Watch a video presentation of this article
Abbreviations
- anti‐HBc
antibody to hepatitis B core antigen
- anti‐HBs
antibody to hepatitis B surface antigen
- CDC
Centers for Disease Control and Prevention
- HAV
hepatitis A virus
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HCW
health care worker
- HEV
hepatitis E virus
- HIV
human immunodeficiency virus
- PWID
persons with injecting drugs
- WHO
World Health Organization
Hepatitis B virus (HBV) infection is a significant cause of morbidity and mortality in the world.1 The results of a study by the Institute for Health Metrics and Evaluation concluded that viral hepatitis is a leading cause of death and disability worldwide.2 In the World Health Organization's (WHO) 2017 report,3 there are an estimated 257 million persons worldwide who are living with chronic HBV infection, of which an estimated 20% will die early of liver failure or hepatocellular carcinoma without management of their infection. More persons worldwide die of chronic viral hepatitis B and C than human immunodeficiency virus (HIV); this is also true in the United States. HBV accounts for 900,000 deaths/year (Fig. 1).3 Most of these persons reside in the WHO African and Western Pacific regions. The WHO has adapted the goal eliminating HBV globally by 2030 defined as a 65% reduction in mortality and a 90% reduction in incidence compared with the baseline levels obtained in 2015 with interim goals set for 2020. The WHO has targeted five synergistic interventions that include widespread vaccination, and detection and linkage to care of 90% of those with chronic HBV infection. The purpose of this review is to examine those goals and discuss what elements will be needed to successfully reach each of them. The US Government and Centers for Disease Control and Prevention (CDC) have endorsed the goals and developed an implementation strategy. The WHO has asked each member state to develop a plan to reach these goals in their respective countries. Table 1 displays the WHO elimination goals. These goals are divided into two main areas: (1) prevention of new HBV infections through vaccination and blood safety; and (2) identification, linkage to care, and treatment of persons with chronic HBV.
Figure 1.
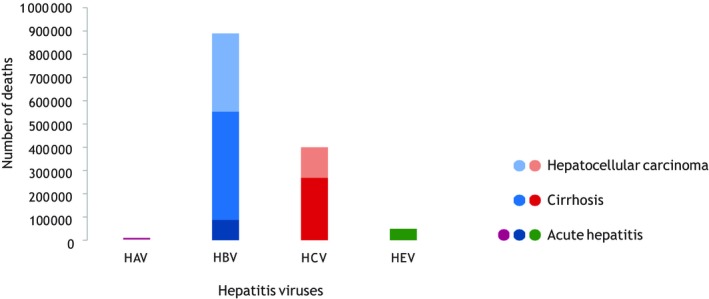
Deaths from viral hepatitis, by virus and type of sequelae, 2015: most viral hepatitis deaths are due to the late complications of HBV and HCV infection. HAV: hepatitis A virus; HEV: hepatitis E virus. Reproduced with permission from Global Health Estimates 2015: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2015. Geneva: World Health Organization; 2016.7
Table 1.
WHO Goals for HBV Elimination
| Goals | Indicator | 2015 Baseline | 2020 Target | 2030 Target | Achievable? |
|---|---|---|---|---|---|
| Hepatitis B vaccination | 3‐Dose coverage in infancy | 84% | 90% | 90% | Yes |
| Prevention perinatal transmission | Birth dose coverage | 39% | 50% | 90% | Likely |
| Blood safety | Donations screened | 97% | 95% | 100% | Yes |
| Injection safety | Proportion of unsafe injections | 5% | 0% | 0% | Yes |
| Harm reduction | Syringes and needle exchange per PWID | 27 | 200 | 200 | Likely |
| Testing services | % with HBV diagnosed | 9% | 30% | 90% | Will need a massive effort and funding support |
| Treatment | % diagnosed with HBV on treatment | 7% | Not specified | 80% | Massive support needed |
Abbreviation: PWID, persons with injecting drugs.
Prevention
Prevention includes prevention of HBV infections by vaccination and reducing transmission. Goals for vaccination are defined by the proportion of infants who have received a full series by 1 year of age, which is already at 84% worldwide (Fig. 2). Unfortunately, funding for the birth dose for prevention of perinatal transmission was not included in the Global Alliance for Vaccine Initiative program, and global birth dose coverage is currently only 39%, ranging from a low of 10% in the Africa region to a high of 72% to 83% in the Americas and Western Pacific, respectively (Fig. 3). In Africa and other low‐income countries, half or more newborns are born outside the hospital, which is a challenge to administering the birth dose even if the vaccine is available and the cost is covered. In many countries, serology is limited to rapid testing for hepatitis B surface antigen (HBsAg). Although access to the HBV DNA test is limited because of cost and laboratory expertise, the Global HIV Program has recently placed smaller, more cost‐effective platforms for molecular testing, such as the Gene X‐pert platform, which can be performed on saliva or fingerstick blood samples using less expensive disposable cartridges that give results in a couple of hours. HIV and hepatitis C virus (HCV) RNA cartridges are now licensed, and licensure of the HBV cartridge system is anticipated by 2019.
Figure 2.
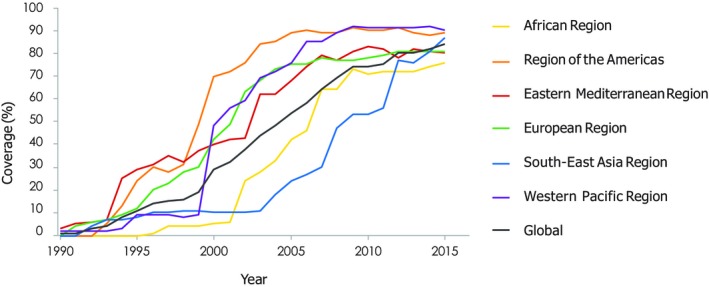
Three‐dose hepatitis B vaccine coverage, by WHO region, 2000–2015.
Figure 3.
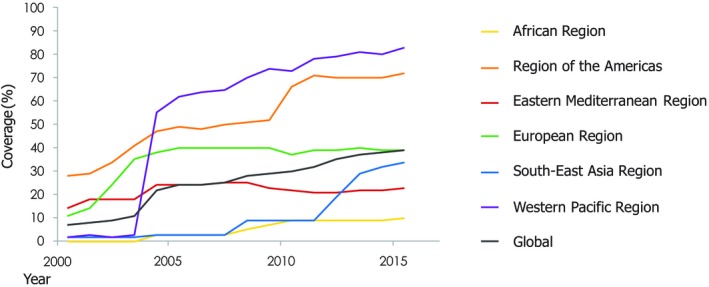
Hepatitis B birth dose coverage, by WHO region, 2000–2015
Reducing transmission includes: (1) blood safety by screening donations with quality assurance, which is already at 97%; (2) injection safety measured by the proportion of unsafe injections in health care settings, which is currently at less than 5% per year; and (3) harm reduction measured by the syringes and needles distributed to persons who inject drugs per year. This latter goal will require a large scale‐up globally of needle exchange programs.
Identification, Linkage to Care, and Treatment
Goals for testing and treatment are displayed in Table 1. The WHO estimates that 9% of persons globally with chronic HBV are currently detected (Fig. 4). To reach the goal of 90% diagnosed by 2030 will take a Herculean effort. What would be necessary is the establishment of massive screening programs of large populations in endemic nations. To meet this goal in the United States, better screening of persons born in endemic countries will have to occur. However, globally, it is feasible to begin this task in incremental steps. Screening is currently taking place in blood donation settings (Intervention 3 in Table 1). Screening could initially be expanded to include household contacts of HBsAg‐positive persons and patients with obvious liver disease in hospitals and clinics. The next step might be to “piggyback” screening onto existing HIV screening programs, so that when HIV screening occurs, screening is also done for HBsAg and HCV antibody using rapid low‐cost tests. Population‐based screening will be a massive undertaking in countries with a more than 2% prevalence rate of HBsAg. In many countries, health care workers (HCWs) are not offered testing or vaccination for HBV. Screening could be done with anti‐HBc (antibody to hepatitis B core antigen), anti‐HBs (antibody to HBsAg) to identify those who are negative for vaccination, but HCWs should be screened for HBsAg at a minimum before vaccination to identify persons at risk for transmission to patients. HCWs who are HBsAg‐positive could then be tested for HBV DNA and offered antiviral therapy if their levels are high, to prevent transmission as is recommended by the CDC.4
Figure 4.
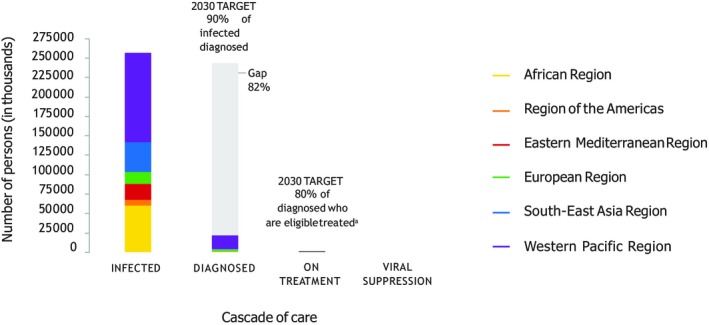
Cascade of care for HBV infection, by WHO region, 2015.
Regarding treatment goals, it is important to note that the current antiviral medications very effectively lower levels of HBV DNA, quiet liver inflammation, and decrease the risk for liver failure and HCC. Tenofovir is now available for as little as $48 per year in most countries. However, not everyone will benefit from these medications, because over time most people's immune system accomplishes the same effect. All major guidelines, including American Association for the Study of Liver Diseases and WHO, recommend monitoring of all HBV‐infected patients on a regular basis.5, 6 Because successful programs to monitor persons with chronic HIV infections are in place in almost all countries, programs to manage chronic HBV infection and select appropriate persons for treatment could be combined with the existing HIV care and treatment programs.
In conclusion, elimination of HBV will take considerable effort globally, but the tools to do this are available now, and with concerted effort, most of the WHO elimination goals could be met by 2030.
Potential conflict of interest: Nothing to report.
References
- 1. McMahon BJ. Chronic hepatitis B virus infection. Med Clin North Am 2014;98:39‐54. [DOI] [PubMed] [Google Scholar]
- 2. Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Abu‐Raddad LJ, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016;388:1081‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Global Hepatitis Report 2017. Geneva: World Health Organization; 2017:1‐83. http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ [Google Scholar]
- 4. Updated CDC recommendations for the management of hepatitis B virus‐infected health‐care providers and students. MMWR Recomm Rep 2012;61:1‐12. [PubMed] [Google Scholar]
- 5. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Guidelines for the Prevention, Care and Treatment of Persons With Chronic Hepatitis B Infection. Geneva: World Health Organization; 2015:1‐166. http://www.who.int/hepatitis/publications/hepatitis-b-guidelines/en/ [PubMed] [Google Scholar]
- 7. Global Health Estimates 2015: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2015. Geneva: World Health Organization; 2016. [Google Scholar]


