Watch a video presentation of this article
Answer questions and earn CME
Abbreviations
- ACEi
angiotensin‐converting enzyme inhibitor
- ARNI
angiotensin receptor neprilysin inhibitor
- JGA
juxtaglomerular apparatus
- LVAD
left ventricular assist device
- MRA
mineralocorticoid receptor antagonist
- RAAS
renin‐angiotensin‐aldosterone system
- SGLT2
sodium‐glucose cotransporter 2
- TIPS
transjugular intrahepatic portosystemic shunting
Diabetes mellitus is common among patients with cirrhosis, affecting approximately one‐third of this population, as a causative factor, comorbid condition, or secondary effect of hepatic dysfunction. Likewise, ascites occurs in about half of patients with cirrhosis and is the most common decompensating event. Both conditions are indicators of advanced disease and are associated with poor clinical outcomes.1, 2 For a number of reasons, the management of diabetes and ascites in individuals with cirrhosis is quite challenging and has remained virtually unchanged for decades.
Currently, the pharmacological treatment of diabetes in the context of advanced liver disease is controversial. Most oral antihyperglycemic agents are hepatically cleared, and due to safety concerns stemming from altered drug metabolism, patients are often preferentially treated with insulin. However, discontinuation of oral therapy and unchecked insulin use may be harmful.3 Induction of insulin‐like growth factor 1 signaling through phosphoinositide 3‐kinase/Akt/mammalian target of rapamycin and nuclear factor‐κB pathways results in a proinflammatory state, favoring fibrosis and tumorigenesis.4 Furthermore, over the past several years, there has been a small but meaningful influx of biochemical and clinical evidence supporting the use of oral agents in cirrhosis.1
The management of cirrhotic ascites also poses a dilemma. Portal hypertension and splanchnic vasodilatation promote the development and maintenance of ascites. Although several therapeutic options exist, including neurohormonal and diuretic management, paracentesis, transjugular intrahepatic portosystemic shunting (TIPS), and transplantation, each modality is associated with significant risks and limitations. Of these strategies, however, neurohormonal modulation is most appealing because it is noninvasive, and effective and affordable medications that target a well‐described maladaptive response are available. In cirrhosis, splanchnic and peripheral vasodilatation decrease the effective circulating volume and induce the renin‐angiotensin‐aldosterone system (RAAS), which promotes salt and water retention. Mineralocorticoid receptor antagonists (MRAs) such as spironolactone, which are often coupled with loop diuretics, are the mainstays of therapy. Although their use is associated with a side‐effect profile that includes electrolyte disturbances, hypotension, renal injury, and hepatic encephalopathy, they are presumably still safer and better tolerated than angiotensin‐converting enzyme inhibitors (ACEis), angiotensin receptor blockers, and nonselective beta‐blockers.5 These drugs have more potent hemodynamic effects than MRAs in advanced cirrhosis, and their use has been associated with poor outcomes in multiple trials.6, 7 In stark contrast with the management of congestive heart failure, for which multiple RAAS‐modifying agents are concurrently used, MRAs are the only neurohormonal modulators that are currently prescribed for cirrhotic ascites (Fig. 1). However, there are promising agents that have yet to be studied in this setting.
Figure 1.
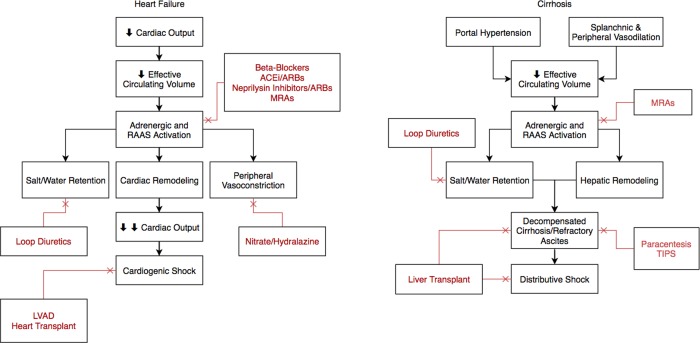
Pathophysiology and management of heart failure and cirrhosis. The RAAS is central to the maladaptive response in both states. In patients with heart failure, multiple RAAS‐modifying agents, including beta‐blockers, ACEis, angiotensin receptor blockers (ARBs), and MRAs, are concurrently used with significant mortality benefit. However, in the setting of vasodilation from excess nitric oxide, the use of neurohormonal modulators has been limited in cirrhosis. Abbreviations: ARNI, angiotensin receptor neprilysin inhibitor; LVAD, left ventricular assist device.
Sodium‐glucose cotransporter 2 (SGLT2) inhibitors represent a relatively new class of medications for the management of type II diabetes mellitus. Dapagliflozin, empagliflozin, and canagliflozin are three drugs that target SGLT2 in the proximal tubule of the nephron, promoting increased excretion of both sodium and glucose in the urine. They have a modest effect on glycemic control, resulting in a hemoglobin A1c reduction of 0.5% to 1%. However, there is also interplay between SGLT2 blockade and the RAAS pathway. When sodium reabsorption is inhibited in the proximal tubule, downstream sodium delivery to the macula densa increases. Consequently, renin secretion is inhibited and RAAS activity is attenuated. SGLT2 inhibitors may have added renoprotective effects either alone or when combined with other RAAS‐modifying agents by quelling the renin‐mediated hyperfiltration injury that occurs through afferent arteriole vasodilation.8 Theoretically, these characteristics make SGLT2 inhibitors ideal for patients with cirrhosis and diabetes with or without ascites (Figs. 2 and 3). Their clinical benefit in heart failure has already been demonstrated in a large, multicenter, randomized control trial that included patients with both diabetes and cardiovascular disease. In this context, treatment with empagliflozin led to significant reductions in heart failure hospitalizations and heart failure–associated mortality.9 Unfortunately, their use in patients with cirrhosis has been quite limited because of uncertainty about their pharmacological profile and general safety in this population.
Figure 2.
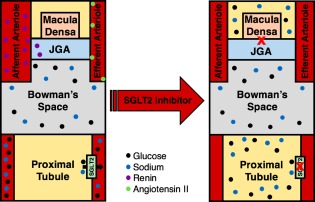
SGLT2 inhibition in the diabetic and cirrhotic kidney. Inhibition of SGLT2 in the proximal tubule promotes both glycosuria and natriuresis, and attenuates renin secretion, thereby improving glycemic control, reducing salt and water retention, and mitigating hepatic and renal fibrosis. Prior to SGLT2 inhibition, the actions of renin and angiotensin II result in afferent arteriolar dilatation and efferent arteriolar constriction, leading to glomerular hyperfiltration injury. SGLT2 inhibitors reduce both renin and angiotensin II levels, restoring vascular tone and glomerular filtration. Abbreviation: JGA, juxtaglomerular apparatus.
Figure 3.
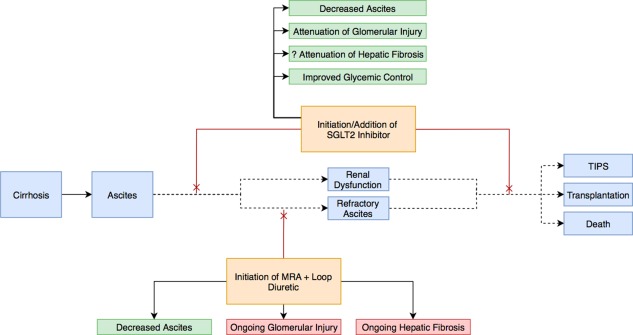
SGLT2 inhibition in the management of ascites. Because of their relatively favorable safety profile, SGLT2 inhibitors have the potential to serve as lone or adjunctive therapy and at both early and late stages of disease progression. They provide the added benefit of likely attenuating hepatic and renal fibrosis and improving glycemic control.
Since their introduction in 2011, SGLT2 inhibitors have been studied in patients with diabetes and a number of other common comorbidities, including cirrhosis, with promising safety data. In the context of chronic liver disease, the most concerning potential adverse effects are hypotension, acute renal injury, and genitourinary tract infections. SGLT2 inhibitors have a small but consistent impact on blood pressure that is not dose dependent. The estimated decrease in systolic blood pressure is 3 to 5 mm Hg, and the magnitude of the effect is most pronounced in patients with preexisting hypertension.10 Furthermore, this impact is less than that seen with some MRAs and is not associated with an increased incidence of adverse events related to intravascular volume depletion. Acute renal injury, although described in some reports, has not been definitively linked to SGLT2 inhibitors in clinical trials. A pooled analysis of 13 studies using dapagliflozin demonstrated no significant increase in the incidence of renal dysfunction. Genitourinary tract infections are perhaps the most common adverse effect, occurring in about 5% of patients treated with dapagliflozin. These infections are mostly uncomplicated genital infections that can be prevented with careful hygiene and antifungal therapy.11 Finally, although SGLT2 inhibitors are cleared by the liver, pharmacokinetic trials involving patients with at least mild or moderate hepatic impairment demonstrated that dapagliflozin and empagliflozin were well tolerated and required no dosing adjustments.12, 13 Therefore, the current body of safety literature supports the continued investigation of this class of medications in patients with chronic liver disease.
Trials examining the role of SGLT2 inhibitors in the comanagement of diabetes and ascites in cirrhosis are necessary to test the hypothesis that these agents can safely attenuate the maladaptive neurohormonal and inflammatory responses. In a potential study focusing on the management of ascites, dapagliflozin or empagliflozin could be compared with standard MRA therapy with or without furosemide. A positive trial with acceptable safety outcomes could be followed up with a multifactorial study combining anti‐SGLT2 therapy with standard diuretics. Additional studies focusing on the impact of SGLT2 inhibitors on diabetes management in cirrhosis are also essential with some emphasis on further exploring the idea that long‐term insulin use can be detrimental. Ultimately, if the benefits of neurohormonal modulation in cirrhosis are at all comparable with the landmark achievements made in the management of congestive heart failure, these trials could pave the way for the targeted management of cirrhosis and its complications.
Acknowledgments
The authors thank Drs. Wassim Fares and Matthew Griffin for assistance with the technical editing of this manuscript.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. García‐Compeán D, González‐González JA, Lavalle‐González FJ, González‐Moreno EI, Maldonado‐Garza HJ, Villarreal‐Pérez JZ. The treatment of diabetes mellitus of patients with chronic liver disease. Ann Hepatol 2015;14:780‐788. [DOI] [PubMed] [Google Scholar]
- 2. Gines P, Quintero E, Arroyo V, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology 1987;7:122‐128. [DOI] [PubMed] [Google Scholar]
- 3. Zhang X, Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology 2014;60:2008‐2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malaguarnera R, Belfiore A. The emerging role of insulin and insulin‐like growth factor signaling in cancer stem cells. Front Endocrinol 2014;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gines P, Arroyo V, Quintero E, Planas R, Bory F, Cabrera J, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology 1987;93:234‐241. [DOI] [PubMed] [Google Scholar]
- 6. Ge P, Runyon B. The changing role of beta‐blocker therapy in patients with cirrhosis. J Hepatol 2014;60:643‐653. [DOI] [PubMed] [Google Scholar]
- 7. Vlachogiannakos J, Tang AK, Patch D, Burroughs AK. Angiotensin converting enzyme inhibitors and angiotensin II antagonists as therapy in chronic liver disease. Gut 2001;49:303‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med 2015;66:255‐270. [DOI] [PubMed] [Google Scholar]
- 9. Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA‐REG OUTCOME® trial. Eur Heart J 2016;37:1526‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes: cardiovascular and kidney effects, potential mechanisms and clinical applications. Circulation 2016;134:752‐772. [DOI] [PubMed] [Google Scholar]
- 11. Ptaszynska A, Johnsson KM, Parikh SJ, de Bruin TW, Apanovitch AM, List JF. Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events. Drug Saf 2014;37:815‐829. [DOI] [PubMed] [Google Scholar]
- 12. Kasichayanula S, Liu X, Zhang W, Pfister M, Lacreta F, Boulton D. Influence of hepatic impairment on the pharmacokinetics and safety profile of dapagliflozin: an open‐label, parallel‐group, single‐dose study. Clin Ther 2011;33:1798‐1808. [DOI] [PubMed] [Google Scholar]
- 13. Heise T, Seewaldt‐Becker E, Macha S, Hantel S, Pinnetti S, Seman L, Woerle HJ. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks' treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab 2013;15:613‐621. [DOI] [PubMed] [Google Scholar]


