Watch a video presentation of this article
Abbreviations
- anti‐HBs
hepatitis B surface antibody
- APOBEC3A
apolipoprotein B mRNA editing enzyme catalytic subunit 3A
- CAS9
CRISPR‐associated protein 9
- cccDNA
covalently closed circular DNA
- CRISPR
clustered regularly interspaced short palindromic repeats
- DAA
direct‐acting antiviral agent
- DHQ
dihydroxyquinoline
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HSP
heat shock protein
- IDAA
indirect‐acting antiviral agent
- NTCP
sodium taurocholate cotransporting polypeptide
- PD‐1
programmed death 1
- PD‐L1
programmed death ligand 1
- RIG‐I
retinoic acid–inducible gene I
- RNAi
RNA interference
- siRNA
small interfering RNA
Hepatitis B virus (HBV) is a small, partially double‐stranded DNA virus that can cause acute and chronic hepatitis. Chronic HBV infection is a major cause of cirrhosis and hepatocellular carcinoma.1 Despite the availability of a protective vaccine for more than 30 years, it is estimated there are 257 million persons worldwide with chronic HBV infection and 887,000 deaths annually from complications of cirrhosis and hepatocellular carcinoma.2 Chronic infection is due to the persistence of the covalently closed circular DNA (cccDNA) as an episomal mini‐chromosome in the hepatocyte nucleus and to the ability of HBV to evade the immune system.
Current therapy consists of two classes of antiviral agents, nucleos(t)ide analogues and interferon.3 Nucleos(t)ide analogues are small‐molecule, competitive inhibitors of the viral reverse transcriptase. They block formation of the nascent HBV DNA, thereby inhibiting HBV replication.4 The exact mode of action of interferon is not known, but it is generally assumed to function by induction of dozens of different host functions with direct antiviral activities and others that stimulate the host adaptive immunological response. There is also recent evidence that interferons cause repression of transcription of the HBV cccDNA5 and may even induce cytidine deaminases (apolipoprotein B mRNA editing enzyme catalytic subunit 3A [APOBEC3A] and APOBEC3B) that cause cccDNA degradation.6, 7, 8 Although current antiviral agents are effective at inhibiting viral replication and reducing complications of chronic HBV infection, they are not curative and in the case of nucleos(t)ide analogues must be administered long term, if not indefinitely, because of persistence of the cccDNA.3
The development of new treatments for HBV has been hampered by the small and compact nature of the HBV genome with relatively few druggable viral targets. However, a better understanding of the viral life cycle together with advancements in in vitro culture systems and animal models have led to the identification of a number of novel therapeutic approaches against HBV, as illustrated in Fig. 1. There are now more than 30 investigational agents in the pipeline of development, targeting specific viral gene products (direct‐acting antiviral agents [DAAs]) and host targets (indirect‐acting antiviral agents [IDAAs]).9 The focus of current drug development is on achieving functional cure, defined as sustained loss of hepatitis B surface antigen (HBsAg) with or without concomitant development of hepatitis B surface antibody (anti‐HBs), without the need for ongoing therapy.10 This brief review will highlight key developments in antiviral and immunomodulatory therapy and the rationale for choosing these approaches.
Figure 1.
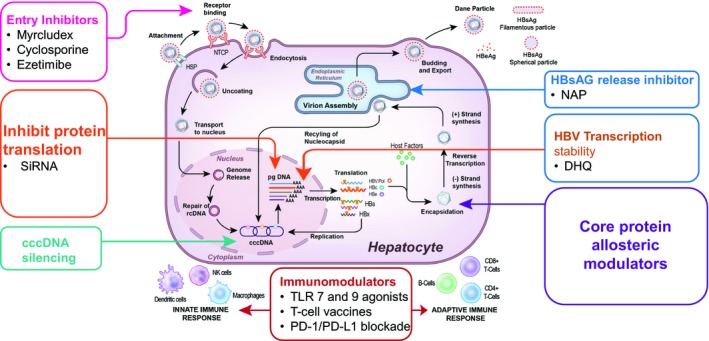
Novel approaches for HBV cure. A better understanding of HBV biology has identified a number of new targets for drug development, and at least seven new classes of compounds are being pursued as therapy for HBV. These include entry inhibitors such as Myrcludex B and monoclonal antibodies. Targeting the viral mRNA using nucleic acid approaches (e.g. siRNA) but delivery to the hepatocyte nucleus remains a challenge. Eliminating or silencing cccDNA is the holy grail of HBV therapy. The availability of gene‐editing technology such as CRISPR/CAS9 now makes this a feasible approach, but the clinical applicability is limited by how to efficiently deliver this to all HBV‐infected cells. Core protein allosteric modulators are an attractive approach because of the importance of the core particle for the viral life cycle, and several compounds are in early‐stage development. The viral polymerase still remains a target for drug development, and agents that target the RNaseH function of the polymerase are being pursued. Releasing inhibitors that block HBsAg or virion secretion are being developed and appear promising, but there is the concern of the effect of retention of viral proteins or complete virions within the hepatocyte. Finally, antiviral approaches alone may not be enough, and strategies to boost the innate or adaptive immune responses using novel immunomodulatory approaches also are being developed including TRL agonists, checkpoint inhibitors, T cell reprogramming, and T cell vaccines. Abbreviations: HSP, heat shock protein; NK, natural killer; PD‐1, programmed death 1; PD‐L1, programmed death ligand 1.
Entry Inhibitors
HBV entry into hepatocytes requires coordinated binding to heparin sulfate proteoglycans, a low‐affinity receptor, to mediate hepatocyte attachment, followed by a high‐affinity interaction with the sodium taurocholate cotransporting polypeptide (NTCP) for viral internalization.11, 12 The NTCP receptor confers the species specificity to HBV.13, 14 Understanding this process has led to the development of several classes of specific and nonspecific inhibitors of viral entry (see Table 1).15, 16, 17, 18 Entry inhibitors may be useful by preventing de novo infection of uninfected hepatocytes.
Table 1.
Viral and Host Targets, Mechanism of Action, and Novel Compounds in Development for Chronic Hepatitis B
| Target | Mechanism of Action | Class | Compounds in Development |
|---|---|---|---|
| Viral entry | Antibodies targeting pre‐S1 or small surface protein | Monoclonal antibodies | GC1102 |
| Attachment inhibitors that prevent viral interaction with entry receptors | Heparin | ||
| Poly‐L‐lysine | |||
| Reversibly/irreversibly block the NTCP receptor | Conjugated bile salts | ||
| Synthetic N‐acylated pre‐S1 | Myrcludex B | ||
| Cyclosporine | |||
| cccDNA | Inactivate cccDNA | Zinc finger nucleases | |
| Transcription activator‐like effector nuclease | |||
| CRISPR/cas9 system | EBT106 HBV CRISPR‐CAS9 lipid nanoparticle | ||
| Degrade cccDNA | Interferon , , | ||
| Tumor necrosis factor‐ | |||
| Lymphotoxin‐ receptor agonists | |||
| Functionally silence cccDNA | Epigenomic modifiers | ||
| Viral transcripts | Degrade mRNA | siRNA |
ARB‐1467 ARB‐1740 ALN‐HBV Hepbarna (BB‐HB‐331) Lunar‐HBV |
| Bind viral mRNA to prevent viral protein production | Antisense oligonucleotides |
IONIS‐HBVRx (GSK3228836) IONIS‐HBVLRx (GSK33389404) |
|
| Cause degradation of HBV RNA in the nucleus | DHQ |
AB452 RG7834 |
|
| Downregulate viral mRNA | Farnesoid X receptor agonist | EYP001 | |
| Core assembly modulators | Inhibit encapsidation of pregenomic RNA or nucleocapsid assembly | Heteroaryldihydropyrimidines (HAPS) | Morphothiadin (GLS4) |
| Phenylpropenamide | AT‐61; AT‐130 | ||
| Pyridazinone derivatives | 3711 | ||
| Sulfamoylbenzamide |
AB‐423 JNJ56136379 NVR 3‐778 |
||
| Isothiafludine | NZ‐4 | ||
| 2‐Amino‐n‐(2,6‐dichloropyridin‐3‐yl) acetamide derivatives | BCM‐599 | ||
| 5,5′‐bis[8‐(phenylamino)‐1‐ naphthalenesulfonate] | Bis‐ANS | ||
| HBsAg release inhibitors | Synthetic oligonucleotides that bind HBsAg | Nucleic acid polymers |
Rep 2139 Rep 2165 |
| Boost innate immunity | Agonists of sensing arm of innate immune system | Toll‐like receptor‐7 agonist | RO6864018 (RG7795, ANA773 |
| Toll‐like receptor‐8 agonist | GS‐9688 | ||
| RIG‐I and NOD2 agonist | Inarigivir (SB9200) | ||
| STING agonists | |||
| TCR‐like antibodies | |||
| Boost humoral immunity | Anti‐HBs | ||
| Boost adaptive immunity | Checkpoint inhibitors | Anti‐CTLA‐4 | |
| Anti‐PD‐1 | Nivolumab | ||
| Engineering new HBV‐specific T cells | TCR gene transfer | LTCR‐H2‐1 | |
| CAR‐T cells | |||
| Therapeutic vaccines | Induction of HBV‐specific B and T cells | T cell vaccines | HepTcell |
| DNA vaccines |
HB‐110 INO‐1800 |
||
| Viral vectors expressing HBV proteins |
TG1050 TomegaVax HBV |
Targeting the cccDNA
cccDNA serves as the template for viral transcription and plays a key role in the viral life cycle. Its persistence in the nucleus of infected hepatocytes is a major reason why cure of HBV is currently not possible. Therefore, approaches to eliminate cccDNA are the holy grail of HBV therapy. However, our understanding of the molecular mechanisms that regulate control of cccDNA activity in hepatocytes is limited. A number of strategies are currently being investigated to eradicate cccDNA, including inactivation through the generation of sequence‐specific endonucleases with zinc finger nucleases or to cleave cccDNA by using transcription activator‐like effector nucleases or clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR‐associated protein 9 (CAS9) system.19, 20, 21 Additional approaches being pursued are to use agents to upregulate APOBEC3A/B deaminases (see Table 1) that noncytolytically degrade cccDNA and to functionally silence cccDNA through modifiers of epigenetic regulation.17 The clinical applicability of all of these approaches is currently limited by efficient and targeted delivery of these molecules to all HBV‐infected cells and concerns for off‐target effects.
Targeting Viral Transcripts
Four viral genes are transcribed from the cccDNA template by host RNA polymerase II in the cell nucleus. These mRNA transcripts (core, polymerase, surface, and X genes) encode all the viral proteins. Technological advancements now permit gene silencing through the use of small interfering RNAs (siRNAs) that target viral transcripts and induce their degradation by the RNA‐induced silencing complex/argonaute 2 and by the use of antisense oligonucleotides that block protein expression through steric hindrance and/or RNA degradation by ribonuclease H cleavage (Table 1).22 Recently, small dihydroxyquinoline (DHQ) molecules have been identified that appear to selectively cause degradation of HBV transcripts, accomplishing much of what siRNA achieves.23, 24 These represent an entirely new approach to HBV antivirals and are in the development pipeline, having been shown to be effective in vitro and in vivo. The precise molecular target of these drugs is not clear but requires that the targeted RNA contain cis‐acting RNA sequences and may involve host degradation pathways. Cellular toxicity and how to efficiently deliver these compounds remain a challenge.
Targeting Nucleocapsid Assembly
The HBV core protein has many important roles in the HBV life cycle including assembly of viral nucleocapsids, it is the site of HBV DNA replication, stability of cccDNA through modification of chromatin, regulation of viral transcription, and modulation of the host innate immune response. Thus, development of modulators of nucleocapsid assembly have long been an attractive therapeutic target. Unfortunately, the first‐generation compounds were limited by hepatotoxicity.25 There are now many newer core protein allosteric modulators that have been shown to inhibit encapsidation of pregenomic RNA or nucleocapsid assembly, or both (Table 1).26, 27, 28, 29, 30, 31 Potential advantages of these compounds include high selectively and broad spectrum of activity against all HBV genotypes.
HBsAg Release Inhibitors
Clearance of HBsAg is one of the goals of a functional cure. HBsAg is made in vast excess of what is needed for enveloping viral nucleocapsids to complete virion assembly, and it has been speculated that HBsAg may contribute to the immune exhaustion observed in patients with chronic HBV infection. Thus, there have been efforts to block release of HBsAg from hepatocytes. A number of compounds have shown promise (see Table 1).32, 33, 34 However, there are concerns about the long‐term consequences of HBsAg retention in the hepatocyte because this has been associated with the development of hepatocellular carcinoma in animal models.35
Immunological Approaches
Chronic hepatitis B is characterized by a weak innate immune response, a defective humoral response with absence of neutralizing antibodies, and an exhausted adaptive response.36 Therefore, the other major effort in HBV therapeutics is to develop strategies to reinvigorate or boost the immune response. Broadly, strategies to induce an innate immune response include use of agonists of the sensing arm of the innate immune response (e.g. toll‐like receptor or retinoic acid–inducible gene I (RIG‐I) agonists) or to augment the response by selectively targeting HBV‐infected hepatocytes using T cell receptor‐like antibodies conjugated with inhibitory cytokines.37 Approaches to enhance the adaptive immune response include strategies to boost the limited HBV‐specific T cells already present by using checkpoint inhibitors,38, 39 or to genetically engineer new HBV‐specific T cells via T cell receptor gene transfer or chimeric antigen receptor T cells that can be adoptively transferred to patients.37, 40 There has also been renewed interest in developing therapeutic vaccines that can break T cell tolerance to HBV proteins and stimulate HBV‐specific T cell responses in patients with chronic infection.37 Protein, DNA, and T cell vaccines have been tested, but studies of therapeutic vaccination have been unsuccessful probably because they targeted only HBsAg. Newer approaches using vaccines incorporating multiple HBV proteins with or without adjuvants are in development.37 Major limitations of these immune‐based approaches are the potential to induce severe hepatitis flares, autoimmunity, and in the case of adoptive T cell therapy, issues with scalability.
Combination Therapy
It is very likely that combinations of antiviral therapy targeting multiple steps in the viral life cycle or combination antiviral/immunomodulatory approaches will be needed to achieve the goal of functional cure. Which specific agents will be required is currently not known, but with the development of new therapeutic agents, a combination of a DAA and host‐acting, perhaps immunorestorative agents, as shown in Fig. 2, will be possible. Although effective suppressive therapy exists for HBV, the development of multiple novel approaches brings achieving the goal of a functional cure into the realm of possibility.
Figure 2.
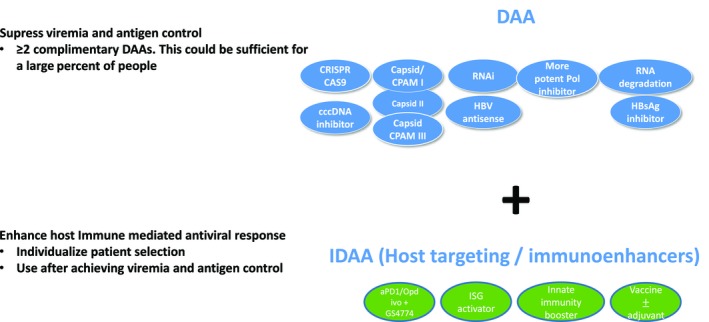
Possible combination therapy strategies to treat chronic HBV. As new therapies to manage HBV become available, it seems likely that combination therapy will be used. Each oval represents a distinct drug in development that is considered either direct acting (blue) or indirect acting (green), based on its mechanism of action. The IDAAs include those that target the host immune systems. One approach will be to first use DAAs to achieve suppression of viremia and antigenemia followed by IDAAs to clear remaining virus and effect functional cure. Abbreviation: RNAi, RNA interference.
Potential conflict of interest
M.G.G. is an employee of the U.S. Government and has no financial conflicts of interest to disclose.
M.G.G. and T.M.B. wrote and revised the manuscript.
This study was supported by Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
References
- 1. GBD 2016 Causes of Death Collaborators . Global, regional, and national age‐sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Hepatitis C. www.who.int/mediacentre/factsheets/fs164/en/. Published October 2, 2017. Accessed May 2, 2018.
- 3. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Severini A, Liu XY, Wilson JS, Tyrrell DL. Mechanism of inhibition of duck hepatitis B virus polymerase by (‐)‐beta‐L‐2′,3′‐dideoxy‐3′‐thiacytidine. Antimicrob Agents Chemother. 1995;39:1430‐1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu F, Campagna M, Qi Y, et al. Alpha‐interferon suppresses hepadnavirus transcription by altering epigenetic modification of cccDNA minichromosomes. PLoS Pathog. 2013;9:e1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alexander GJ, Nouri‐Aria KT, Neuberger J, et al. In vitro effects of lymphoblastoid interferon on lymphocyte activation and cell‐mediated cytolysis in patients with chronic hepatitis B virus infection. J Hepatol. 1986;3(suppl 2):S269‐S277. [DOI] [PubMed] [Google Scholar]
- 7. Peters M, Walling DM, Waggoner J, Avigan MI, Sjogren M, Hoofnagle JH. Immune effects of alpha‐interferon in chronic liver disease. J Hepatol. 1986;3(suppl 2):S283‐S289. [DOI] [PubMed] [Google Scholar]
- 8. Gill US, Peppa D, Micco L, et al. Interferon alpha induces sustained changes in NK cell responsiveness to hepatitis B viral load suppression in vivo. PLoS Pathog. 2016;12:e1005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liang TJ, Block TM, McMahon BJ, et al. Present and future therapies of hepatitis B: from discovery to cure. Hepatology. 2015;62:1893‐1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: from discovery to regulatory approval. Hepatology. 2017;66:1296‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schulze A, Gripon P, Urban S. Hepatitis B virus infection initiates with a large surface protein‐dependent binding to heparan sulfate proteoglycans. Hepatology. 2007;46:1759‐1768. [DOI] [PubMed] [Google Scholar]
- 12. Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhong G, Yan H, Wang H, et al. Sodium taurocholate cotransporting polypeptide mediates woolly monkey hepatitis B virus infection of Tupaia hepatocytes. J Virol. 2013;87:7176‐7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lempp FA, Wiedtke E, Qu B, et al. Sodium taurocholate cotransporting polypeptide is the limiting host factor of hepatitis B virus infection in macaque and pig hepatocytes. Hepatology. 2017;66:703‐716. [DOI] [PubMed] [Google Scholar]
- 15. Sankhyan A, Sharma C, Dutta D, et al. Inhibition of preS1‐hepatocyte interaction by an array of recombinant human antibodies from naturally recovered individuals. Sci Rep. 2016;6:21240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ying C, Van Pelt JF, Van Lommel A, et al. Sulphated and sulphonated polymers inhibit the initial interaction of hepatitis B virus with hepatocytes. Antivir Chem Chemother. 2002;13:157‐164. [DOI] [PubMed] [Google Scholar]
- 17. Lucifora J, Esser K, Protzer U. Ezetimibe blocks hepatitis B virus infection after virus uptake into hepatocytes. Antiviral Res. 2013;97:195‐197. [DOI] [PubMed] [Google Scholar]
- 18. Volz T, Allweiss L, Ben MM, et al. The entry inhibitor Myrcludex‐B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J Hepatol. 2013;58:861‐867. [DOI] [PubMed] [Google Scholar]
- 19. Ely A, Moyo B, Arbuthnot P. Progress with developing use of gene editing to cure chronic infection with hepatitis B virus. Mol Ther. 2016;24:671‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moyo B, Bloom K, Scott T, Ely A, Arbuthnot P. Advances with using CRISPR/Cas‐mediated gene editing to treat infections with hepatitis B virus and hepatitis C virus. Virus Res. 2018;244:311‐320. [DOI] [PubMed] [Google Scholar]
- 21. Seeger C, Sohn JA. Targeting hepatitis B virus with CRISPR/Cas9. Mol Ther Nucleic Acids. 2014;3:e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wooddell CI, Yuen MF, Chan HL, et al. RNAi‐based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci Transl Med 2017;9:eaan0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mueller H, Wildum S, Luangsay S, et al. A novel orally available small molecule that inhibits hepatitis B virus expression. J Hepatol. 2018;68:412‐420. [DOI] [PubMed] [Google Scholar]
- 24. Zhou T, Block T, Liu F, et al. HBsAg mRNA degradation induced by a dihydroquinolizinone compound depends on the HBV posttranscriptional regulatory element. Antiviral Res. 2018;149:191‐201. [DOI] [PubMed] [Google Scholar]
- 25. Deres K, Schroder CH, Paessens A, et al. Inhibition of hepatitis B virus replication by drug‐induced depletion of nucleocapsids. Science. 2003;299:893‐896. [DOI] [PubMed] [Google Scholar]
- 26. Mani N, Cole AG, Phelps JR, Ardzinski A, Cobarrubias KD, Cuconati A, et al. Preclinical profile of AB‐423, an inhibitor of Hepatitis B virus pregenomic RNA encapsidation. Antimicrob Agents Chemother. 2018;62:e00082‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu G, Liu B, Zhang Y, et al. Preclinical characterization of GLS4, an inhibitor of hepatitis B virus core particle assembly. Antimicrob Agents Chemother. 2013;57:5344‐5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berke JM, Dehertogh P, Vergauwen K, Van Damme E, Mostmans W, Vandyck K, Pauwels F. Capsid assembly modulators have a dual mechanism of action in primary human hepatocytes infected with hepatitis B virus. Antimicrob Agents Chemother. 2017;61:e00560‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klumpp K, Shimada T, Allweiss L, et al. Efficacy of NVR 3‐778, alone and in combination with pegylated interferon, vs entecavir in uPA/SCID mice with humanized livers and HBV infection. Gastroenterology. 2018;154:652‐662.e8. [DOI] [PubMed] [Google Scholar]
- 30. Delaney WE 4th, Edwards R, Colledge D, Shaw T, Furman P, Painter G, Locarnini S. Phenylpropenamide derivatives AT‐61 and AT‐130 inhibit replication of wild‐type and lamivudine‐resistant strains of hepatitis B virus in vitro. Antimicrob Agents Chemother. 2002;46:3057‐3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang L, Lu M. Small molecule inhibitors of hepatitis B virus nucleocapsid assembly: a new approach to treat chronic HBV infection. Curr Med Chem. 2018;25:802‐813. [DOI] [PubMed] [Google Scholar]
- 32. Noordeen F, Vaillant A, Jilbert AR. Nucleic acid polymers inhibit duck hepatitis B virus infection in vitro. Antimicrob Agents Chemother. 2013;57:5291‐5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Al‐Mahtab M, Bazinet M, Vaillant A. Safety and efficacy of nucleic acid polymers in monotherapy and combined with immunotherapy in treatment‐naive Bangladeshi patients with HBeAg+ chronic hepatitis B infection. PLoS One. 2016;11:e0156667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baugh SDP. Inhibiting the secretion of hepatitis B surface antigen (HBsAg) to treat hepatitis B infection: a review. Infect Disord Drug Targets. 2017;17:24‐35. [DOI] [PubMed] [Google Scholar]
- 35. Chisari FV, Klopchin K, Moriyama T, et al. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell. 1989;59:1145‐1156. [DOI] [PubMed] [Google Scholar]
- 36. Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut. 2012;61:1754‐1764. [DOI] [PubMed] [Google Scholar]
- 37. Bertoletti A, Bert NL. Immunotherapy for chronic hepatitis B virus infection. Gut Liv.; doi: 10.5009/gnl17233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu J, Zhang E, Ma Z, et al. Enhancing virus‐specific immunity in vivo by combining therapeutic vaccination and PD‐L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014;10:e1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fisicaro P, Valdatta C, Massari M, et al. Antiviral intrahepatic T‐cell responses can be restored by blocking programmed death‐1 pathway in chronic hepatitis B. Gastroenterology 2010;138:682‐693.e1‐4. [DOI] [PubMed] [Google Scholar]
- 40. Fisicaro P, Boni C, Barili V, Laccabue D, Ferrari C. Strategies to overcome HBV‐specific T cell exhaustion: checkpoint inhibitors and metabolic re‐programming. Curr Opin Virol. 2018;30:1‐8. [DOI] [PubMed] [Google Scholar]


