Watch a video presentation of this article
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ALT
alanine aminotransferase
- DNL
de novo lipogenesis
- ER
endoplasmic reticulum
- FFA
free fatty acids
- HOMA‐IR
homeostatic model assessment‐insulin resistance
- LFT
liver function test
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TAG
triglycerides
- UDCA
ursodeoxycholic acid
Nonalcoholic steatohepatitis (NASH) is the inflammatory sequelae of fatty liver disease that is characterized histologically by steatosis with ballooning degeneration and lobular hepatitis. NASH and particularly fibrosis development are significantly associated with increased all‐cause and liver‐related mortality.1, 2 By 2030, NASH is projected to affect more than 27 million patients in the United States and be attributed to 10.9% of all patient deaths.3 Despite this major public health burden, no U.S. Food and Drug Administration–approved medications exist for the specific treatment of NASH, identifying an unmet need for a further understanding of disease‐causing processes.
Since the initial proposal of the “two‐hit” mechanism of NASH by Berson et al.,4 great progress has been made in understanding the role of oxidant stress in the development of steatohepatitis (reviewed by Singal et al.5 and Kawano and Cohen6). Basic and translational data have culminated in the current understanding of the pathogenesis of NASH summarized briefly, whereby environmental factors and nutrient excess in predisposed individuals favor the development of obesity, insulin resistance, and the accumulation of intrahepatic triglyceride (Fig. 1). Reactive oxygen species (ROS) and reactive nitrogen species (RNS) production are required for normal metabolic functioning, but in NASH, pathological elevation of intracellular oxidant stress overwhelms antioxidant defenses and leads to oxidized DNA damage, protein malfunctioning, and enhanced peroxidation of intracellular lipids. These events spur hepatocyte apoptosis with release of proinflammatory cytokines, triggering inflammatory hepatic infiltrates that initiate stellate cell activation and elevated extracellular collagen deposition. Thus, interventions that abate hepatic oxidant stress are hypothesized to be beneficial in patients with NASH.
Figure 1.
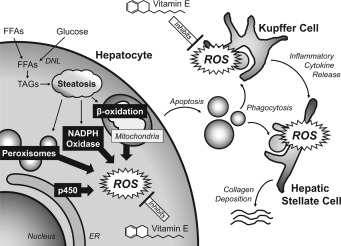
Potential mechanism of action of vitamin E in NASH. Hepatocyte steatosis results from dysregulated lipid metabolism that leads to elevated oxidant stress. Major sources of intracellular ROS are shown in black boxes, where, in steatotic hepatocytes, pathological ROS formation overwhelms antioxidant defenses. The damaged hepatocytes undergo apoptosis and are phagocytosed by Kupffer and stellate cells, further increasing ROS formation and stellate cell activation. Current hypotheses are that vitamin E reduces oxidative stress by either scavenging ROS or augmenting hepatic antioxidant capacity. Abbreviations: DNL, de novo lipogenesis; ER, endoplasmic reticulum; FFA, free fatty acids; TAG, triglycerides.
Efficacy of Vitamin E in Adults and Children With NASH
Vitamin E in the bioactive α‐tocopherol form is a fat‐soluble compound, abundant in certain foods such as vegetable oil, that helps maintain intracellular redox status by extinguishing free radical chain propagation. Vitamin E is inexpensive and safe in adults at dosages of less than 1500 IU/day. Select clinical trials investigating the therapeutic potential of vitamin E therapy in adults with NASH are listed in Table 1. Of these, the PIVENS trial by Sanyal et al.7 comparing vitamin E 800 IU/day or pioglitazone 30 mg/day with placebo in nondiabetic patients with biopsy‐proven NASH was a landmark study. PIVENS reported significant improvements in NASH histology including decreased steatosis and lobular inflammation in patients treated with vitamin E for 96 weeks versus placebo, which was not met in patients treated with pioglitazone. There was no regression in liver fibrosis with either treatment. The PIVENS trial was notable because it was the first intervention shown to be beneficial in patients with NASH. These results prompted the American Association for the Study of Liver Diseases (AASLD) to recommend vitamin E 800 IU/day in nondiabetic adult patients with biopsy‐proven NASH in their 2012 guidelines on the management of nonalcoholic fatty liver disease (NAFLD).8 Updated AASLD guidance statements released in 2017 now also recommend pioglitazone be considered in patients with type 2 diabetes or nondiabetic patients with biopsy‐proven NASH.9 The risks and benefits of these interventions, along with recommendations for exercise and dietary modifications, should be considered in patients who satisfy the earlier conditions. Patients should also be counseled on the risk for vitamin E–mediated vitamin K deficiency, which is rare but can cause deficits in coagulation.
Table 1.
Select Clinical Trials Investigating Vitamin E Therapy in Adult Patients With Nonalcoholic Fatty Liver Disease Spectrum
| Reference (year) | Study Design | Study Population | Treatment Arms | Treatment Length | Primary or Relevant Endpoints | Summary of Relevant Major Findings |
|---|---|---|---|---|---|---|
| Harrison (2003)13 | Double‐blind randomized control trial | Biopsy‐proven NASH (N = 49) | Nutritional and dietary counseling and either vitamin E 1000 IU/day plus vitamin C 1000 mg/day or placebo | 6 months | Histology | Improvement in fibrosis with vitamin E and vitamin C treatment |
| Bugianesi (2005)14 | Open‐label, randomized trial | Nondiabetic NAFLD (N = 55) | Nutritional counseling plus either metformin 2 g/day or vitamin E 800 IU/day or prescriptive diet | 12 months | LFTs, HOMA‐IR, histology, anthropomorphic measures of metabolic syndrome | Metformin more effective than other treatments in improving histology, HOMA‐IR, and LFTs |
| Dufour (2006)15 | Randomized placebo‐controlled trial | Biopsy‐proven NASH (N = 48) | UDCA 12–15 mg/kg/day and vitamin E 800 IU/day versus UDCA and placebo versus neither (placebo) | 24 months | Histology | Improvement in steatosis after 2‐year treatment only in UDCA plus vitamin E group |
| Sanyal (2010)7 | Randomized control trial | Nondiabetic biopsy‐proven NASH (N = 247) | Vitamin E 800 IU/day versus pioglitazone 30 mg/day versus placebo | 96 weeks | Histology (primary); various secondary endpoints including NASH activity, LFTs anthropomorphic, HOMA‐IR, dyslipidemia, and quality‐of‐life measures | Improvement in histology with vitamin E, but not pioglitazone, versus placebo (primary); both treatments effective in reaching some secondary endpoints such as resolution of NASH; neither treatment improved fibrosis |
Abbreviations: HOMA‐IR, homeostatic model assessment‐insulin resistance; LFT, liver function test; UDCA, ursodeoxycholic acid.
Table 2 lists select clinical trials investigating the effect of vitamin E therapy in pediatric patients with NAFLD/NASH. Notable among these is the TONIC trial by Lavine et al.,10 who reported vitamin E 800 IU/day was superior to placebo in reaching resolution of NASH histology. These data resulted in the AASLD recommending vitamin E therapy be considered, in addition to intensive lifestyle modifications, in pediatric patients with biopsy‐proven NASH.9 Several other reports have also investigated the potential of vitamin E therapy for adult and pediatric populations with NASH. However, these studies are hampered by significant design flaws including underpowered studies, differences in baseline patient parameters, inconsistencies in patient entry criteria, lack of standardized endpoints, and poor long‐term patient follow‐up, limiting interpretations of study findings. Moreover, due to shared clinical risk factors, the presence of NASH is associated with increased incidence of many other comorbid medical conditions such as diabetes and metabolic syndrome. These individuals are also the most at risk for disease progression or to experience an adverse outcome. Thus, future studies in NASH should emphasize a robust and diverse patient cohort to best capture the real‐world patient with NASH presenting with a number of complex medical conditions.
Table 2.
Select Clinical Trials Investigating Vitamin E Therapy in Pediatric Patients With Nonalcoholic Fatty Liver Disease Spectrum
| Reference (year) | Study Design | Study Population | Treatment Arms | Treatment Length | Primary or Relevant Endpoints | Summary of Relevant Major Findings |
|---|---|---|---|---|---|---|
| Vajro (2004)16 | Randomized single‐blind | Obesity‐related liver dysfunction (N = 28) | Low‐calorie diet plus vitamin E 400 mg/day (×2 months) and 100 mg/day (×3 months) or placebo | 5 months | LFTs, ultrasound, weight loss | Improvement with vitamin E versus placebo only in patients who could not adhere to low‐calorie diet |
| Nobili (2008)17 | Double‐blind placebo‐controlled study | Biopsy‐proven NAFLD (N = 90) | Nutritional program ± vitamin E 600 IU/day and vitamin C 500 mg/day | 24 months | Histology, ALT, HOMA‐IR | Minimal benefit with addition of antioxidant therapy over nutritional intervention alone |
| Lavine (2011)10 | Randomized double‐blind, double‐dummy placebo‐controlled trial | Biopsy‐confirmed NAFLD (or NASH subset; N = 173 total) | Vitamin E 800 IU/day or metformin 1 g/day versus placebo | 96 weeks | ALT (primary); various secondary endpoints including histology (in NASH subset), LFTs, anthropomorphic, HOMA‐IR, dyslipidemia, quality‐of‐life measures | Neither vitamin E nor metformin improved ALT versus placebo (primary); resolution of NASH greater with vitamin E versus placebo (secondary) |
Abbreviations: ALT, alanine aminotransferase; HOMA‐IR, homeostatic model assessment‐insulin resistance; LFT, liver function test.
Unresolved Controversies Regarding Vitamin E Therapy
Despite the proven benefits of vitamin E therapy, the use of antioxidants in patients with NASH has not been without controversy. In addition to its role as a potent peroxyl scavenger, therapy with vitamin E also alters intracellular signaling pathways and patterns of gene expression. The long‐term effects of these changes on cellular metabolism are unclear. Further, several meta‐analyses investigating the relationship between antioxidants and patient survival suggested a possible link between vitamin E therapy alone or in combination with other antioxidants and elevated all‐cause mortality.11, 12 The reasons for this are unclear but may be related to suppression of beneficial ROS activity on cell division and repair mechanisms, leaving cells more vulnerable to malignant transformation. The long‐term effects of vitamin E therapy need further study. The AASLD states that following a discussion with the patient regarding the risks and benefits of long‐term antioxidant therapy, vitamin E at 800 IU/day may be considered in nondiabetic patients with biopsy‐proven NASH.9
Clearly, targeted inhibition of the specific mitochondrial or enzymatic sources responsible for elevated oxidant stress in NASH would be an attractive pharmacological strategy that avoids the nonspecific effects of vitamin E therapy. However, the source of increased ROS production in NASH is still unclear. Basic investigations studying the molecular mechanisms of NASH are needed.
Conclusions
NASH is a major public health burden in the United States. Patient counseling should emphasize that the most effective and best tolerated interventions in NASH are weight loss, exercise, and dietary modifications. In select populations, current pharmacological strategies that improve patient outcomes include vitamin E and pioglitazone. Because the incidence of NASH in the United States is projected to increase exponentially,3 additional basic and translational studies on potential NASH interventions are urgently needed.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease: meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389–397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology; doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berson A, De Beco V, Letteron P, Robin MA, Moreau C, El Kahwaji J, et al. Steatohepatitis‐inducing drugs cause mitochondrial dysfunction and lipid peroxidation in rat hepatocytes. Gastroenterology 1998;114:764–774. [DOI] [PubMed] [Google Scholar]
- 5. Singal AK, Jampana SC, Weinman SA. Antioxidants as therapeutic agents for liver disease. Liver Int 2011;31:1432–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non‐alcoholic fatty liver disease. J Gastroenterol 2013;48:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005–2023. [DOI] [PubMed] [Google Scholar]
- 9. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology; doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 10. Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 2011;305:1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta‐analysis. JAMA 2007;297:842–857. [DOI] [PubMed] [Google Scholar]
- 12. Miller ER 3rd, Pastor‐Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta‐analysis: high‐dosage vitamin E supplementation may increase all‐cause mortality. Ann Intern Med 2005;142:37–46. [DOI] [PubMed] [Google Scholar]
- 13. Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2003;98:2485–2490. [DOI] [PubMed] [Google Scholar]
- 14. Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol 2005;100:1082–1090. [DOI] [PubMed] [Google Scholar]
- 15. Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, et al. Randomized placebo‐controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2006;4:1537–1543. [DOI] [PubMed] [Google Scholar]
- 16. Vajro P, Mandato C, Franzese A, Ciccimarra E, Lucariello S, Savoia M, et al. Vitamin E treatment in pediatric obesity‐related liver disease: a randomized study. J Pediatr Gastroenterol Nutr 2004;38:48–55. [DOI] [PubMed] [Google Scholar]
- 17. Nobili V, Manco M, Devito R, Di Ciommo V, Comparcola D, Sartorelli MR, et al. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology 2008;48:119–128. [DOI] [PubMed] [Google Scholar]


