Watch a video presentation of this article
Abbreviations
- anti‐HBc+
hepatitis B core antibody–positive
- AWP
average wholesale price (US $)
- CMV
cytomegalovirus
- DAA
direct‐acting antiviral
- DNH
de novo hepatitis
- FW
follow‐up week
- HCV+
hepatitis C virus–infected
- HCV−
hepatitis C virus‐uninfected
- Ig
immunoglobulin
- IU
international unit
- NAT
nucleic acid test
- POD
postoperative day
- SVR
sustained virological response
- TW
treatment week
Key Points
Hundreds of high‐quality deceased‐donor organs are discarded each year because of detection of hepatitis C infection, delaying lifesaving transplants.
Multiple studies have shown that direct‐acting antiviral (DAA) therapy is both safe and highly effective in preventing or treating donor‐derived hepatitis C infection in patients with solid organ transplant.
Pan‐genotypic DAA therapy is cost‐effective, and insurance coverage has not proven to be a major issue for patients post transplant.
There are more than 125,000 individuals in need of solid organ transplant in the United States.1 Depending on the organ type, national data indicate that approximately half of these patients will undergo transplantation within 1 year, whereas nearly 20% will be removed from the wait list because of clinical deterioration or death.1 Expanding the donor pool to include hepatitis C virus–infected (HCV+) donor organs is an important means to bridge this gap.
HCV+ Organs Are Increasingly Available Yet Frequently Discarded
Due to the catastrophic opiate epidemic, the proportion of deceased HCV+ donors has risen significantly, with an overall prevalence rate of 8.5% among potential donors and more than 30% prevalence rate among those dying of drug overdose.2 Nearly 4% of donors may be viremic by screening nucleic acid test (NAT) at donation. These potential donors are younger and have little comorbidity.2, 3 Despite these qualities, HCV+ organs are discarded at high rates. A 2018 study showed 3.7‐fold higher discard of HCV+ kidneys compared with matched HCV‐uninfected (HCV−) donor kidneys, including discard of 388 HCV+ kidneys in 2017 alone.3
DAA Therapy Safely and Effectively Cures HCV Infection in Transplant Recipients
Discard of HCV+ donor organs should be reconsidered because DAA therapy has revolutionized HCV treatment via well‐tolerated, highly effective regimens (Table 1) exhibiting sustained virological response rates (SVR12) greater than 95%.4 Several large series demonstrate that DAA therapy is equally effective among HCV+ transplant recipients, with cure rates near 100% predominately using 12‐week, interferon‐ and ribavirin‐free regimens.5 As a result of these data, national guidelines clearly support the use of DAAs to cure HCV post transplant.4
Table 1.
Relative Costs of Common Therapies Used for Transplant Recipients
| Indication | Therapy | Treatment Cost (AWP*) |
|---|---|---|
| HCV infection | Glecaprevir/pibrentasvir (Mavyret) | $47,520/12 weeks |
| Elbasvir/grazoprevir (Zepatier) | $65,520/12 weeks | |
| Sofosbuvir/velpatasvir (Epclusa) | $89,712/12 weeks | |
| Ledipasvir/sofosbuvir (Harvoni) | $113,400/12 weeks | |
| Daclatasvir (Daklinza) + sofosbuvir (Sovaldi) | $176,400/12 weeks | |
| Hepatitis B virus infection | Entecavir (Baraclude) | $15,996.60/12 months |
| Lamivudine (Epivir) | $5,799.96/12 months | |
| Cytomegalovirus infection | Valganciclovir (Valcyte) | $47,684.52/6 months |
| End organ support | Hemodialysis (Maintenance) | $250,000/12 months |
| Left ventricular assist device (Placement) | $732,000/once | |
| Left ventricular assist device (Maintenance) | $30,000‐$580,000/12 months |
Estimates based on Lexicomp drug data (https://online.lexi.com) and UnitedHealth Group Analysis (2009‐2015).
(US $).
The organ shortage, increase in availability of HCV+ donors, and success with DAAs have prompted a series of single‐center trials of HCV NAT+ donor organs into HCV− recipients (HCV D+/R−), with excellent outcomes. First, in the Transplanting Hepatitis C Kidneys into Negative Kidney Recipients (THINKER) trial,6 10 HCV D+/R− transplants using genotype 1 NAT+ kidneys were performed with preemptive elbasvir/grazoprevir for 12 weeks after detection of viremia (seen in all patients by day 3). Our center conducted the Exploring Renal Transplants Using Hepatitis C Infected Donors for HCV Negative Recipients (EXPANDER) trial,7 performing 10 HCV D+/R− transplants using prophylactic elbasvir/grazoprevir started preoperatively and continued for 12 to 16 weeks, plus sofosbuvir for genotype 2 or 3 donor infections (Fig. 1). No recipients in either trial experienced chronic hepatitis C infection, significant hepatopathy, rejection, graft loss, or death. In both trials, wait times were short (1‐2 months), and organ quality was excellent (kidney donor profile index 42%‐45%). There were no definite treatment‐attributable adverse events, although one THINKER patient with pretransplant immunoglobulin A (IgA) nephropathy had proteinuria and focal segmental glomerulosclerosis after SVR12, of uncertain significance.
Figure 1.
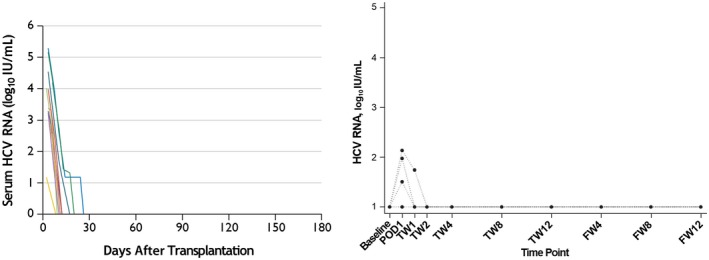
HCV RNA serum levels in HCV‐uninfected recipients of kidneys from HCV‐infected donors. (Left) Data from the THINKER trial6 in which 10 participants received preemptive posttransplant DAA therapy: 12 weeks of elbasvir/grazoprevir once HCV RNA was detected. (Right) Data from the EXPANDER trial7 in which 10 participants received prophylactic pretransplant and posttransplant DAA therapy: elbasvir/grazoprevir +/− sofosbuvir for 12 to 16 weeks. Abbreviations: FW, follow‐up week; IU, international unit; POD, postoperative day; TW, treatment week. Adapted with permission from The New England Journal of Medicine 6 and Annals of Internal Medicine 7.
Since then, other centers have presented or published results of 124 HCV D+/R− transplants (97 HCV NAT+) now totaling 55 heart, 40 liver, 20 kidney, 7 lung, and 2 heart/kidney grafts (Table 2).8, 9, 10, 11, 12, 13 Several DAA regimens were used against multiple HCV genotypes, resulting in universal prevention or SVR12 in all treated patients with sufficient follow‐up. This included observational studies in which patients received DAAs several weeks after transplantation, obtained outside of the trial setting. Neither treatment‐attributable adverse effects nor insurmountable insurance barriers were reported, and wait times were brief after consent to accept HCV+ donors.
Table 2.
Summary of Recent Studies of HCV+ Donor Organ Transplantation Into HCV‐Uninfected Recipients
| Author | Center | Transplant Recipients (n) | HCV Donor NAT+ (n, %) | Genotype (n) | Prophylactic or Preemptive Design | DAA Therapy (n) | SVR12 or Virological Suppression * (n, %) |
|---|---|---|---|---|---|---|---|
| Goldberg et al. (2017)6 | University of Pennsylvania | Kidney (10) | 10/10 (100) | 1a (9) | Preemptive | Elbasvir/grazoprevir × 12 weeks (10) | 10/10 (100) |
| Not typed (1) | |||||||
| Durand et al. (2018)7 | Johns Hopkins University | Kidney (10) | 10/10 (100) | 1a/3 (1) | Prophylactic | Elbasvir/grazoprevir × 12 weeks (7) | 10/10 (100) |
| 1a (3) | Elbasvir/grazoprevir + Sofosbuvir × 12 weeks (3) | ||||||
| 2 (1) | |||||||
| 3 (1) | |||||||
| Not detected | |||||||
| (4) | |||||||
| Bari et al. (2018)11 | University of Cincinnati | Liver (26) | 4/26 (15) † | 1a (2) | Preemptive | Ledipasvir/sofosbuvir + Ribavirin × 12 weeks (1) | 3/3 (100) |
| 3 (2) | Velpatasvir/sofosbuvir × 12 weeks (1) | ||||||
| Sofosbuvir/daclatasvir × 12 weeks (1) | |||||||
| Ledipasvir/sofosbuvir × 12 weeks (1) | |||||||
| Schlendorf et al. (2018)8 | Vanderbilt University | Heart (13) | 9/13 (69) | 1a (6) | Preemptive | Ledipasvir/sofosbuvir × 12 weeks (7) | 9/9 (100) |
| 1b (1) | Sofosbuvir/velpatasvir × 12‐24 weeks (2) | ||||||
| 3 (2) | |||||||
| Not detected (2) | |||||||
| Alonso et al. (2018)9 | Intermountain Healthcare | Liver (12) | 12/12 (100) | 1a/1b (5) | Preemptive | Sofosbuvir/ledipasvir × 12 weeks (4) | 5/5 (100) |
| 3a (5) | Sofosbuvir/velpatasvir × 12 weeks (1) | ||||||
| 2b (1) | |||||||
| Not typed (1) | |||||||
| Aslam et al. (2018)12 | University of California, San Diego | Heart (10) | 10/12 (83) | 1a (6) | Preemptive | Sofosbuvir/velpatasvir × 12 weeks (2) | 10/10 (100) |
| Heart/kidney (2) | 2 (1) | Glecaprevir/pibrentasvir × 12 weeks (6) | |||||
| 2b (1) | Elbasvir/grazoprevir × 12 weeks (2) | ||||||
| 3 (2) | |||||||
| Kwong et al. (2018)10 | Stanford University | Heart (8) | 9/10 (90) | 1a (5) | Preemptive | Not reported in abstract | 5/5 (100) |
| Liver (2) | 1b (1) | ||||||
| 3 (4) | |||||||
| Woolley et al. (2018)13 | Brigham and Women’s Hospital | Heart (24) | 31/31 (100) | Not reported in abstract | Prophylactic | Sofosbuvir/velpatasvir × 4‐6 weeks (31) | 20/20 (100) |
| Lung (7) |
*
By the end of the study period. Patients who did not receive the intended DAA treatment were excluded. One recurrence was noted.
†
All donors were HCV antibody–positive and NAT‐negative, yet four recipients developed HCV viremia post transplant.
Transitioning From Studies To Standards In Transplant Medicine
Controversy surrounding the use of HCV D+/R− transplantation remains in determining whether the field can now transition from research studies to standard clinical care. Practice guidelines are ideally achieved through graded, high‐quality studies such as multicenter, blinded, randomized controlled trials. Multiple factors limit this process in transplantation. Populations of interest are smaller and exhibit significant heterogeneity with respect to underlying disease processes and immune suppression protocols. In addition, fully blinded interventions are rarely feasible or ethical. As such, less robust data appropriately drive standard clinical practice and guideline development. For example, as acknowledged in the 2013 American Society of Transplantation Infectious Diseases Guidelines,14 which inform current transplant infectious diseases practice, most recommendations are based on evidence level II (ie, “non‐randomized trials, cohort or case‐control analyses, uncontrolled experiments”) or III (ie, “consensus opinion”). The HCV D+/R− studies to date already meet or surpass data for these current standards.
“Acceptable” Donor‐Derived Infections: The Hazards Of Cytomegalovirus And Hepatitis B Virus
Additional reticence to proceed with HCV D+/R− transplantation centers on dangers of donor‐derived infection, extrapolating from experience in the pre‐DAA era when medications were poorly tolerated and often ineffective. Ironically, however, transmission of higher‐risk, incurable, donor‐derived viral infections are currently standards of care in transplantation. For example, transplantation of a hepatitis B core antibody–positive (anti‐HBc+) graft into the nonimmune, unexposed recipient occurs in ~3% of liver transplants each year.15 This standard practice occurs despite historical data of high rates of de novo hepatitis (DNH) associated with increased graft fibrosis and a 2.5‐fold higher risk for death by 5 years post transplant.16 DNH may occur despite antiviral therapy and vaccination, thus requiring lifelong treatment to suppress this incurable donor‐derived infection. A more widespread and perhaps more hazardous intervention is the use of cytomegalovirus (CMV) mismatched grafts (ie, donor IgG+, recipient IgG− [D+/R−]). More than 50% of the CMV antibody–negative recipients in 2016 to 2017 received a CMV D+ organ, accounting for more than 5000 transplants.15 CMV D+/R− transplantation is associated with high rates of viremia, even after antiviral prophylaxis, which itself incurs significant cost (Table 2) and serious negative side effects.17 Posttransplant mortality is higher in CMV D+/R− transplantation, and late‐onset CMV disease post prophylaxis is associated with increased rejection, graft loss, and opportunistic infection.18 Notwithstanding, both anti‐HBc+ D+/R− and CMV D+/R− transplantation remain acceptable clinical practice given the survival benefit of transplantation.
HCV D+/R− Transplantation Should Become Standard of Care
In summary, accumulated data and experience indicate that HCV D+/R− transplantation is an underused strategy and a mode to safely expand the donor pool to include lifesaving, high‐quality organ transplants immediately for patients in need. With DAA therapy, HCV infection is readily curable in transplant recipients, with minimal side effects. Restricting HCV D+/R− transplantation to research protocols would result in the unnecessary deaths of hundreds of wait‐list patients each year. Thus, we propose that transplant teams consider HCV D+ organs for all prospective recipients as part of clinical care.
Potential conflict of interest: Nothing to report.
References
- 1. National Data: Organ Procurement and Transplantation Network . 2018. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. Accessed June 24, 2018.
- 2. Durand CM, Bowring MG, Thomas AG, et al. The drug overdose epidemic and deceased‐donor transplantation in the United States: A national registry study. Ann Intern Med 2018;168:702‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bowring MG, Kucirka LM, Massie AB. Changes in utilization and discard of HCV‐antibody positive deceased‐donor kidneys in the era of direct‐acting antiviral therapy. Transplantation 2018;1‐10. doi: 10.1097/TP.0000000000002323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Association for the Study of Liver Diseases‐Infectious Diseases Society of America . HCV guidance: recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org. Accessed June 18, 2018. [DOI] [PMC free article] [PubMed]
- 5. Selzner N, Berenguer M. Should organs from hepatitis C‐positive donors be used in hepatitis C‐negative recipients for liver transplantation? Liver Transpl 2018;24:831‐840. [DOI] [PubMed] [Google Scholar]
- 6. Goldberg D, Abt PL, Blumberg EA, et al. Trial of transplantation of HCV‐infected kidneys into uninfected recipients. N Engl J Med 2017;376:2394‐2395. [DOI] [PubMed] [Google Scholar]
- 7. Durand CM, Bowring MG, Brown DM, et al. Direct‐acting antiviral prophylaxis in kidney transplantation from hepatitis C virus‐infected donors to noninfected recipients: An open‐label nonrandomized trial. Ann Intern Med 2018;168:533‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schlendorf KH, Zalawadiya S, Shah AS, et al. Early outcomes using hepatitis C–positive donors for cardiac transplantation in the era of effective direct‐acting anti‐viral therapies. J Heart Lung Transpl 2018;37:763‐769. [DOI] [PubMed] [Google Scholar]
- 9. Alonso D, Fujita S, Zendejas I, Rodriguez M, Harmston G, Jones R, et al. Liver transplantation utilizing donor livers from HCV RNA positive donors into HCV RNA negative recipients. ASTS Winter Symposium, Miami, USA: January 13th, 2018. [Google Scholar]
- 10. Kwong A, Wall A, Melcher M, et al. Antiviral therapy for donor‐derived hepatitis C virus infection after solid organ transplantation [Abstract 570]. American Transplant Congress; Seattle, WA; June 5, 2018. [Google Scholar]
- 11. Bari K, Luckett K, Kaiser T, et al. Hepatitis C transmission from seropositive, nonviremic donors to non‐hepatitis C liver transplant recipients. Hepatology 2018;67:1673‐1682. [DOI] [PubMed] [Google Scholar]
- 12. Aslam S, Mariski G, Pretorius G, Adler E. Heart and heart‐kidney transplantation from hepatitis C virus (HCV) positive donors into HCV‐negative recipients. J Heart Lung Transpl 2018;37:S76‐S77. [Google Scholar]
- 13. Woolley AE, Singh SK, Mallidi HR, et al. Transplanting thoracic organs from hepatitic C positive donors to hepatitis C uninfected patients. J Heart Lung Transpl 2018;37:S142. [Google Scholar]
- 14. Blumberg EA, Danziger‐Isakov L, Kumar D, Michaels MG, Razonable RR. Foreword: Guidelines 3. Am J Transplant 2013;13:1‐2. [DOI] [PubMed] [Google Scholar]
- 15. United Network for Organ Sharing . Data. https://unos.org/data/. Accessed June 24, 2018.
- 16. Huprikar S, Danziger‐Isakov L, Ahn J, et al. Solid organ transplantation from hepatitis B virus–positive donors: consensus guidelines for recipient management. Am J Transplant 2015;15:1162‐1172. [DOI] [PubMed] [Google Scholar]
- 17. Hakimi Z, Aballea S, Ferchichi S, et al. Burden of cytomegalovirus disease in solid organ transplant recipients: a national matched cohort study in an inpatient setting. Transpl Infect Dis 2017;19:e12732. [DOI] [PubMed] [Google Scholar]
- 18. Arthurs SK, Eid AJ, Pedersen RA, et al. Delayed‐onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis 2008;46:840‐846. [DOI] [PubMed] [Google Scholar]


