Abstract
The modification of the mouse genome by site-specific gene insertion of transgenes and other genetic elements allows the study of gene function in different developmental stages and in the pathogenesis of diseases. Here, we generated a “genomic safe harbor” Hipp11 (H11) locus-specific knock-in transgenic mouse line in which the albumin promoter is used to drive the expression of the reverse tetracycline transactivator (rtTA) in the liver. The newly generated H11-albumin-rtTA transgenic mice were bred with tetracycline-operator-Histone-2B-green fluorescent protein (TetO-H2BGFP) mice to assess inducibility and tissue-specificity. Expression of the H2BGFP fusion protein was observed exclusively upon doxycycline (Dox) induction in the liver of H11-albumin-rtTA/TetO-H2BGFP double transgenic mice. To further analyze the ability of the Dox-inducible H11-albumin-rtTA mice to implement conditional DNA recombination, H11-albumin-rtTA transgenic mice were crossed with TetO-Cre and Ai14 mice to generate H11-albumin-rtTA/TetO-Cre/Ai14 triple transgenic mice. We successfully confirmed that the Cre-mediated recombination efficiency was as strong in Dox-induced H11-albumin-rtTA /TetO-Cre/Ai14 mice as in the control albumin-Cre/A14 mice. Finally, to characterize the expression-inducing effects of Dox in H11-albumin-rtTA/TetO-H2BGFP mice in detail, we examined GFP expression in embryos at different developmental stages and found that newly conceived H11-albumin-rtTA/TetO-H2BGFP embryos of Dox-treated pregnant female mice were expressing reporter GFP by E16.5. Our study demonstrates that these new H11-albumin-rtTA transgenic mice are a powerful and efficient tool for the temporally and spatially conditional manipulation of gene expression in the liver, and illustrates how genetic crosses with these new mice enable the generation of complex multi-locus transgenic animals for mechanistic studies.
Keywords: Hipp11, albumin promoter, doxycycline, Tet-on system
Mouse genome modification via the stable insertion of functional transgenes and other genetic elements is currently of great significance to biological and medical research. There are two broad strategies that can be applied to generate transgenic mice: random or site-specific integration. Notably, randomly inserted genes are subject to position effects, and can result in unstable phenotypes, gene silencing, and/or unexpected gene expression (Sadelain et al. 2011). With site-specific gene integration, alternatively, particular locations in the host genome are selected based on particular research goals. As site-specific integration of DNA requires the identification of a “genomic safe harbor” locus that will allow for gene expression but that will not perturb surrounding endogenous gene function (Ruan et al. 2015). At present, the ROSA26 locus is widely used as a transgene insertion site, owing to its ubiquitous gene expression and few reported insertion site side effects (Zambrowicz et al. 1997; Ma et al. 2017; Movahedi et al. 2018).
Consider that functional studies based on genetically modified mice often require the generation of complex genotypes (double-hybridization, triple-, etc.). When researchers need to make crosses with mice that already harbor engineered cassettes at their Rosa26 locus, the ability to introduce any additional modifications would depend on the use of an alternative genomic safe harbor locus. One potential alternative safe harbor, the H11 locus, was first described by Hippenmeyer et al. (2010), and has been used in human stem cells (Zhu et al. 2014) and in transgenic mice (Tasic et al. 2011). In mice, the H11 locus occurs in an intergenic region near the chromosome 11 centromere that is positioned between the Eif4enif1 and Drg1 genes, and in vivo experiments have established that the integration of targeting cassettes from the H11 locus does not affect mouse viability or fertility and that biallelic expression of targeting cassettes was possible (Hippenmeyer et al. 2010; Tasic et al. 2011).
Unlike the Rosa26 locus, the H11 locus does not have an endogenous promoter (Hippenmeyer et al. 2010; Ruan et al. 2015). We reasoned that this would make the H11 locus particularly suitable for the conditional expression of transgenes engineered to be driven by a variety of tissue-specific promoters or otherwise inducible expression systems. The tetracycline on (Tet-on) system, which is also known as the rtTA-dependent system, has been widely-used in genetic studies to enable the conditional regulation of gene expression in a wide variety of cells, tissue cultures, and transgenic animals (Zhou et al. 2006; Das et al. 2016; Bijlani et al. 2018). It is composed of two elements: the ligand-dependent transactivator tetracycline rtTA as the effector and a TetO-cytomegalovirus (TetO-CMV) minimal promoter cassette regulating the expression of the transgene as the responder. In the presence of tetracycline, or one of its analogs like doxycycline (Dox), rtTA binds to TetO-sequence and activates the transcription of target genes (Burger et al. 2011; Cox et al. 2014; Thiem et al. 2016).
Researchers have combined the Tet-on inducible expression system with tissue-specific promoters (e.g., major urinary protein promoter, albumin promoter for liver-specific expression (Gandhi et al. 2015)) to achieve both temporal and spatial control of the expression of genes-of-interest for functional studies in various stages of embryonic development and adulthood (Chen et al. 2011; Gandhi et al. 2015). For example, Zhou et al. (2012) crossed albumin-rtTA mice with TetO-urokinase plasminogen activator (uPA) transgenic mice to produce double transgenic albumin-rtTA/TetO-uPA mice in which both Dox-inducible and liver-specific over-expression of a uPA transgene was achieved (Zhou et al. 2012).
Here, seeking to expand the toolbox available for targeted knock-in experimental strategies by generating mice with an additional and highly flexible genomic safe harbor locus, we generated a new site-specific knock-in transgenic mouse model in which an albumin-rtTA cassette was inserted into the H11 locus. Note that our selection of the albumin promoter also offers a new experimental system both basic and medical researchers of hepatocytes. To initially assess the inducibility and tissue-specificity of transgene expression, H11-albumin-rtTA transgenic mice were bred with TetO-H2BGFP mice to generate H11-albumin-rtTA/TetO-H2BGFP double transgenic mice. We confirmed that the administration of Dox induced the liver-specific expression of an H2BGFP reporter transgene, and cessation of Dox administration halted transgene expression in these double transgenic mice. Subsequently, H11-albumin-rtTA transgenic mice were crossed with TetO-Cre and Ai14 mice to generate H11-albumin-rtTA/TetO-Cre/Ai14 triple transgenic mice and we successfully confirmed very strong Dox-inducible conditional Cre-mediated recombination efficiency in these H11-albumin-rtTA/TetO-Cre/Ai14 mice. Finally, a study of embryos conceived by Dox-treated H11-albumin-rtTA/TetO-H2BGFP mothers revealed that embryos were expressing the reporter by E16.5. Collectively, this study demonstrates that our H11-albumin-rtTA transgenic mice are a powerful and efficient tool for conditional gene expression in the liver and illustrates how crosses with these mice can generate complex genotypes to facilitate mechanistic studies.
Materials and Methods
H11-albumin-rtTA plasmid construction
For the liver-specific expression of rtTA at the H11 locus, the transgenic construct H11-albumin-rtTA was generated. The H11 locus homologous arms were amplified by PCR using genomic DNA extracted from a C57BL/6 mouse as the template. The albumin promoter and enhancer fragment was amplified by PCR using pALB-GFP as the template. A coding-sequence-improved version of the rtTA-advanced element was amplified by PCR using plasmid pPB-CAG-rtTA-IN as the template. The Bovine Growth Hormone Polyadenylation Signal (BGHpolyA) element was amplified by PCR using plasmid vpCRII-TOPO CMV-cGFP-BGH poly(A) as the template. All the PCR donor template sequences were cloned into the pBS plasmid using Gibson assembly reactions (E2611S/L; New England Biolabs), and incorporated into the H11-albumin-rtTA donor plasmid. We generated H11-albumin-rtTA mice via Cas9/sgRNA mediated gene targeting in zygotes (see Figure 1A, below). The primers for the donor template sequences and the sgRNA sequences used in this study are listed in the Supplemental Materials (Table S1).
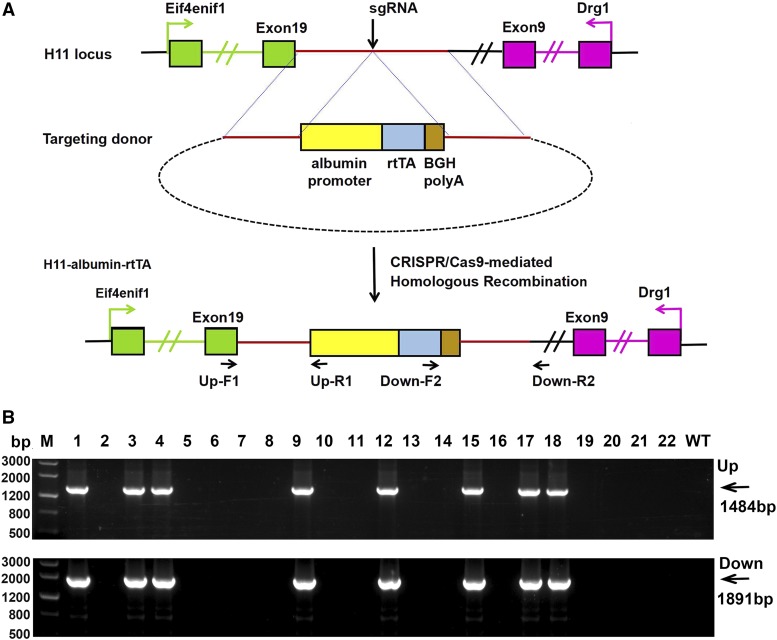
Figure 1.
Generation of H11-albumin-rtTA knock-in mice using the CRISPR/Cas9 system. (A) Schematic overview of CRISPR/Cas9-mediated knock-in of the albumin-rtTA cassette at the H11 locus. Top panel shows the organization of the H11 genomic locus. Green boxes indicate exons 1 and 19 of the flanking gene Eif4enif1, and pink boxes indicate exons 1 and 9 of Drg1. The middle panel shows the design of the H11-albumin-rtTA targeting donor. The bottom panel shows the design for how the targeting donor is recombined into the H11 genomic locus via CRISPR/Cas9-mediated homologous recombination in mice. (B) PCR amplification of the sgRNA:Cas9-mediated albumin-rtTA cassette knock-in at the endogenous H11 locus. Upper panel: PCR amplification of the genomic junction with the left homologous arm and part of the albumin cassette. Lower panel: PCR amplification of the genomic junction with the right homologous arm and part of the rtTA cassette. Lanes 1, 3, 4, 9, 12, 15, 17, and 18 were transgene+ mice; lanes 2, 5, 6, 7, 8, 10, 11, 13, 14, 16, 19, 20, 21, and 22 were transgene- mice. M, Marker; Up, upstream;Down, downstream.
Cas9/sgRNA production and injection
The sgRNAs were prepared using a MEGAshortscript T7 Transcription kit (AM1354; Ambion) according to the manufacturer’s instructions. TheT7-Cas9 DNA was amplified by PCR using plasmid hCas9 as the template and in vitro transcribed using a T7 Ultra Kit (AM1345; Ambion). Cas9 mRNA was purified using an RNeasy Mini Kit (74104; Qiagen). For microinjection, the Cas9 mRNA and sgRNAs were diluted into injection buffer (0.25 mM EDTA/10 mM TrisHCl, pH 7.4) and incubated for 10 min at 37° before injection. The final concentration of Cas9 was 80 ng/µl, and that of sgRNA was 30 ng/µl. The H11-albumin-rtTA donor plasmid concentration was 10 ng/μl for the injection.
The microinjection process was performed as described previously (Yang et al. 2014). Briefly, zygotes were obtained from female donor mice mated with C57BL/6 males after treatment with pregnant mare serum gonadotropin (PMSG; Sigma-Aldrich) and human chorionic gonadotropin (hCG; Sigma-Aldrich). Microinjection was performed under an inverted microscope (OLYMPUS IX71) equipped with a microinjector system (Eppendorf FemtoJet 4i). Microinjections were into the larger male pronucleus of fertilized oocytes. After microinjection, the injected zygotes were transferred to pseudopregnant C57BL/6 mice (20-30 zygotes per pseudopregnant mice). The primers used for genotyping the H11-albumin-rtTA mice are listed in Supplemental Materials, Table S2.
Mouse lines and Dox treatment
Ai14 (Rosa26-CAG-loxp-stop-loxp-tdTomato) (Guenthner et al. 2013), TetO-Cre (Uemura et al. 2014), TetO-H2BGFP (Chakkalakal et al. 2012) and Albumin-Cre (Cui et al. 2016) mice were obtained from The Jackson Laboratory. All mice were housed in an SPF environment. This study was carried out in accordance with the Animal Care and Use Committee of the National Institute of Biological Sciences, Beijing, which follow the governmental regulations of China. Adult mice were 6-to 8-weeks-old for this study. The age of mouse embryos was determined by the appearance of the vaginal plug, which was taken to be E0.5. The birth day of new pups was denoted as P1 for these experiments.
To examine how Dox treatment affected transgene expression, we administrated the tetracycline derivative Dox (D9891; Sigma) in the adult mouse drinking water at a concentration of 1 mg/ml for 48 h and then ceased treatment. Dox was dissolved in 5% sucrose (pH 6.0) to mask the bitter taste. It was kept in aluminum foil-wrapped bottles to prevent light-induced degradation.
Western blotting
Livers were lysed with RIPA buffer (9806; Cell Signaling) containing 1 mM phenylmethylsulfonyl fluoride (8553S; Cell Signaling). The protein concentration of each sample was determined using a bicinchoninic acid (BCA) assay reagent (Vigorous Biotechnology) according to the manufacturer’s recommendations. An equal amount of each protein sample was electrophoresed on a 10% acrylamide gel and the bands were then transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad). The membrane was blocked with 5% non-fat dry milk for 3 h and incubated with GFP antibody (#2555; Cell Signaling) and internal control β-actin antibody (ab8227; Abcam) overnight at 4°. The PVDF membrane was then washed three times for 30 min in 0.1% Tween-20 in Tris-buffered saline (TBST) and incubated for 1 h with horseradish peroxidase-conjugated goat anti-rabbit IgG (Zhongshan). After washing for 30 min with three changes of TBST, the membrane was treated with the Pierce ECL 2 western blotting Substrate (Thermo Scientific).
Hematoxylin and eosin (HE) staining
Hematoxylin and eosin staining was performed as previously described (Li et al. 2014). Briefly, sections were dewaxed, rehydrated, stained with hematoxylin, incubated in bluing solution, counterstained with eosin, dehydrated, and equilibrated with xylene. Glass coverslips were mounted with Permount Mounting Media (SP15-100; ThermoFisher Scientific). Sections were photographed using a bright-field microscope system (Leica Microsystems).
Immunofluorescence
Liver were fixed in 4% paraformaldehyde (PFA) overnight, and then dehydrated in 30% sucrose/PBS. Tissue was embedded in Cryo-gel OCT compound (62806-01; Tissue-Tek) and frozen on dry ice. Fixed livers were sectioned at 10 μm using cryostat. Cryosections were washed 5 min×3 in PBS, counterstained with DAPI (10236276001; Roche Applied Science) for 10 min, and mounted on plus-coated slides that were cover-slipped using Vectashield (H-1000; Vector Laboratories). Finally, sections were photographed under a fluorescence microscope (Leica Microsystems).
For the labeling of albumin, cryosections were pretreated as above. Sections were blocked using 10% donkey serum in PBS, and incubated with anti-albumin antibody (Ab207327; Abcam) overnight at 4°. Subsequently, sections were washed and incubated with DAR-555 (A-31572; ThermoFisher Scientific) at 37° for 1 h.
PCR and quantitative real-time PCR (qRT-PCR)
Genomic DNA was isolated from tail biopsies following the HotSHOT method (Truett et al. 2000) and genotyping was performed using standard PCR methods with sequence-specific primers (listed in Supplemental Materials, Table S3). Total RNA was extracted from the liver and various tissues using Trizol reagent (Vigorous Biotechnology) according to the manufacturer’s protocols. RNA was converted to cDNA using M-MLV reverse transcriptase (M170A; Promega) according to the manufacturer’s protocols. qRT-PCR was performed with SYBR Green master mix (DRR420A; Takara) using an ABI PRISM 7500 Sequence Detection System (Applied Biosystems). Relative RNA quantifications were normalized to the endogenous control (Gapdh). Data were analyzed using the 2–△△Ct method. All of the experiments were repeated independently at least three times. The primers used are listed in the Supplemental Materials (Table S4).
Statistical analysis
Data are expressed as means ± SEM. Statistical analysis was performed with GraphPad Prism 6.0 software. Comparisons between two groups were analyzed by Student’s t-test. More than two groups were compared using a one-way factorial analysis of variance (ANOVA), followed by Student’s t-test. The nature of errors bars in graphical representations and the number of biological replicates (n) is indicated in the corresponding figure legend.
Data availability
All data necessary for confirming the conclusions presented are within the article. File S1 contains supplemental data to support the conclusions in this article. Supplemental material available at Figshare: https://doi.org/10.25387/g3.7502405.
Results
Generation of transgenic H11-albumin-rtTA mice
The H11 locus is located in an intergenic region, flanked by the two genes Eif4enif1 and Drg1 (Figure 1A). The H11-albumin-rtTA donor plasmid, in which the albumin-rtTA cassette was flanked by two 1.3 kb H11 homologous arms, was used as the template for CRISPR/Cas9-mediated homologous recombination (HR). The albumin-rtTA cassette contained rtTA coding sequence and poly A elements directed by the albumin promoter. sgRNA was designed to target the H11 locus. Then, the Cas9 mRNAs, sgRNA, and H11-albumin-rtTA donor plasmid were co-microinjected into zygotes, which were subsequently transferred to pseudopregnant females (Figure 1A).
After microinjection and embryo transfer, we obtained 22 pups. Two pairs of primers (upstream and downstream) were designed to detect HR-mediated albumin-rtTA cassette insertion. The primers used for PCR amplification are indicated (Figure 1A) and are detailed in supplementary Table 2. The primers were designed outside of the homologous arm to exclude random insertion. Thus, only genomic DNA with correct insertion of the albumin-rtTA cassette produced the desired PCR products (Figure 1B). PCR and sequencing showed that 8 pups (36%) harbored the correct albumin-rtTA insertion. We named the HR-mediated albumin-rtTA insertion mice as H11-albumin-rtTA mice.
Liver-specific inducible transgene expression in Dox-treated H11-albumin-rtTA mice
To examine the suitability of using this newly introduced construct for the inducible and tissue-specific expression of transgenes, the H11-albumin-rtTA mice were crossed with TetO-H2BGFP mice to generate H11-albumin-rtTA/TetO-H2BGFP double-transgenic mice (Figure 2A). The genotypes of all mice were confirmed by PCR analysis of tail DNA (Figure 2B). Adult H11-albumin-rtTA/TetO-H2BGFP mice were given drinking water supplemented with 1mg/ml Dox for two days, and qRT-PCR and whole mount fluorescence microscopy were used to characterize the induction of H2BGFP reporter expression in a variety of mouse tissue types (Figure 2C-D). In the absence of Dox treatment, no H2BGFP mRNA expression was detected in the liver of H11-albumin-rtTA/TetO-H2BGFP mice. The expression of both H2BGFP mRNA transcripts and H2BGFP protein was restricted to the liver of Dox-treated H11-albumin-rtTA/TetO-H2BGFP mice (i.e., no expression was detected in heart, spleen, colon, lungs, or kidneys).
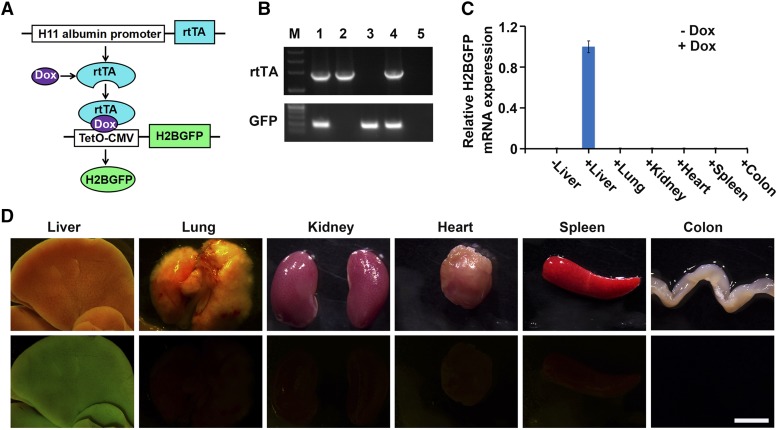
Figure 2.
Dox-inducible H2BGFP reporter gene expression in different organs of H11-albumin-rtTA/TetO-H2BGFP double-transgenic mice. (A) Schematic diagram of induced H2BGFP expression in the H11-albumin-rtTA/TetO-H2BGFP mice after Dox treatment. The rtTA protein binds with Dox and forms a complex that initiates transcription of a H2BGFP fusion protein construct positioned downstream of a tetracycline inducible promoter (TetO-CMV). (B) PCR genotyping of mice. The positive transgenic mice had a 1.9 kb product for rtTA; H2BGFP generated a 191 bp product. Lanes 1 and 4 were transgene+ mice; lanes 2, 3, and 5 were transgene- mice. (C) qRT-PCR analysis of the relative expression of H2BGFP mRNA in different organs dissected from double transgenic mice treated with 1mg/ml Dox (+Dox) or water (-Dox) for 48 h (H2BGFP expression was normalized to Gapdh (n = 4)). Error bars show mean ± SEM (n = 4). (D) Light images (top panels) and whole mount fluorescence microscopy (bottom panels) analyses of H2BGFP protein in the Dox treated H11-albumin-rtTA/TetO-H2BGFP mice. Scale bar: 5 mm.
In addition, we examined expression of hepatocyte differentiation marker albumin and of GFP in the liver of Dox-treated H11-albumin-rtTA/TetO-H2BGFP mice. Our immunofluorescence results demonstrated that the GFP and albumin proteins were co-expressed within the same liver cells (Figure S1). Moreover, the expression of GFP was restricted to well-differentiated hepatocytes. Notably, HE staining revealed no obvious histological or morphological differences between untreated and Dox-treated H11-albumin-rtTA/TetO-H2BGFP mice (Figure S2). These results collectively establish that our new site-specific knock-in H11-albumin-rtTA transgenic mouse model can be used as a valuable tool for the Dox-inducible and liver-specific expression of a given transgene-of-interest.
Reporter gene induction and extinction in Dox-treated H11-albumin-rtTA mice
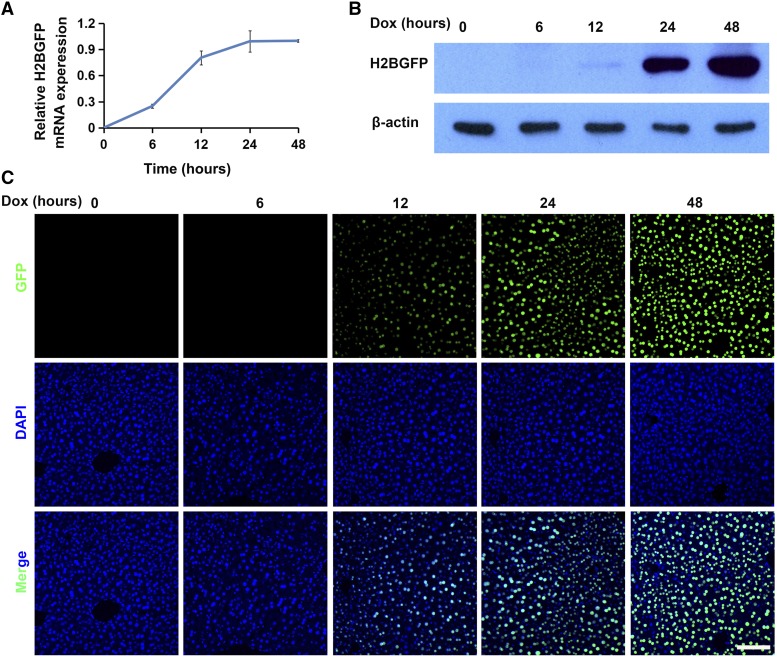
We measured reporter gene expression in Dox-treated H11-albumin-rtTA/TetO-H2BGFP mice over a 48 h period. qRT-PCR analysis of H2BGFP mRNA accumulation following Dox exposure revealed that transgene expression had started by 6 h, continued rising to 24 h, and then remained steady up to end of the experiment at 48 h (Figure 3A). Western blotting and immunofluorescence results demonstrated that obvious H2BGFP protein expression could be detected at 12 h and reached its maximum detected level at 48 h (Figure 3B-C).
Figure 3.
H2BGFP expression in Dox-treated H11-albumin-rtTA/TetO-H2BGFP mice. (A) qRT-PCR analysis of the relative expression of H2BGFP mRNA in response to Dox exposure (H2BGFP expression was normalized to Gapdh (n = 4)). Error bars show mean ± SEM (n = 4). (B-C) Western blotting and immunofluorescence analyses of H2BGFP protein obtained from livers of mice treated with Dox over a 48 h period. GFP staining (green); DAPI nuclear counterstaining of DNA (blue). Scale bar: 100 μm.
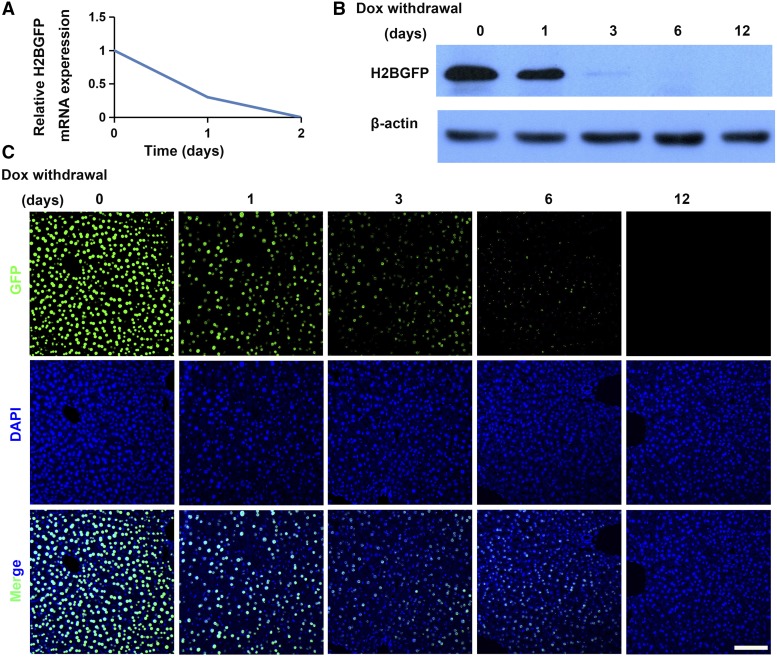
We also examined how cessation of Dox treatment affected transgene expression in H11-albumin-rtTA/TetO-H2BGFP mice that had already been treated with Dox for 48 h. qRT-PCR analysis showed that H2BGFP mRNA levels diminished rapidly within 1 day of stopping Dox treatment and no H2BGFP transcripts could be detected by the second day (Figure 4A). Western blotting and immunofluorescence analyses showed stopping Dox treatment resulted in a significant decrease in H2BGFP protein levels by day 3, with H2BGFP being nearly undetectable by day 6; note that GFP is highly stable, so the low levels observed on day 6 may represent residual protein that was translated during the Dox-induction phase (Figure 4B-C). Collectively, these results highlight the tight temporal regulation of Dox-inducible transgene expression that can be achieved using the albumin-rtTA element that we introduced in the H11 locus in our new site-specific knock-in transgenic mouse model.
Figure 4.
Extinction of H2BGFP expression after stopping Dox-treatment of H11-albumin-rtTA/TetO-H2BGFP mice. (A) qRT-PCR analysis of the relative expression of H2BGFP mRNA in response to Dox cessation (H2BGFP expression was normalized to Gapdh (n = 4)). (B-C) Western blotting and immunofluorescence analyses of protein obtained from livers of treated mice (from start of Dox withdrawal to day 12). GFP staining (green); DAPI nuclear counterstaining of DNA (blue). Scale bar: 100 μm.
Combining the Cre/loxP recombination system with H11-albumin-rtTA mice
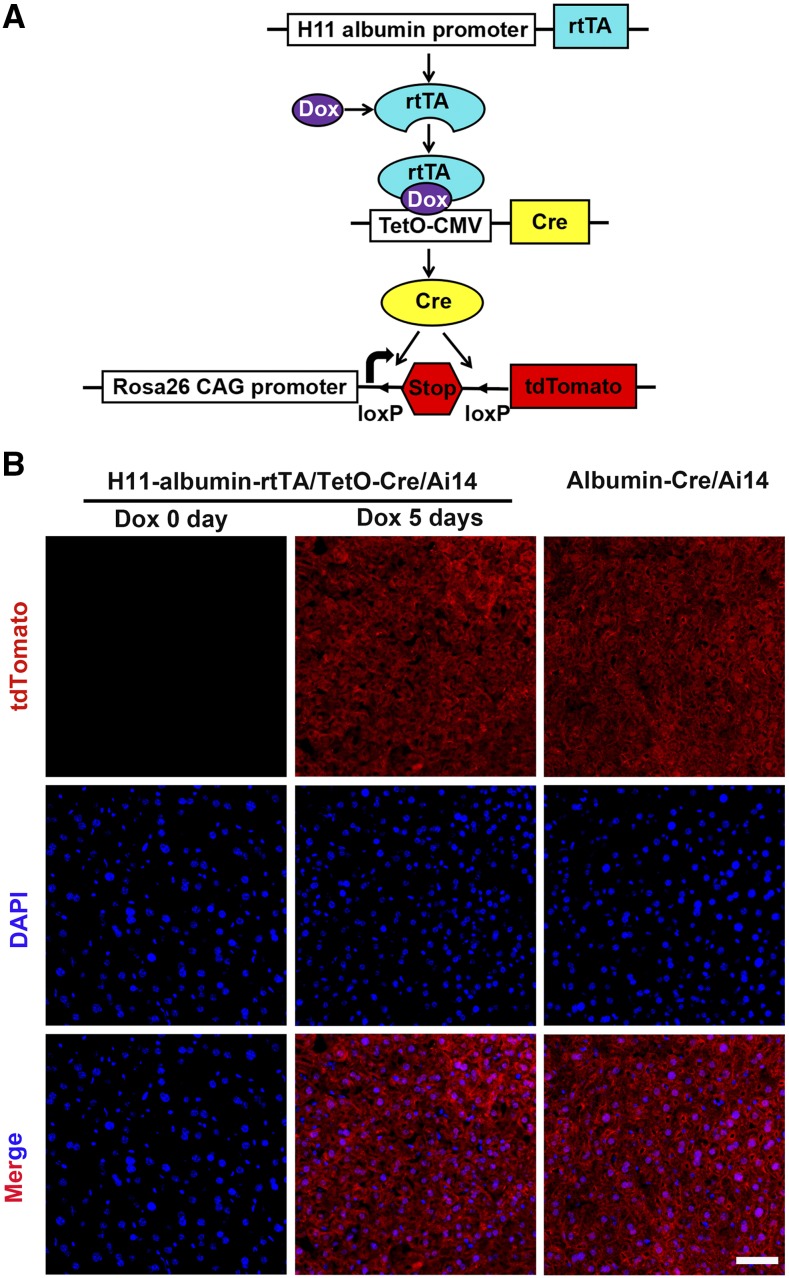
The Cre/loxP recombination system, which enables conditional DNA recombination, is a well-established research tool that is especially popular for experiments with transgenic mice (e.g., albumin-Cre mice ubiquitously in studies of liver development and disease (Cui et al. 2016)). Thus, we tested if we could implement Cre/loxP conditional DNA recombination technology with our mice by crossing Dox-inducible H11-albumin-rtTA mice with TetO-Cre and Ai14 mice to generate H11-albumin-rtTA/TetO-Cre/Ai14 triple transgenic mice. Specifically (Figure 5A), Ai14, a knock-in allele of the Rosa26 locus that allows high-level ubiquitous expression of the red fluorescent protein tdTomato downstream of a loxP-flanked transcriptional stop signal: in the presence of Cre, the stop codon is excised, and tdTomato expression proceeds (Madisen et al. 2010).
Figure 5.
Dox-inducible tdTomato expression in the livers of H11-albumin-rtTA/TetO-Cre/Ai14 mice. (A) Schematic diagram of induced tdTomato expression in the H11-albumin-rtTA/TetO-Cre/Ai14 triple transgenic mice after Dox treatment. The rtTA protein binds Dox and forms a complex that initiates transcription of Cre recombinase downstream of a TetO-CMV. Subsequently, Cre excises the loxP-flanked Stop sequence from the Rosa26 locus, inducing tdTomato expression. (B) Immunofluorescence analysis of tdTomato protein expression in the adult H11-albumin-rtTA/TetO-Cre/Ai14 mice which were treated with or without 1 mg/ml Dox for 5 days (Dox 0 day, left panel; Dox 5 days, middle panel) and in the Albumin-Cre/Ai14 mice (right panel). tdTomato staining (red); DAPI nuclear counterstaining of DNA (blue). Scale bar: 50 μm.
Adult H11-albumin-rtTA/TetO-Cre/Ai14 triple transgenic mice were given drinking water supplemented with 1 mg/ml Dox for 0, 3, or 5 days (Figure S2). Whole mount fluorescence microscopy and immunofluorescence analysis were used to characterize the induction of dTomato reporter expression (Figure 5B and Figure S2), and we found that, in the absence of Dox treatment, no dTomato signal was detected in the livers of H11-albumin-rtTA/TetO-Cre/Ai14 mice. The mount fluorescence analysis showed that the dTomato signal levels in the livers H11-albumin-rtTA/TetO-Cre/Ai14 mice became stronger as the length of Dox treatment increased (Figure S3). Notably, highlighting the very high Cre-mediated recombination efficiency achieved with this Dox-inducible system for conditional transgene expression in our H11-albumin-rtTA/TetO-Cre/Ai14 mice, we found that the tdTomato signal of the H11-albumin-rtTA /TetO-Cre/Ai14 mice treated with Dox for 5 days was apparently as strong as the signal in the albumin-Cre/A14 mice, which express tdTomato in the liver constitutively (Figure 5B and Figure S3).
Inducible and tissue-specific transgene expression in embryonic and neonatal mice
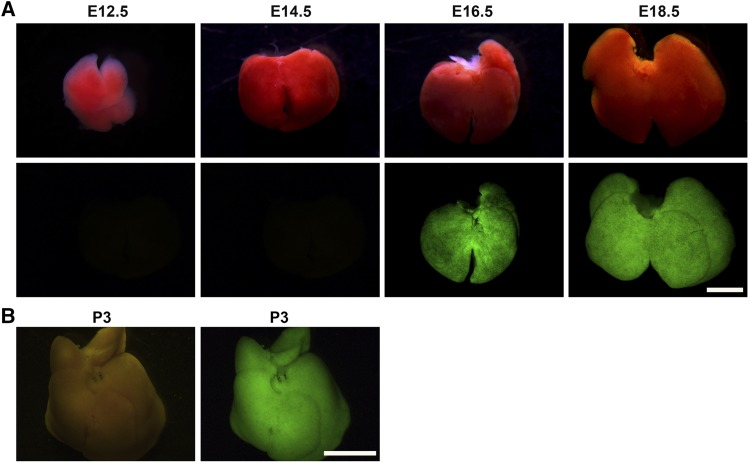
Having determined that our new site-specific knock-in H11-albumin-rtTA transgenic mouse model can be used with adult mice, we next explored its use in developing mouse embryos. Dox treatment of (1mg/ml) pregnant female mice was initiated upon detection of the vaginal plug, and embryos were analyzed by whole mount fluorescence microscopy analysis at E12.5, E14.5, E16.5, and E18.5 in H11-albumin-rtTA/TetO-H2BGFP mice. No GFP signal was detected before E16.5; strong GFP signal was observed at E18.5 (Figure 6A). We also conducted an experiment in which neonatal H11-albumin-rtTA/TetO-H2BGFP mice were administered an oral gavage of 20 μl of Dox (1 mg/ml) at P1 for two consecutive days (12 hr a time), which revealed strong liver-specific GFP expression at P3 (Figure 6B). Together, these results demonstrate that our new site-specific knock-in H11-albumin-rtTA transgenic mouse model can be used for Dox-inducible and tissue-specific expression of transgenes-of interest in both embryos and neonatal pups.
Figure 6.
Dox-inducible H2BGFP reporter gene expression in the livers of embryonic and neonatal H11-albumin-rtTA/TetO-H2BGFP double-transgenic mice. (A) Light images (top panels) and whole mount fluorescence microscopy (bottom panels) analysis of H2BGFP protein expression in the livers of embryonic mice at successive developmental stages. H2BGFP expression in the liver at E12.5, E14.5, E16.5, and E18.5. Scale bar: 2 mm. (B) Light image (left panel) and whole mount fluorescence microscopy (right panel) analysis of H2BGFP protein expression in the livers of P3 mice. Scale bar: 5 mm.
Discussion
In this study, we developed a new H11 site specific knock-in transgenic mouse model in which albumin-rtTA elements were inserted into the endogenous H11 locus via homologous recombination. The use of this locus to drive generalized gene expression addresses many of the problems associated with random DNA insertion integration, such as unstable insertion, insertion-site side effects, and low insertion efficiency (Sadelain et al. 2011). To date, the Rosa26 locus has been popularly used as a transgene insertion site; it causes no apparent adverse effects and permits stable gene expression (Sukup-Jackson et al. 2014). Our study confirms the previously reported findings about H11 as an attractive locus for transgene insertion (Hippenmeyer et al. 2010; Zhu et al. 2014; Ruan et al. 2015). Previous studies in mice have established that genes inserted into the H11 locus can display robust, ubiquitous expression that is apparently higher than the expression levels achieved with other commonly used ubiquitously loci, including Rosa26 (Hippenmeyer et al. 2010; Tasic et al. 2011; Tasic et al. 2012).
Our H11-albumin-rtTA mice will be particularly useful for studies of the liver, as we confirmed that H2BGFP expression is specifically localized to the liver of Dox-treated H11-albumin-rtTA/TetO-H2BGFP double-transgenic mice and verified that albumin promoter can activate rtTA expression in a Dox-dependent manner. Previous studies of albumin expression in embryonic mice reported that albumin mRNA was initially detected at E10.5 and then increased continuously and reached maximal expression in adult livers (Jochheim et al. 2004). However, and indicating an obvious discrepancy, we found in the H11-albumin-rtTA/TetO-H2BGFP mice the GFP expression level driven by the albumin promoter was undetectable at E12.5 and E14.5; we found that newly conceived H11-albumin-rtTA embryos that were treated with Dox were expressing GFP reporter protein by E16.5. Further, Hayhurst et al. (2001) reported that albumin-Cre transgenic mice express Cre exclusively in the postpartum liver (Yakar et al. 1999; Hayhurst et al. 2001). Thus, it appears that the strength of albumin promoter activity in embryonic Dox-inducible H11-albumin-rtTA mice is significantly higher than in albumin-Cre transgenic mice. These discrepancies may be related to the higher efficiency of inducing gene expression in transgenic mice generated by site-specific insertion. Nevertheless, it is clear that our H11-albumin-rtTA mice can be widely used to manipulate target gene expression in the livers of later embryonic and neonatal mice.
In some mechanistic studies, researchers need to manipulate gene expression in more than one cell type or in the same cell type but at different ages. This can be achieved for example by combining Tet-on inducible mouse models and the widely deployed Cre/loxP system, which has become a standard method of choice for cell-type or tissue-specific gene knockout in mice (Xue et al. 2014). In recent years, there have been many studies that used targeted Cre-mediated conditional gene expression in the liver (He et al. 2017; Chao et al. 2018). Our new Dox-inducible H11-albumin-rtTA mice add a flexible new option for researchers to use in their experimental designs: we successfully confirmed that Dox-induced albumin-driven Cre expression in the is very high in transgenic mice. When researchers need to study a liver-specific gene in mice that already harbor engineered sequences at their Rosa26 locus, H11-albumin-rtTA mice will be an excellent experimental option, especially considering the hepatocyte-specific expression driven by the albumin promoter. For example, for liver studies that need to use some particular line of knock-in mice (e.g., Rosa26-LSL (loxP-Stop-loxP)- given-gene-of-interest) and that also want to use TetO-Cre mice, we could use our new H11-albumin-rtTA mice to generate H11-albumin-rtTA/TetO-Cre/Rosa26-LSL-given-gene-of-interest mice. Transgenic animals are now recognized as invaluable research tools for studying human disease, and beyond highlighting the utility of the H11 locus generally, our study establishes that these new H11-albumin-rtTA mice enable unprecedently temporal and spatial control of the expression for genes-of-interest in the liver.
Acknowledgments
We thank John Hugh Snyder for helpful discussions in preparing the article. This work was supported by the National Institute of Biological Sciences, Beijing and Xinxiang Medical University (XYBSKYZZ201802).
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25387/g3.7502405.
Communicating editor: A. McCallion
Literature Cited
- Bijlani S., Nahar A. S., Ganesan K., 2018. Improved Tet-On and Tet-Off systems for tetracycline-regulated expression of genes in Candida. Curr. Genet. 64: 303–316. 10.1007/s00294-017-0720-9 [DOI] [PubMed] [Google Scholar]
- Burger A., Koesters R., Schafer B. W., Niggli F. K., 2011. Generation of a novel rtTA transgenic mouse to induce time-controlled, tissue-specific alterations in Pax2-expressing cells. Genesis 49: 797–802. 10.1002/dvg.20701 [DOI] [PubMed] [Google Scholar]
- Chakkalakal J. V., Jones K. M., Basson M. A., Brack A. S., 2012. The aged niche disrupts muscle stem cell quiescence. Nature 490: 355–360. 10.1038/nature11438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao X., Wang S., Zhao K., Li Y., Williams J. A., et al. , 2018. Impaired TFEB-Mediated Lysosome Biogenesis and Autophagy Promote Chronic Ethanol-Induced Liver Injury and Steatosis in Mice. Gastroenterology 155: 865–879.e12. 10.1053/j.gastro.2018.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Q., Song X. Q., Wang Y. L., Zhou T. Y., Bai L., et al. , 2011. Construction of a highly-active, liver-specific transcriptional regulatory element through combination of the albumin promoter and alpha-fetoprotein enhancer. Plasmid 65: 125–131. 10.1016/j.plasmid.2010.11.006 [DOI] [PubMed] [Google Scholar]
- Cox B. C., Dearman J. A., Brancheck J., Zindy F., Roussel M. F., et al. , 2014. Generation of Atoh1-rtTA transgenic mice: a tool for inducible gene expression in hair cells of the inner ear. Sci. Rep. 4: 6885 10.1038/srep06885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Chen Q., Dong Z., Xu L., Lu T., et al. , 2016. Inactivation of Sirt1 in mouse livers protects against endotoxemic liver injury by acetylating and activating NF-kappaB. Cell Death Dis. 7: e2403 10.1038/cddis.2016.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A. T., Tenenbaum L., Berkhout B., 2016. Tet-On Systems For Doxycycline-inducible Gene Expression. Curr. Gene Ther. 16: 156–167. 10.2174/1566523216666160524144041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi C. R., Chaillet J. R., Nalesnik M. A., Kumar S., Dangi A., et al. , 2015. Liver-specific deletion of augmenter of liver regeneration accelerates development of steatohepatitis and hepatocellular carcinoma in mice. Gastroenterology 148: 379–391 e374. 10.1053/j.gastro.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenthner C. J., Miyamichi K., Yang H. H., Heller H. C., Luo L., 2013. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 78: 773–784 (erratum: Neuron 79: 1257). 10.1016/j.neuron.2013.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayhurst G. P., Lee Y. H., Lambert G., Ward J. M., Gonzalez F. J., 2001. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 21: 1393–1403. 10.1128/MCB.21.4.1393-1403.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Li Y., Li Y., Pu W., Huang X., et al. , 2017. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat. Med. 23: 1488–1498. 10.1038/nm.4437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer S., Youn Y. H., Moon H. M., Miyamichi K., Zong H., et al. , 2010. Genetic mosaic dissection of Lis1 and Ndel1 in neuronal migration. Neuron 68: 695–709. 10.1016/j.neuron.2010.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochheim A., Hillemann T., Kania G., Scharf J., Attaran M., et al. , 2004. Quantitative gene expression profiling reveals a fetal hepatic phenotype of murine ES-derived hepatocytes. Int. J. Dev. Biol. 48: 23–29. 10.1387/ijdb.15005571 [DOI] [PubMed] [Google Scholar]
- Li Y., Pan J., Wei C., Chen J., Liu Y., et al. , 2014. LIM homeodomain transcription factor Isl1 directs normal pyloric development by targeting Gata3. BMC Biol. 12: 25 10.1186/1741-7007-12-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Yu L., Pan S., Gao S., Chen W., et al. , 2017. CRISPR/Cas9-mediated targeting of the Rosa26 locus produces Cre reporter rat strains for monitoring Cre-loxP-mediated lineage tracing. FEBS J. 284: 3262–3277. 10.1111/febs.14188 [DOI] [PubMed] [Google Scholar]
- Madisen L., Zwingman T. A., Sunkin S. M., Oh S. W., Zariwala H. A., et al. , 2010. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13: 133–140. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahedi K., Wiegmann R., De Vlaminck K., Van Ginderachter J. A., Nikolaev V. O., 2018. RoMo: An efficient strategy for functional mosaic analysis via stochastic Cre recombination and gene targeting in the ROSA26 locus. Biotechnol. Bioeng. 115: 1778–1792. 10.1002/bit.26594 [DOI] [PubMed] [Google Scholar]
- Ruan J., Li H., Xu K., Wu T., Wei J., et al. , 2015. Highly efficient CRISPR/Cas9-mediated transgene knockin at the H11 locus in pigs. Sci. Rep. 5: 14253 10.1038/srep14253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadelain M., Papapetrou E. P., Bushman F. D., 2011. Safe harbours for the integration of new DNA in the human genome. Nat. Rev. Cancer 12: 51–58. 10.1038/nrc3179 [DOI] [PubMed] [Google Scholar]
- Sukup-Jackson M. R., Kiraly O., Kay J. E., Na L., Rowland E. A., et al. , 2014. Rosa26-GFP direct repeat (RaDR-GFP) mice reveal tissue- and age-dependence of homologous recombination in mammals in vivo. PLoS Genet. 10: e1004299 10.1371/journal.pgen.1004299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B., Hippenmeyer S., Wang C., Gamboa M., Zong H., et al. , 2011. Site-specific integrase-mediated transgenesis in mice via pronuclear injection. Proc. Natl. Acad. Sci. USA 108: 7902–7907. 10.1073/pnas.1019507108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B., Miyamichi K., Hippenmeyer S., Dani V. S., Zeng H., et al. , 2012. Extensions of MADM (mosaic analysis with double markers) in mice. PLoS One 7: e33332 (erratum: PLoS One 7: 10.1371/annotation/e4275a34-48e1-42b8-8615-f59aacaf3733). 10.1371/journal.pone.0033332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiem S., Eissmann M. F., Stuart E., Elzer J., Jonas A., et al. , 2016. Inducible gene modification in the gastric epithelium of Tff1-CreERT2, Tff2-rtTA, Tff3-luc mice. Genesis 54: 626–635. 10.1002/dvg.22987 [DOI] [PubMed] [Google Scholar]
- Truett G. E., Heeger P., Mynatt R. L., Truett A. A., Walker J. A., et al. , 2000. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29: 52, 54. [DOI] [PubMed] [Google Scholar]
- Uemura S., Nagaoka T., Yokoyama M., Igarashi M., Kishi M., 2014. A simple and highly efficient method to identify the integration site of a transgene in the animal genome. Neurosci. Res. 80: 91–94. 10.1016/j.neures.2013.11.007 [DOI] [PubMed] [Google Scholar]
- Xue W., Chen S., Yin H., Tammela T., Papagiannakopoulos T., et al. , 2014. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature 514: 380–384. 10.1038/nature13589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S., Liu J. L., Stannard B., Butler A., Accili D., et al. , 1999. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. USA 96: 7324–7329. 10.1073/pnas.96.13.7324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wang H., Jaenisch R., 2014. Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat. Protoc. 9: 1956–1968. 10.1038/nprot.2014.134 [DOI] [PubMed] [Google Scholar]
- Zambrowicz B. P., Imamoto A., Fiering S., Herzenberg L. A., Kerr W. G., et al. , 1997. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc. Natl. Acad. Sci. USA 94: 3789–3794. 10.1073/pnas.94.8.3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Vink M., Klaver B., Berkhout B., Das A. T., 2006. Optimization of the Tet-On system for regulated gene expression through viral evolution. Gene Ther. 13: 1382–1390. 10.1038/sj.gt.3302780 [DOI] [PubMed] [Google Scholar]
- Zhou X. J., Sun S. H., Wang P., Yu H., Hu J. Y., et al. , 2012. Over-expression of uPA increases risk of liver injury in pAAV-HBV transfected mice. World J Gastroenterol 18: 1892–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Gamboa M., Farruggio A. P., Hippenmeyer S., Tasic B., et al. , 2014. DICE, an efficient system for iterative genomic editing in human pluripotent stem cells. Nucleic Acids Res. 42: e34 10.1093/nar/gkt1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data necessary for confirming the conclusions presented are within the article. File S1 contains supplemental data to support the conclusions in this article. Supplemental material available at Figshare: https://doi.org/10.25387/g3.7502405.