Watch a video presentation of this article
Answer questions and earn CME
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ALT
alanine aminotransferase
- CVD
cardiovascular disease
- HR
hazard ratio
- IDEAL
Initiating Dialysis Early and Late
- LDL
low‐density lipoprotein
- NAFLD
nonalcoholic fatty liver disease
- NAS
NASH histological activity score
- NASH
nonalcoholic steatohepatitis
- OR
odds ratio
- RCT
randomized controlled trial
- T2DM
type 2 diabetes mellitus
Cardiovascular disease (CVD) and nonalcoholic fatty liver disease (NAFLD) share a bidirectional relationship in which one condition could potentiate the other; however, the causal relationship independent of other metabolic risk factors remains uncertain.1, 2, 3, 4 Although rates of incident CVD have been shown to be higher in patients with NAFLD,5, 6, 7, 8, 9 CVD has been established as the most common cause of mortality in patients with NAFLD.10 Because the prevalence of NAFLD is high among patients with metabolic risk factors for CVD, including obesity, type 2 diabetes mellitus (T2DM), and dyslipidemia, aggressive modification of CVD risk factors could potentially reduce cardiovascular and liver‐related morbidity in patients with NAFLD.
Effect of NAFLD on CVD
Growing literature suggests that NAFLD could potentially lead to excess risk for CVD‐related morbidity and mortality. A systematic review and meta‐analysis of 34 studies showed that NAFLD was associated with an increased risk for incident CVD (hazard ratio [HR], 1.37) and specifically an increased risk for coronary artery disease (HR, 2.31) and hypertension (1.16) but was not associated with CVD‐related mortality and overall mortality when compared with patients without NAFLD.8 The majority of these studies included patients with underlying risk factors for CVD, including smoking and T2DM, yet the significant associations of NAFLD persisted despite controlling for these risk factors.8 A large prospective cohort study by Wong et al.5 revealed a higher prevalence of coronary artery stenosis (84.6% versus 64.1%; P < 0.001) and need for percutaneous coronary intervention (68.3% versus 43.4%; P < 0.001) in patients with NAFLD versus without NAFLD. However, no significant difference was observed between patients with and without NAFLD in the composite cardiovascular outcome, which was primarily associated with T2DM (HR, 1.37; P = 0.03).5 A meta‐analysis of 16 cohort studies with median 7‐year follow‐up revealed that patients with NAFLD were 64 times more likely than patients without NAFLD to have fatal or nonfatal cardiovascular events, such as myocardial infarction, stroke, angina, or coronary revascularization.6 An additional analysis of 1051 Framingham Heart Study participants revealed that patients with increased liver fat on multidetector computed tomography at baseline experienced higher incident cardiovascular risk factors including hypertension (odds ratio [OR], 1.42; P < 0.001) and T2DM (OR, 1.43; P < 0.001).7 A recent retrospective cohort study of 134,368 people with T2DM showed that patients with NAFLD were not only more likely to have incident or recurrent CVD events (HR, 1.70), they were also more likely to have greater all‐cause mortality (HR, 1.60) compared with patients without liver disease.9 Therefore, although a causal mechanism by which CVD contributes to NAFLD has yet to be elucidated, NAFLD has been identified as an independent risk factor for increased CVD‐related morbidity and all‐cause mortality.
Effect of CVD on NAFLD
Patients with NAFLD bear higher CVD‐related mortality than the general population.1, 2 Two large cohort studies have confirmed this observation, including a report by Adams et al.,1 which revealed that 25% of all deaths in 420 patients with NAFLD were due to ischemic heart disease, and a report by Ekstedt et al.2 in which the leading cause of death in a cohort of patients with NAFLD was CVD, which was significantly greater than the general population (15.5% versus 7.5%; P = 0.04). A third report of 132 patients with histologically proven NAFLD confirmed CVD and malignancy as the leading causes of mortality in this cohort with more than 18 years of follow‐up.3 The effect of CVD on NAFLD is closely associated with T2DM; both studies by Adams et al.1 and Ekstedt et al.2 identified T2DM as an important covariate among patients with NAFLD. In the study by Adams et al.,1 T2DM or impaired glucose tolerance was an independent predictor of increased mortality in patients with NAFLD versus the general population (HR, 2.6). Ekstedt et al.2 suggested that, because 78% of patients with NAFLD were diagnosed with impaired glucose at follow‐up, progression of NAFLD was closely associated with type 2 diabetes. The central role of CVD and its associated metabolic cofactors (e.g., diabetes) in mortality in patients with NAFLD represents an important contrast with patients with other chronic liver diseases, such as hepatitis C infection, in whom mortality is driven by liver‐related outcomes.11
Modification of Metabolic Risk Factors to Improve Liver and Cardiovascular‐Related Morbidity in NAFLD
The strong association between CVD and NAFLD supports the need to aggressively modify metabolic risk factors as recommended by both American and European hepatology guidelines.10, 12 Guidelines of the European Association for the Study of the Liver have recommended all individuals with NAFLD undergo screening for metabolic risk factors, including obesity, diabetes mellitus, dyslipidemia, and hypertension.12
Weight loss of up to 10% has been shown to improve steatosis and necroinflammation. A trial of 31 obese patients with nonalcoholic steatohepatitis (NASH) demonstrated that those who were randomized to the intensive lifestyle changes arm (200 minutes of moderate physical activity per week for 48 weeks, diet, and behavior modification) had greater weight loss than those in the dietary counseling alone arm (9.3% versus 0.2%; P= 0.003).13 The primary outcome, which was the change in NASH histological activity score (NAS) after 48 weeks of intervention, was greater in the intensive versus control group (72% versus 30%; P = 0.03).13 In addition, those who lost ≥7% of their baseline body weight compared with those who achieved less than 7% hadsignificant improvements in NAS (−3.45 versus −1.18; P < 0.001).13
Limited studies have investigated the use of metformin in patients with diabetes in the setting of NAFLD. A trial of 48 patients with histologically proven NAFLD (27% had T2DM), who were randomized to receive metformin versus placebo while adhering to a similar diet and exercise intervention in both arms for 6 months, showed no significant effect on liver histology but did demonstrate improvement in body weight (P < 0.001), hemoglobin A1c (P = 0.02), and cholesterol levels (P = 0.004).14 Although metformin can modify CVD risk in patients with NAFLD, it is not currently recommended to specifically treat NAFLD.
The role of statins to treat NASH has not been studied using randomized controlled trials (RCTs) with histological endpoints; however, some post hoc analyses have demonstrated improvement in CVD‐related mortality in patients with NAFLD who receive statins.15, 16 A post hoc analysis of the Greek Atorvastatin and Coronary Heart Disease Evaluation Study compared improvement in liver tests and cardiovascular events in 437 patients with coronary artery disease and presumed NAFLD based on moderately abnormal liver enzymes.15 Patients with presumed NAFLD, who were treated with atorvastatin, had improvement in liver enzymes (P < 0.001) and a 68% relative risk reduction (P < 0.001) in cardiovascular events compared with those who did not receive a statin.15 The beneficial effect of statins for patients with presumed NAFLD was interestingly greater than the effect for patients without NAFLD (39% relative risk reduction for cardiovascular events in non‐NAFLD patients with versus without statin; P < 0.001).15 Another post hoc analysis described the value of intensive management of dyslipidemia in patients with possible NAFLD.16 The Initiating Dialysis Early and Late (IDEAL) trial compared high‐intensity versus moderate‐intensity statin therapy for the prevention of cardiovascular events in patients with coronary artery disease with normal versus abnormal alanine aminotransferase (ALT) levels.16 The IDEAL study revealed that major cardiovascular events were reduced in the high‐intensity versus moderate‐intensity arm in patients with elevated baseline ALT (HR, 0.56; P = 0.0056).16
Therefore, patients with NAFLD could potentially have a greater reduction in liver‐related or cardiovascular‐related morbidity from intense modification of metabolic risk factors in light of limited proposed treatments of NAFLD (Table 1). Patients with NAFLD who meet criteria for treatment of obesity, T2DM, dyslipidemia, and hypertension should receive vigilant follow‐up to ensure adherence to and tolerability of treatment given the potential benefits. Notably, because there is no evidence to show increased drug‐related liver injury from statins in patients with NAFLD, statins are not contraindicated in the treatment of dyslipidemia.10
Table 1.
Proposed Treatments of NAFLD
| Treatment | Study | Patient Population | Significant Outcomes | Current AASLD Recommendation10 |
|---|---|---|---|---|
| Pioglitazone | RCT17: pioglitazone 45 mg/day versus placebo + hypocaloric diet for 6 months | 55 NASH patients with T2DM or impaired glucose tolerance |
• Pioglitazone improved glycemic control, aminotransferases, steatosis, balloon necrosis, and inflammation compared with placebo • No difference in reducing fibrosis • Significant weight gain with pioglitazone |
Pioglitazone can be used to treat histologically proven NASH with the caveat that the pioglitazone trials studied mostly nondiabetic patients and do not have long‐term data on safety and efficacy. |
| RCT18: pioglitazone 30 mg/day versus placebo + lifestyle intervention for 12 months | 74 nondiabetic patients with NASH |
• Pioglitazone improved glucose levels, aminotransferases, hepatocellular injury, and fibrosis compared with placebo • No difference in steatosis • Significant weight gain with pioglitazone |
||
| RCT19: pioglitazone 30 mg/day versus placebo for 96 weeks | 247 nondiabetic patients with NASH |
• Pioglitazone improved aminotransferases • Pioglitazone versus placebo had no difference in NASH histology, fibrosis, and portal inflammation • Significant weight gain with pioglitazone |
||
| Meta‐analysis of 19 pioglitazone trials that identified CVD outcomes20 | 16,390 patients with T2DM |
• Pioglitazone was associated with reduction in death, myocardial infarction, or stroke • Pioglitazone had a higher rate of congestive heart failure |
||
| Vitamin E | RCT19: vitamin E 800 IU/day versus placebo for 96 weeks | 247 nondiabetic patients with NASH |
• Vitamin E versus placebo improved histological features of NASH and aminotransferases • No difference in fibrosis and portal inflammation |
Vitamin E 800 IU/day is considered first‐line therapy for patients with histologically proven NASH without diabetes and cirrhosis. |
| Meta‐analysis of 19 vitamin E trials that assessed mortality21 | 135,967 patients with NASH | • High‐dosage vitamin E ≥400 IU/day showed increased risk for all‐cause mortality in comparisons of vitamin E versus control | ||
| Bariatric surgery | Prospective cohort study to evaluate fibrosis and NASH outcomes 5 years after bariatric surgery22 | 381 severely obese patients |
• Percentage of patients with steatosis and percentage of patients with probable or definite NASH decreased • Inflammation remained unchanged • Fibrosis slightly increased |
Bariatric surgery is not recommended to specifically treat NASH. However, bariatric surgery can be considered in eligible obese patients with NASH in the absence of cirrhosis. |
| Systematic review to assess the benefits and harms of bariatric surgery for NASH in obese patients23 | 21 prospective, retrospective cohort studies |
• The benefits and harms of bariatric surgery for treatment of NASH cannot be determined in the absence of RCTs • Steatosis or inflammation scores improved, but fibrosis worsened in some prospective and retrospective cohort studies |
Abbreviation: AASLD, American Association for the Study of Liver Diseases.
Conclusion
The causal relationship of CVD and NAFLD remains under investigation, but the strong bidirectional association between CVD and NAFLD warrants clinical intervention in patients with NAFLD to modify metabolic risk factors, including T2DM, dyslipidemia, hypertension, and obesity. Although current cardiovascular society guidelines have not identified NAFLD as an independent risk factor for CVD despite recent studies suggesting NAFLD's role in incident CVD, vigilant age‐appropriate screening and treatment for associated risk factors, including weight loss for obesity, glycemic control for T2DM, and treatment of hypertension and hyperlipidemia, remain prudent strategies that should be supported by clinicians managing patients with NAFLD. A screening and management algorithm for associated metabolic risk factors in patients with NAFLD is proposed in Figure 1. Additional research is needed tofurther define the independent contribution of NAFLD to cardiovascular risk to inform future evidence‐based guidelines for clinical practice.
Figure 1.
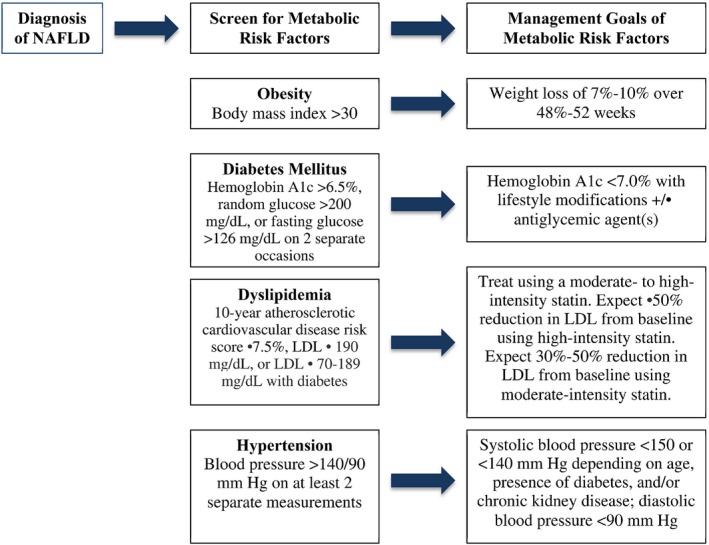
Proposed algorithm to screen for and manage metabolic risk factors in patients with NAFLD. Abbreviation: LDL, low‐density lipoprotein.
Further Educational NAFLD Resources can be found through the Following
Fundamentals of Liver Disease online program, Non‐Alcoholic Fatty Liver Disease & Non‐Alcoholic Steatohepatitis unit: https://liverlearning.aasld.org/aasld/2017/fold_2017/193371/aasld.html?f=c_id=193371
Program Overview
The Fundamentals of Liver Disease Non‐Alcoholic Fatty Liver Disease educational unit consists of 9 online interactive, narrated modules 30 to 40 minutes in length. The overall goal of the Fundamentals of Liver Disease program is to improve patient care by increasing learner competence and confidence in both proper patient identification and assessment and increasing learner performance in therapeutic options and on‐treatment management strategies for patients. Patient outcomes will be improved as a result of the improvements in more providers understanding these key components in the management and care of patients with liver diseases.
Key Topics Included in the Fundamentals of Liver Disease Non Alcoholic Fatty Liver Disease Unit:
NAFLD ‐ Diagnosis and Identification of Those at Risk of Disease Progression.
Pathogenesis of Non‐alcoholic Steatohepatitis.
Non‐Invasive Determination of Advanced Disease in NAFLD.
Lifestyle Interventions: Setting Goals and Recommendations.
Comorbidities in NAFLD: Cardiovascular Disease, Sleep Apnea, and Chronic Kidney Disease.
Bariatric Surgery: Is the Cure for NASH in the OR?
Managing NASH Cirrhosis and Assessment for Liver Transplant.
Current Pharmacological Treatment Options for Non‐Alcoholic Steatohepatitis (NASH).
Emerging Treatments for Non‐Alcoholic Steatohepatitis.
Potential conflict of interest: Nothing to report.
References
- 1. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: A population‐based cohort study. Gastroenterology 2005;129:113‐121. [DOI] [PubMed] [Google Scholar]
- 2. Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long‐term follow‐up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865‐873. [DOI] [PubMed] [Google Scholar]
- 3. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413‐1419. [DOI] [PubMed] [Google Scholar]
- 4. Targher G, Day CP, Bonora B. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341‐1350. [DOI] [PubMed] [Google Scholar]
- 5. Wong VW‐S, Wong GL, Yeung JC, Fung CY, Chan JK, Chang ZH, et al. Long‐term clinical outcomes after fatty liver screening in patients undergoing coronary angiogram: A prospective cohort study. Hepatology 2016;63:754‐763. [DOI] [PubMed] [Google Scholar]
- 6. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non‐alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta‐analysis. JHepatol 2016;65:589‐600. [DOI] [PubMed] [Google Scholar]
- 7. Ma J, Hwang SJ, Pedley A, Massaro JM, Hoffmann U, Chung RT, et al. Bi‐directional analysis between fatty liver and cardiovascular disease risk factors. JHepatol 2017;66:390‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Association of non‐alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta‐analysis. Sci Rep 2016;6:33386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wild SH, Walker JJ, Morling JR, McAllister DA, Colhoun HM, Farran B, et al. Cardiovascular disease, cancer, and mortality among people with type 2 diabetes and alcoholic or nonalcoholic fatty liver disease hospital admission. Diabetes Care 2018;41:341‐347. [DOI] [PubMed] [Google Scholar]
- 10. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- 11. Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: An international collaborative study. Hepatology 2011;54:1208‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), and European Association for the Study of Obesity (EASO) . EASL–EASD–EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. JHepatol 2016;64:1388‐1402. [DOI] [PubMed] [Google Scholar]
- 13. Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010;51:121‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, et al. Metformin in patients with non‐alcoholic fatty liver disease: A randomized, controlled trial. Scand J Gastroenterol 2009;44:853‐860. [DOI] [PubMed] [Google Scholar]
- 15. Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, et al.; GREACE Study Collaborative Group . Safety and efficacy of long‐term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: A post‐hoc analysis. Lancet 2010;376:1916‐1922. [DOI] [PubMed] [Google Scholar]
- 16. Tikkanen MJ, Fayyad R, Faergeman O, Olsson AG, Wun CC, Laskey R, et al.; IDEAL Investigators . Effect of intensive lipid lowering with atorvastatin on cardiovascular outcomes in coronary heart disease patients with mild‐to‐moderate baseline elevations in alanine aminotransferase levels. Int J Cardiol 2013;168:3846‐3852. [DOI] [PubMed] [Google Scholar]
- 17. Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo‐controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;22:2297‐2307. [DOI] [PubMed] [Google Scholar]
- 18. Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, et al. Randomized, placebo‐controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 2008;135:1176‐1184. [DOI] [PubMed] [Google Scholar]
- 19. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lincoff A, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: A meta‐analysis of randomized trials. JAMA 2007;298:1180‐1188. [DOI] [PubMed] [Google Scholar]
- 21. Miller ER 3rd, Pastor‐Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta‐analysis: high‐dosage vitamin E supplementation may increase all‐cause mortality. Ann Intern Med 2005;142:37‐46. [DOI] [PubMed] [Google Scholar]
- 22. Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, et al. Prospective study of the long‐term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology 2009;137:532‐540. [DOI] [PubMed] [Google Scholar]
- 23. Chavez‐Tapia NC, Tellez‐Avila FI, Barrientos‐Gutierrez T, Mendez‐Sanchez N, Lizardi‐Cervera J, Uribe M. Bariatric surgery for non‐alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev 2010;1:CD007340. [DOI] [PMC free article] [PubMed] [Google Scholar]


