regulated by ubiquitination of lysyl residues in two distinct receptor domains. J Biol Chem 2011;286:12785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hislop JN, Henry AG, von Zastrow M. Ubiquitination in the First Cytoplasmic Loop of Œ°-Opioid Receptors Reveals a Hierarchical Mechanism of Lysosomal Down-regulation. Journal of Biological Chemistry 2011;286:40193–40204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukai A, Yamamoto-Hino M, Awano W, Watanabe W, Komada M, Goto S. Balanced ubiquitylation and deubiquitylation of Frizzled regulate cellular responsiveness to Wg/Wnt. EMBO J 2010;29:2114–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooray SN, Guasti L, Clark AJL. The E3 Ubiquitin Ligase Mahogunin Ubiquitinates the Melanocortin 2 Receptor. Endocrinology 2011;152:4224–4231. [DOI] [PubMed] [Google Scholar]
- 28.Grimsey NJ, Aguilar B, Smith TH, Le P, Soohoo AL, Puthenveedu MA, Nizet V, Trejo J. Ubiquitin plays an atypical role in GPCR-induced p38 MAP kinase activation on endosomes. J Cell Biol 2015;210:1117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El Ayadi A, Prasai A, Wang Y, Herndon DN, Finnerty CC. β-Adrenergic Receptor Trafficking, Degradation, and Cell Surface Expression Are Altered in Dermal Fibroblasts from Hypertrophic Scars. J Invest Dermatol 2018;138:1645–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peeler JC, Schedin-Weiss S, Soula M, Kazmi MA, Sakmar TP. Isopeptide and ester bond ubiquitination both regulate degradation of the human dopamine receptor 4. J Biol Chem 2017;292:21623–21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berthouze M, Venkataramanan V, Li Y, Shenoy SK. The deubiquitinases USP33 and USP20 coordinate [beta]2 adrenergic receptor recycling and resensitization. EMBO J [Internet] 2009;advanced online publication. Available from: 10.1038/emboj.2009.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasdemir B, Murphy JE, Cottrell GS, Bunnett NW. Endosomal deubiquitinating enzymes control ubiquitination and down-regulation of protease-activated receptor 2. J Biol Chem 2009;M109.025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mines MA, Goodwin JS, Limbird LE, Cui FF, Fan GH. Deubiquitination of CXCR4 by USP14 is critical for both CXCL12-induced CXCR4 degradation and chemotaxis but not ERK ativation. J Biol Chem 2009;284:5742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen B, Dores MR, Grimsey N, Canto I, Barker BL, Trejo J. Adaptor protein complex-2 (AP-2) and epsin-1 mediate protease-activated receptor-1 internalization via phosphorylation- and ubiquitination-dependent sorting signals. J Biol Chem 2011;286:40760–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dores MR, Paing MM, Lin H, Montagne WA, Marchese A, Trejo J. AP-3 regulates PAR1 ubiquitin-independent MVB/lysosomal sorting via an ALIX-mediated pathway. Mol Biol Cell 2012;23:3612–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanowitz M, von Zastrow M. Ubiquitination-independent trafficking of G protein-coupled receptors to lysosomes. J Biol Chem 2002;277:50219–22. [DOI] [PubMed] [Google Scholar]
- 37.Cho DI, Zheng M, Min C, Kwon KJ, Shin CY, Choi HK, Kim KM. ARF6 and GASP-1 are post-endocytic sorting proteins selectively involved in the intracellular trafficking of dopamine D(2) receptors mediated by GRK and PKC in transfected cells. British journal of pharmacology 2013;168:1355–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang Y, Zhou XE, Gao X, He Y, Liu W, Ishchenko A, Barty A, White TA, Yefanov O, Han GW, Xu Q, de Waal PW, Ke J, Tan MHE, Zhang C, Moeller A, West GM, Pascal BD, Van Eps N, Caro LN, Vishnivetskiy SA, Lee RJ, Suino-Powell KM, Gu X, Pal K, Ma J, Zhi X, Boutet S, Williams GJ, Messerschmidt M, Gati C, Zatsepin NA, Wang D, James D, Basu S, Roy-Chowdhury S, Conrad CE, Coe J, Liu H, Lisova S, Kupitz C, Grotjohann I, Fromme R, Jiang Y, Tan M, Yang H, Li J, Wang M, Zheng Z, Li D, Howe N, Zhao Y, Standfuss J, Diederichs K, Dong Y, Potter CS, Carragher B, Caffrey M, Jiang H, Chapman HN, Spence JCH, Fromme P, Weierstall U, Ernst OP, Katritch V, Gurevich VV, Griffin PR, Hubbell WL, Stevens RC, Cherezov V, Melcher K, Xu HE. Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 2015;523:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheerer P, Sommer ME. Structural mechanism of arrestin activation. Curr Opin Struct Biol 2017;45:160–169. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen SGF, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah STA, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 2011;477:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shenoy SK, Barak LS, Xiao K, Ahn S, Berthouze M, Shukla AK, Luttrell LM, Lefkowitz RJ. Ubiquitination of beta-Arrestin Links Seven-transmembrane Receptor Endocytosis and ERK Activation. J Biol Chem 2007;282:29549–29562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shenoy SK, Modi AS, Shukla AK, Xiao K, Berthouze M, Ahn S, Wilkinson KD, Miller WE, Lefkowitz RJ. Beta-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc Natl Acad Sci U S A 2009;106:6650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shenoy SK, Xiao K, Venkataramanan V, Snyder PM, Freedman NJ, Weissman AM. Nedd4 Mediates Agonist-dependent Ubiquitination, Lysosomal Targeting, and Degradation of the beta2-Adrenergic Receptor. J Biol Chem 2008;283:22166–22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han SO, Kommaddi RP, Shenoy SK. Distinct roles for beta-arrestin2 and arrestin-domain-containing proteins in beta(2) adrenergic receptor trafficking. EMBO Rep [Internet] 2012;Available from: http://www.ncbi.nlm.nih.gov/pubmed/23208550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malik R, Marchese A. Arrestin-2 Interacts with the Endosomal Sorting Complex Required for Transport Machinery to Modulate Endosomal Sorting of CXCR4. Mol Biol Cell 2010;21:2529–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marchese A, Raiborg C, Santini F, Keen JH, Stenmark H, Benovic JL. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev Cell 2003;5:709–722. [DOI] [PubMed] [Google Scholar]
- 47.Bhandari D, Trejo J, Benovic JL, Marchese A. Arrestin-2 Interacts with the Ubiquitin-Protein Isopeptide Ligase Atrophin-interacting Protein 4 and Mediates Endosomal Sorting of the Chemokine Receptor CXCR4. J Biol Chem 2007;282:36971–36979. [DOI] [PubMed] [Google Scholar]
- 48.Holleman J, Marchese A. The ubiquitin ligase deltex-3l regulates endosomal sorting of the G protein-coupled receptor CXCR4. Molecular biology of the cell 2014;25:1892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hislop JN, Marley A, von Zastrow M. Role of Mammalian Vacuolar Protein-sorting Proteins in Endocytic Trafficking of a Non-ubiquitinated G Protein-coupled Receptor to Lysosomes. J Biol Chem 2004;279:22522–22531. [DOI] [PubMed] [Google Scholar]
- 50.Hasdemir B, Bunnett NW, Cottrell GS. Hepatocyte Growth Factor-regulated Tyrosine Kinase Substrate (HRS) Mediates Post-endocytic Trafficking of Protease-activated Receptor 2 and Calcitonin Receptor-like Receptor. J Biol Chem 2007;282:29646–29657. [DOI] [PubMed] [Google Scholar]
- 51.Dores MR, Lin H, N JG, Mendez F, Trejo J. The alpha-arrestin ARRDC3 mediates ALIX ubiquitination and G protein-coupled receptor lysosomal sorting. Mol Biol Cell 2015;26:4660–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yi X, Bouley R, Lin HY, Bechoua S, Sun T, del Re E, Shioda T, Raychowdhury MK, Lu HAJ, Abou-Samra AB, Brown D, Ausiello DA. Alix (AIP1) is a vasopressin receptor (V2R)-interacting protein that increases lysosomal degradation of the V2R. Am J Physiol Renal Physiol 2007;292:F1303–1313. [DOI] [PubMed] [Google Scholar]
- 53.Zhan L, Liu B, Jose-Lafuente M, Chibalina MV, Grierson A, Maclean A, Nasir J. ALG-2 interacting protein AIP1: a novel link between D1 and D3 signalling. Eur J Neurosci 2008;27:1626–33. [DOI] [PubMed] [Google Scholar]
- 54.Dowlatshahi DP, Sandrin V, Vivona S, Shaler TA, Kaiser SE, Melandri F, Sundquist WI, Kopito RR. ALIX Is a Lys63-Specific Polyubiquitin Binding Protein that Functions in Retrovirus Budding. Dev Cell 2012;23:1247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jean-Charles P-Y, Snyder JC, Shenoy SK. Chapter One - Ubiquitination and Deubiquitination of G Protein-Coupled Receptors. Prog Mol Biol Transl Sci 2016;141:1–55. [DOI] [PubMed] [Google Scholar]
- 56.Jacob C, Cottrell GS, Gehringer D, Schmidlin F, Grady EF, Bunnett NW. c-Cbl Mediates Ubiquitination, Degradation, and Down-regulation of Human Protease-activated Receptor 2. J Biol Chem 2005;280:16076–16087. [DOI] [PubMed] [Google Scholar]
- 57.Buetow L, Huang DT. Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat Rev Mol Cell Biol 2016;17:626–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol 2009;10:398–409. [DOI] [PubMed] [Google Scholar]
- 59.Bhandari D, Robia SL, Marchese A. The E3 ubiquitin ligase atrophin interacting protein 4 binds directly to the chemokine receptor CXCR4 via a novel WW domain-mediated interaction. Mol Biol Cell 2009;20:1324–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated b2- adrenergic receptor and b-arrestin. Science 2001;294:1307–1313. [DOI] [PubMed] [Google Scholar]
- 61.Groer CE, Schmid CL, Jaeger AM, Bohn LM. Agonist-directed interactions with specific beta-arrestins determine mu-opioid receptor trafficking, ubiquitination, and dephosphorylation. J Biol Chem 2011;286:31731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian X, Irannejad R, Bowman SL, Du Y, Puthenveedu MA, von Zastrow M, Benovic JL. The α-Arrestin ARRDC3 Regulates the Endosomal Residence Time and Intracellular Signaling of the β2-Adrenergic Receptor. J Biol Chem 2016;291:14510–14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grimsey NJ, Narala R, Rada CC, Mehta S, Stephens BS, Kufareva I, Lapek J, Gonzalez DJ, Handel TM, Zhang j., Trejo J. A tyrosine switch on NEDD4–2 E3 ligase transmits GPCR inflammator signaling. Cell reports 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petaja-Repo UE, Hogue M, Laperriere A, Bhalla S, Walker P, Bouvier M. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem 2001;276:4416–4423. [DOI] [PubMed] [Google Scholar]
- 65.Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R. An Unfolded Putative Transmembrane Polypeptide, which Can Lead to Endoplasmic Reticulum Stress, Is a Substrate of Parkin. Cell 2001;105:891–902. [DOI] [PubMed] [Google Scholar]
- 66.Berger BS, Acebron SP, Herbst J, Koch S, Niehrs C. Parkinson’s disease-associated receptor GPR37 is an ER chaperone for LRP6. EMBO Rep 2017;18:712–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, Pascua A, Lu Q, Wang X, Villar VAM, Jones JE, Wang Z, Periasamy A, Lau Y-S, Soares-da-Silva P, Creswell K, Guillemette G, Sibley DR, Eisner G, Felder RA, Jose PA. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteosome pathway in mice and human cells. J Clin Invest 2008;118:2180–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kommaddi RP, Jean-Charles P-Y, Shenoy SK. Phosphorylation of the deubiquitinase USP20 by protein kinase A regulates post-endocytic trafficking of β2 adrenergic receptors to autophagosomes during physiological stress. J Biol Chem 2015;290:8888–8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He C, Wei Y, Sun K, Li B, Dong X, Zou Z, Liu Y, Kinch LN, Khan S, Sinha S, Xavier RJ, Grishin NV, Xiao G, Eskelinen EL, Scherer PE, Whistler JL, Levine B. Beclin 2 functions in autophagy, degradation of G protein-coupled receptors, and metabolism. Cell 2013;154:1085–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong X, Cheng A, Zou Z, Yang Y-S, Sumpter RM, Huang C-L, Bhagat G, Virgin HW, Lira SA, Levine B. Endolysosomal trafficking of viral G protein-coupled receptor functions in innate immunity and control of viral oncogenesis. Proc Natl Acad Sci USA 2016;113:2994–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paing MM, Johnston CA, Siderovski DP, Trejo J. Clathrin Adaptor AP2 Regulates Thrombin Receptor Constitutive Internalization and Endothelial Cell Resensitization. Mol Cell Biol 2006;26:3231–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grimsey NJ, Coronel LJ, Cordova IC, Trejo J. Recycling and Endosomal Sorting of Protease-activated Receptor-1 Is Distinctly Regulated by Rab11A and Rab11B Proteins. Journal of Biological Chemistry 2016;291:2223–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y-W, Yang T, Zhao L, Ni Z, Yang N, He F, Dai S-S. Activation of Adenosine 2A receptor inhibits neutrophil apoptosis in an autophagy-dependent manner in mice with systemic inflammatory response syndrome. Sci Rep 2016;6:33614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coly P-M, Perzo N, Le Joncour V, Lecointre C, Schouft M-T, Desrues L, Tonon M-C, Wurtz O, Gandolfo P, Castel H, Morin F. Chemotactic G protein-coupled receptors control cell migration by repressing autophagosome biogenesis. Autophagy 2016;12:2344–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li X, Wang Y, Wang Q, Liu Y, Bao W, Wu S. Exosomes in cancer: Small transporters with big functions. Cancer Lett 2018;435:55–65. [DOI] [PubMed] [Google Scholar]
- 76.Li M, Lu Y, Xu Y, Wang J, Zhang C, Du Y, Wang L, Li L, Wang B, Shen J, Tang J, Song B. Horizontal transfer of exosomal CXCR4 promotes murine hepatocarcinoma cell migration, invasion and lymphangiogenesis. Gene 2018; [DOI] [PubMed] [Google Scholar]
- 77.El Buri A, Adams DR, Smith D, Tate RJ, Mullin M, Pyne S, Pyne NJ. The sphingosine 1-phosphate receptor 2 is shed in exosomes from breast cancer cells and is N-terminally processed to a short constitutively active form that promotes extracellular signal regulated kinase activation and DNA synthesis in fibroblasts. Oncotarget 2018;9:29453–29467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kajimoto T, Mohamed NNI, Badawy SMM, Matovelo SA, Hirase M, Nakamura S, Yoshida D, Okada T, Ijuin T, Nakamura S-I. Involvement of Gβγ subunits of Gi protein coupled with S1P receptor on multivesicular endosomes in F-actin formation and cargo sorting into exosomes. J Biol Chem 2018;293:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]