Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- AKT
protein kinase B
- ER
endoplasmic reticulum
- HbA1c
hemoglobin A1c
- HCV
hepatitis C virus
- HOMA2‐IR
Homeostatic Model Assessment‐Insulin Resistance
- IRS‐1
insulin receptor substrate 1
- ROS
reactive oxygen species
- SOCS3
suppressor of cytokine signaling 3
- T2DM
type 2 diabetes mellitus
- TNF‐α
tumor necrosis factor α
In the United States, an estimated 9.4% of the population, or 30.3 million people, have diabetes, and 1.4% of the population, or 3.5 million people, have hepatitis C virus (HCV).1, 2 With the obesity epidemic and diabetes rates rising as a result, the metabolic effects associated with HCV and its eradication are important to consider.
Epidemiological Link Between HCV and Diabetes Mellitus
HCV infection is associated with a higher prevalence of type 2 diabetes mellitus (T2DM). In 2000, Mehta and colleagues3 used cross‐sectional data from the National Health and Nutrition Examination Survey of nearly 10,000 patients from 1988 to 1994 and found that adults aged 40 years or older with HCV infection were nearly four times more likely to have concurrent diabetes than those without HCV infection. In a follow‐up case–control study of >1000 patients, Mehta and colleagues4 demonstrated that in patients with risk factors for metabolic syndrome, the presence of chronic HCV infection increased the risk for development of T2DM by 11‐fold over a 9‐year follow‐up. Multiple smaller studies throughout the world have shown a higher prevalence of T2DM in patients with concurrent HCV infection. In France, Grimbert and colleagues5 investigated 152 patients with HCV and 152 controls hospitalized during the same period with hepatitis B virus (n = 51) or alcohol‐induced (n = 101) liver diseases matched for age, sex, and the presence of cirrhosis, and found that T2DM was present in 24% of patients with HCV and 9% in the control group (P < 0.002). In Italy, Antonelli and colleagues6 studied 564 noncirrhotic HCV‐infected patients control‐matched with noncirrhotic hepatitis B virus–infected patients, and demonstrated an increased prevalence rate of T2DM of 12% versus 4.9% in controls (P = 0.008). These studies and many others show an epidemiological association between HCV and T2DM.
HCV Infection Causes Insulin Resistance
Molecular mechanisms provide explanations for which HCV infection might increase the risk for development of T2DM or worsen glycemic control in patients with established T2DM. In normal circumstances, when insulin attaches to the insulin receptor on a hepatocyte, the nearby insulin receptor substrate 1 or 2 (IRS‐1/ IRS‐2) is phosphorylated and associates with the insulin receptor, this in turn causes downstream activation of protein kinase B (AKT). The activated AKT causes the translocation of glucose transporter‐4 to the cell surface, which then brings glucose into the hepatocyte. AKT also causes increased protein synthesis, increased glycogen synthesis, inhibition of lipolysis, and inhibition of hepatic gluconeogenesis7 (Fig. 1). When HCV core protein is in the hepatocyte, it upregulates tumor necrosis factor α (TNF‐α), which causes the phosphorylation of the IRS‐1/IRS‐2 at a serine amino acid and marks the IRS‐1/IRS‐2 for degradation, thus preventing it from associating with the insulin receptor and preventing the downstream activation of AKT. The HCV core protein also upregulates suppressor of cytokine signaling 3 (SOCS3). SOCS3 leads to the ubiquitination of IRS1/IRS‐2, which marks the substrate for degradation, again preventing it from interacting with the insulin receptor and preventing the downstream effects of activated AKT8, 9 (Fig. 2). HCV core protein leads to oxidative stress by causing dysfunction at the mitochondria and endoplasmic reticulum (ER) of hepatocytes. The oxidative stress increases inflammatory cytokines such as TNF‐α, which promotes a state of hyperinsulinemia, decreases apolipoprotein B‐100, enhances triglyceride accumulation in the liver, and leads to steatosis, which in turn creates more oxidative stress. Oxidative stress from HCV and inflammatory cytokines also increases leptin, a profibrogenic hormone, and decreases adiponectin, an antifibrogenic hormone, eventually contributing to fibrosis of the liver10 (Fig. 3).
Figure 1.
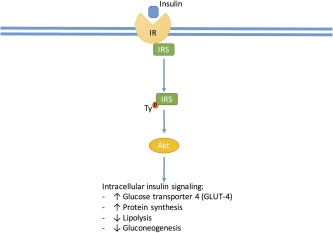
Insulin interacts at the insulin receptor at the hepatocyte membrane that causes tyrosine phosphorylation at the IRS allowing metabolic signaling through phosphoinositide 3‐kinase, which stimulates the activation of AKT, which regulates a diverse set of metabolic functions.
Figure 2.
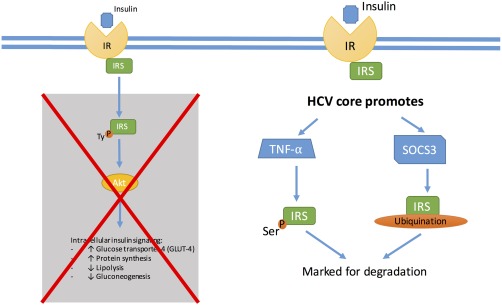
HCV leads to the upregulation of inflammatory cytokines such as TNF‐α, which leads to serine phosphorylation of IRS and marks the protein for degradation. HCV core protein also promotes cytokine signaling 3 (SOCS3), causing ubiquitination of IRS and marking it for degradation. These mechanisms prevent the downstream effects of AKT activation, thus leading to insulin resistance.
Figure 3.
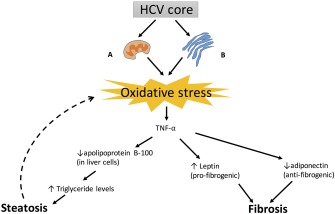
(A) At the mitochondria of hepatocytes, HCV core protein promotes intracellular calcium, which increases the reactive oxygen species (ROS) production through the electron transport chain. (B) At the ER of hepatocytes there is viral replication causing assembly chaperones to be overloaded, and misfolded proteins lead to ER dysfunction and increased ROS. The oxidative stress of HCV leads to triglyceride accumulation within hepatocytes by upregulating apolipoprotein B‐100, causing a cycle of steatosis and more oxidative stress. Leptin is also upregulated and adiponectin downregulated, eventually leading to fibrosis of the liver.
Improvement in Insulin Resistance After Treatment of HCV
Earlier studies examined the improvement of insulin resistance after treating HCV. In the HALT‐C (Hepatitis C Antiviral Long‐Term Treatment Against Cirrhosis) study of 96 patients without a diagnosis of T2DM, Homeostatic Model Assessment‐Insulin Resistance (HOMA2‐IR), a measure of insulin resistance, improved from baseline to 20 weeks after successful treatment with interferon and ribavirin therapies. Investigators found mean HOMA2‐IR differences of −2.23 for complete responders, −0.90 for partial responders, and +0.18 for nonresponders (P = 0.036). HOMA2‐IR improvement with HCV clearance was independent of potential confounders, including age, body mass index, sex, infection duration, and fibrosis.11
Achievement of SVR also reduces the risk for development of impaired fasting glucose. Simó and colleagues12 studied 234 patients with chronic HCV infection after treatment. Over a mean follow‐up period of 5.7 years, 14.6% of SVR‐achieving patients and 34.1% of nonresponders experienced glucose abnormalities (P = 0.001).12 In patients without T2DM prior to treatment for HCV, the development of T2DM was reduced by two‐thirds after obtaining SVR in a study by Arase and colleagues13 of a cohort of 2842 patients treated for HCV infection over a mean period of 6.4 years.
Recently, in a study of direct antiviral treatment of HCV in patients with T2DM, the eradication of HCV led to the improvement in hemoglobin A1c (HbA1c) percentages and overall insulin usage. For patients with diabetes with an average starting HbA1c >7.2% before treatment for HCV, there was nearly a 1% improvement to the average HbA1c in the year after treatment. Overall, patients with diabetes also had fewer prescriptions for insulin in the year after successful eradication of HCV.14 These finding suggest an improvement to glycemic control after successful treatment of HCV with the newer direct antivirals.
Conclusion
In patients with HCV, there is a higher prevalence of T2DM, which likely is a consequence of the alteration of insulin sensitivity by the HCV viral particle itself and via inflammatory cytokines. Multiple studies have now shown an improvement to insulin resistance and decrease in development of T2DM after the eradication of HCV. Given high sustained viral response rates with direct antiviral medications, it is important to consider the added extrahepatic benefits of treatment.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Center for Disease Control . National Diabetes Statistics Report. 2014. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed January 3, 2018.
- 2. Center for Disease Control . Viral hepatitis—hepatitis C information. 2016. https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Accessed December 23, 2016.
- 3. Mehta SH, Brancati FL, Sulkowski MS, et al. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med 2000;133:592‐599. [DOI] [PubMed] [Google Scholar]
- 4. Mehta SH, Brancati FL, Strathdee SA, et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatology 2003;38:50‐56. [DOI] [PubMed] [Google Scholar]
- 5. Grimbert S, Valensi P, Levy‐Marchal C, et al. High prevalence of diabetes mellitus in patients with chronic hepatitis C. A case‐control study. Gastroenterol Clin Biol 1996;20:544‐548. [PubMed] [Google Scholar]
- 6. Antonelli A, Ferri C, Fallahi P, et al. Hepatitis C virus infection: evidence for an association with type 2 diabetes. Diabetes Care 2005;28:2548‐2550. [DOI] [PubMed] [Google Scholar]
- 7. Banerjee S, Saito K, Ait‐Goughoulte M, et al. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate‐1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J Virol 2008;82:2606‐2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012;55:2565‐2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kawaguchi T, Yoshida T, Harada M, et al. Hepatitis C virus down‐regulates insulin receptor substrates 1 and 2 through up‐regulation of suppressor of cytokine signaling 3. Am J Pathol 2004;165:1499‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sheikh MY, Choi J, Qadri I, et al. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology 2008;47:2127‐2133. [DOI] [PubMed] [Google Scholar]
- 11. Delgado‐Borrego A, Jordan SH, Negre B, et al. Reduction of insulin resistance with effective clearance of hepatitis C infection: results from the HALT‐C trial. Clin Gastroenterol Hepatol 2010;8:458‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simó R, Lecube A, Genesca J, et al. Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care 2006;29:2462‐2466. [DOI] [PubMed] [Google Scholar]
- 13. Arase Y, Suzuki F, Suzuki Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology 2009;49:739‐744. [DOI] [PubMed] [Google Scholar]
- 14. Hum J, Jou JH, Green PK, et al. Improvement in glycemic control of type 2 diabetes after successful treatment of hepatitis C virus. Diabetes Care 2017;40(9):1173‐1180. [DOI] [PubMed] [Google Scholar]


