Abstract
Background
Hospitalized patients with cancer experience a high symptom burden, which is associated with poor health outcomes and increased health care utilization. However, studies investigating symptom monitoring interventions in this population are lacking. We conducted a pilot randomized trial to assess the feasibility and preliminary efficacy of a symptom monitoring intervention to improve symptom management in hospitalized patients with advanced cancer.
Patients and methods
We randomly assigned patients with advanced cancer who were admitted to the inpatient oncology service to a symptom monitoring intervention or usual care. Patients in both arms self-reported their symptoms daily (Edmonton Symptom Assessment System and Patient Health Questionnaire-4). Patients assigned to the intervention had their symptom reports presented graphically with alerts for moderate/severe symptoms during daily team rounds. The primary end point of the study was feasibility. We defined the intervention as feasible if >75% of participants hospitalized >2 days completed >2 symptom reports. We observed daily rounds to determine whether clinicians discussed and developed a plan to address patients’ symptoms. We used regression models to assess intervention effects on patients’ symptoms throughout their hospitalization, readmission risk, and hospital length of stay (LOS).
Results
Among 150 enrolled patients (81.1% enrollment), 94.2% completed >2 symptom reports. Clinicians discussed 60.4% of the symptom reports and developed a plan to address the symptoms highlighted by the symptom reports 20.8% of the time. Compared with usual care, intervention patients had a greater proportion of days with lower psychological distress (B = 0.12, P = 0.008), but no significant difference in the proportion of days with improved Edmonton Symptom Assessment System-physical symptoms (B = 0.07, P = 0.138). Intervention patients had lower readmission risk (hazard ratio = 0.68, P = 0.224), although this difference was not significant. We found no significant intervention effects on hospital LOS (B = 0.16, P = 0.862).
Conclusions
This symptom monitoring intervention is feasible and demonstrates encouraging preliminary efficacy for improving patients’ symptoms and readmission risk.
ClinicalTrials.gov identifier NCT02891993
Keywords: symptoms, randomized controlled trial, mood, cancer, outcomes research, hospital readmissions
Key Message
In this pilot randomized controlled trial, we demonstrated that a symptom monitoring intervention can be feasibly integrated into the inpatient care for hospitalized patients with advanced cancer. Importantly, we found that patients assigned to the symptom monitoring intervention experienced improvements in their symptom burden throughout their hospital stay compared with usual care.
Introduction
Patients with cancer experience many physical and psychological symptoms that are often under-recognized by their clinicians [1, 2]. Symptoms such as pain, dyspnea, fatigue, and nausea lead to poor quality of life (QOL) and psychological distress [3, 4]. However, research demonstrates that clinicians often fail to reliably detect their patients’ symptoms and frequently underestimate their severity [5]. While much of the existing literature regarding symptom prevalence and severity is based on reports of patients in ambulatory cancer care settings, little work has focused on the symptoms of patients with cancer in the inpatient setting. Notably, hospitalized patients often experience worse symptom burden than those in the outpatient setting, and therefore interventions have potential for a larger impact in this population [6].
Patients’ symptom burden also contributes to their use of health care services [2, 7, 8]. Specifically, patients with cancer often require hospitalizations for symptoms related to pain, fatigue, and nausea [9]. Furthermore, prior work has shown that patients with higher symptom burden have longer hospital lengths of stay and higher risk for hospital readmissions [2, 7, 8]. Notably, patients with cancer prefer to avoid time in the hospital, which underscores the need for efforts targeting modifiable risk factors, such as patients’ symptoms, that contribute to prolonged hospital stays and readmissions [10].
Patients’ symptom burden represents a modifiable risk factor that, if properly addressed, may improve patient outcomes. Importantly, symptom monitoring interventions can improve symptom management, enhance QOL, and prevent hospitalizations among patients with cancer [11–14]. Prior investigations have focused primarily on patients in the outpatient setting, yet a recent study showed that a pain assessment and management intervention for hospitalized patients with cancer could improve pain outcomes in the inpatient setting [14]. However, this study did not address other important physical and psychological symptoms, which can impact patient outcomes in the inpatient setting [2, 7, 8]. Thus, further research is needed to recognize and address patients’ global symptom burden in the inpatient setting.
We conducted a pilot randomized controlled trial of an electronic symptom monitoring intervention for hospitalized patients with advanced cancer, which we called ‘Improving Management of Patient-Reported Outcomes Via Electronic Data’ (IMPROVED). Specifically, we sought to assess the feasibility and preliminary efficacy of IMPROVED for improving symptom burden and health care utilization among hospitalized patients with advanced cancer.
Methods
Study procedures
From 26 October 2016 to 30 June 2017, we enrolled patients with advanced cancer and an unplanned hospital admission at Massachusetts General Hospital (MGH) in a nonblinded, pilot randomized controlled trial of an electronic symptom monitoring intervention (IMPROVED) versus usual care (ClinicalTrials.gov identifier NCT02891993). We identified and recruited consecutive patients with an unplanned hospital admission during the study period by screening the daily inpatient oncology service census. Trained study staff obtained written, informed consent from eligible patients within 36 hours of their admission. Following consent, participants completed baseline study measures. We randomized participants to the IMPROVED intervention or usual care using computer-generated 1 : 1 ratio block randomization with block size of two, which was concealed until after group assignment. The Dana-Farber/Harvard Cancer Center Institutional Review Board approved the study.
Participants
Patients eligible for study participation included those who were at least 18 years old and admitted to the MGH oncology service with known diagnosis of advanced cancer. We defined patients with advanced cancer as those not being treated with curative intent, ascertained from the chemotherapy order entry treatment intent designation (palliative versus curative), or using documentation in the oncology clinic notes for those not receiving chemotherapy. Study participants had to be able to read and respond to study questionnaires in English or with minimal assistance from an interpreter. We excluded patients with elective or planned hospital admissions, defined as hospitalizations for chemotherapy administration, scheduled procedures, or chemotherapy desensitization. We also excluded patients with leukemia and those admitted for stem cell transplantation.
IMPROVED intervention
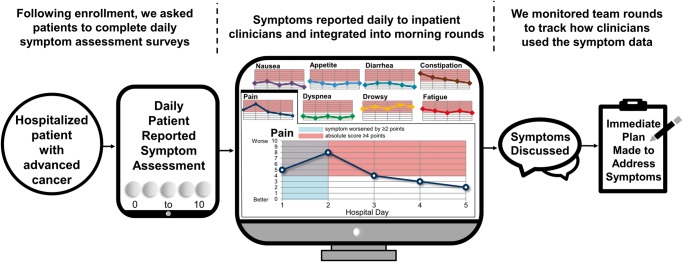
Patients assigned to IMPROVED reported their symptoms daily using tablet computers (Figure 1). Patients who were unable to complete the computerized symptom assessment could use paper versions. During morning rounds each day, study staff presented patients’ symptom reports to the clinical team (nurses, advanced practice nurses, and physicians) on a computer-based projection screen as patients were being discussed. Before study start, we provided information to the clinical team about the study and oriented them to the intervention and study outcomes. For morning rounds, the clinical team meets in a central meeting room on the inpatient oncology ward to discuss each patient. The detailed symptom reports contained patients’ numeric symptom scores, as well as an alert whenever any specific symptom worsened by two or more points from the previous assessment or if a symptom reached an absolute score of four or greater. Additionally, these detailed symptom reports contained a graphical depiction of patients’ daily symptom trajectory for the hospital admission. Study staff did not provide guidance about addressing or managing patients’ symptoms but rather the oncology team made decisions regarding symptom management per their clinical judgment.
Figure 1.
IMPROVED intervention.
Usual care
Participants assigned to usual care also reported their symptoms each day using tablet computers. However, these patients’ clinicians did not receive their symptom reports. Study staff instructed patients in both study groups to report their symptoms to their clinicians as they usually would, at their own discretion.
Study measures
Sociodemographic and clinical characteristics
Study staff administered baseline measures to participants before randomization. Participants completed a sociodemographic questionnaire to report their sex, race, relationship status, employment, and education. We obtained information about participants’ age and cancer history from the medical record.
Physical and psychological symptom burden
Each day of their hospital admission and before morning rounds, study staff asked patients in both study arms to independently complete symptom assessments using REDCap (Research Electronic Data Capture), a free, secure, HIPAA compliant web-based application. We evaluated physical symptoms with the modified, revised Edmonton Symptom Assessment System (ESAS-r), which assesses pain, fatigue, drowsiness, nausea, appetite, dyspnea, depression, anxiety, and well-being over the previous 24 hours [15]. We also included constipation and diarrhea, as these are highly prevalent symptoms among patients with cancer [16]. Each specific symptom is scored on a 0–10 scale (0 reflecting absence of the symptom and 10 reflecting the worst possible severity), with minimal clinically important differences of one point for each [17]. Consistent with prior research, we scored the severity of ESAS scores as 0 (none), 1–3 (mild), 4–6 (moderate), and 7–10 (severe) [2]. We computed a composite ESAS-physical variable, which includes a summated score of physical symptoms (pain, fatigue, drowsiness, nausea, appetite, dyspnea, constipation, and diarrhea).
We used the Patient Health Questionnaire-4 (PHQ-4) to assess participants’ psychological symptoms [18]. The PHQ-4 is a four-item tool, scored as 0–12, evaluating depression and anxiety symptoms. The composite PHQ-4 can be evaluated continuously, with higher scores indicating worse psychological distress, and categorically with scores of 6 and greater indicating moderate/severe symptoms of psychological distress [18].
Health care utilization
We evaluated hospital length of stay and unplanned hospital readmissions. To account for mortality, given that patients who die following hospital discharge have less time at risk for readmission, we used time to first unplanned readmission within 30 days of hospital discharge as the outcome measure, consistent with prior work [1, 2]. We censored patients without a readmission at their 30-day post-discharge date and those who died within 30 days at their death date.
Statistical analysis
The preplanned, primary end point of the study was feasibility. We chose the sample size for the study based on the feasibility of completing the project during the proposed timeframe and achieving the feasibility end point. To assess whether patients were willing and able to self-report their symptoms, we defined the intervention as feasible if ≥75% of participants hospitalized >2 days completed >2 symptom reports. We also observed daily team rounds to determine how often clinicians discussed the symptom reports and developed a plan to address patients’ symptoms (e.g. adjust medications or request consultative support) for those assigned to the intervention.
Secondary end points included an evaluation of preliminary efficacy of the intervention to improve patient-reported symptom burden and health care utilization. To assess the effect of IMPROVED on symptom burden, we used linear regression, controlling for baseline symptom score and patient age (given that age differed significantly between study arms), to model the proportion of days that patients’ symptoms improved by at least one point in a post hoc analysis. For each patient, we calculated the proportion of days that patients’ symptoms improved by at least one point by summing the total days that patients had an improvement in their symptom score and dividing by the total number of symptom assessments completed. To further assess the day-to-day change in patients’ symptoms, we used linear regression to evaluate the average change in symptom scores on a daily basis during patients’ hospitalization, adjusted for baseline symptom score and patient age, in a post hoc analysis. We conducted available case analyses and did not use imputation methods given low rates of missing data. To investigate intervention effects on hospital length of stay, we computed linear regression, adjusted for patient age. To evaluate intervention effects on time to first unplanned readmission within 30 days, we used a Cox proportional hazards regression model, adjusted for patient age and length of stay during the index hospitalization. We used conservative (α = 0.05) and liberal (α = 0.25) values to assess statistical significance, consistent with recommendations for interpreting pilot trials [19, 20].
Results
Participant characteristics
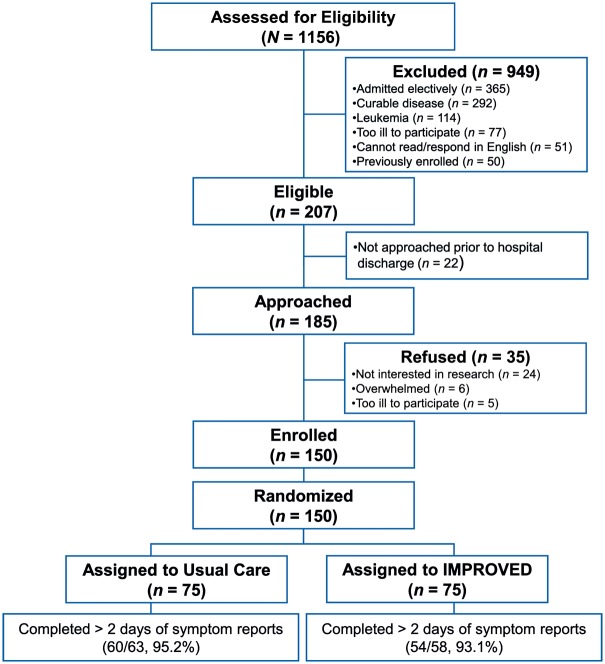
Of 185 patients approached for the study, we enrolled and randomized 150 (81.1% enrollment rate) (Figure 2). Patients were mostly white (84.0%), with a median age of 63.96 years (range: 22.68–92.76 years), and 59.3% male. Intervention patients had a younger mean age than usual care patients (60.44 versus 64.87 years) (Table 1). The mean hospital length of stay for the entire sample was 6.45 days (SD, 5.57 days). Readmission and death rates within 30 days of hospital discharge were 30.0% and 19.3%, respectively.
Figure 2.
CONSORT diagram.
Table 1.
Participant characteristics
| Characteristic | Usual care (N = 75) | IMPROVED (N = 75) |
|---|---|---|
| Age, mean (SD) | 64.87 (12.35) | 60.44 (14.61) |
| Sex, n (%) | ||
| Male | 44 (58.7) | 45 (60.0) |
| Female | 31 (41.3) | 30 (40.0) |
| Race, n (%) | ||
| White | 67 (89.3) | 59 (78.7) |
| African American | 2 (2.7) | 8 (10.7) |
| Asian | 3 (4.0) | 2 (2.7) |
| Other | 3 (4.0) | 6 (8.0) |
| Cancer type, n (%) | ||
| Gastrointestinal | 24 (32.0) | 31 (41.3) |
| Lung | 16 (21.3) | 17 (22.7) |
| Head and neck | 7 (9.3) | 8 (10.7) |
| Lymphoma | 8 (10.7) | 4 (5.3) |
| Breast | 5 (6.7) | 5 (6.7) |
| Genitourinary | 5 (6.7) | 4 (5.3) |
| Skin | 7 (9.3) | 1 (1.3) |
| Sarcoma | 3 (4.0) | 5 (6.7) |
| Relationship status, n (%) | ||
| Married or living with partner | 54 (72.0) | 57 (76.0) |
| Widowed | 7 (9.3) | 5 (6.7) |
| Divorced | 3 (4.0) | 6 (8.0) |
| Never married | 11 (14.7) | 7 (9.3) |
| Employed, n (%) | 20 (26.7) | 21 (28.0) |
| Education, n (%) | ||
| Less than college graduate | 34 (45.3) | 34 (45.3) |
| College graduate and above | 41 (54.7) | 41 (54.7) |
| Symptoms | Mean (SD) | Mean (SD) |
| ESAS physical symptoms | ||
| Number of moderate/severe physical symptoms | 3.68 (1.76) | 4.03 (1.52) |
| ESAS Physical Symptom Score | 27.03 (13.91) | 30.53 (12.53) |
| Individual ESAS symptoms | ||
| Pain | 4.25 (3.56) | 5.27 (3.33) |
| Fatigue | 6.23 (3.00) | 6.55 (2.77) |
| Drowsiness | 4.52 (3.56) | 4.91 (3.64) |
| Nausea | 1.97 (3.11) | 2.03 (2.99) |
| Lack of appetite | 4.23 (3.86) | 4.73 (3.69) |
| Shortness of breath | 2.67 (3.50) | 2.72 (3.41) |
| Constipation | 1.53 (2.84) | 2.60 (3.64) |
| Diarrhea | 1.63 (2.84) | 1.73 (3.33) |
| PHQ-4 psychological symptoms | ||
| PHQ-4 total score | 2.73 (3.20) | 2.14 (2.44) |
IMPROVED, Improving Management of Patient-Reported Outcomes Via Electronic Data; ESAS, Edmonton Symptom Assessment System; PHQ-4, Patient Health Questionnaire-4.
Baseline physical and psychological symptom burden
Of the eight ESAS-physical symptoms we evaluated, patients reported experiencing a mean of 3.85 (SD, 1.64) moderate/severe symptoms at baseline; only four patients (2.7%) reported experiencing no moderate/severe symptoms. On the PHQ-4, 17.4% of participants reported moderate/severe symptoms of psychological distress.
Feasibility
For patients hospitalized >2 days, 94.2% (114/121) completed >2 symptom reports. Overall, patients completed the symptom assessments 89.4% (753/842) of the time. Additionally, for patients assigned to IMPROVED, the inpatient clinicians discussed 60.4% (177/293) of all symptom reports and developed a plan during rounds to address patients’ symptoms for 20.8% (61/293) of all symptom reports.
Intervention effect on patients’ symptom burden
In multivariable models, we found that patients assigned to IMPROVED had a greater proportion of days with lower psychological distress [B = 0.12, 95% confidence interval (CI): 0.03–0.21; P = 0.008]. With a liberal α of 0.25, intervention patients also had a greater proportion of days with improved ESAS-physical symptoms (B = 0.07, 95% CI: −0.02 to 0.16; P = 0.138) (supplementary Figure, available at Annals of Oncology online).
We assessed the average day-to-day change in patients’ symptoms (Table 2). These analyses demonstrated that patients assigned to IMPROVED experienced improvements in their average symptom scores for drowsiness (B = −0.54, 95% CI: −1.04 to −0.05; P = 0.033) and shortness of breath (B = −0.43, 95% CI: −0.75 to −0.11; P = 0.009), but not for other individual physical symptoms.
Table 2.
The effect of IMPROVED on the average change in symptom burden on a daily basis
| IMPROVED effecta | B | 95% Confidence interval |
P value | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Pain | 0.019 | −0.510 | 0.547 | 0.945 |
| Fatigue | −0.185 | −0.742 | 0.372 | 0.513 |
| Drowsiness | −0.543 | −1.039 | −0.046 | 0.033 |
| Nausea | −0.105 | −0.562 | 0.353 | 0.651 |
| Lack of appetite | 0.133 | −0.454 | 0.721 | 0.655 |
| Shortness of breath | −0.430 | −0.754 | −0.107 | 0.009 |
| Constipation | 0.100 | −0.442 | 0.641 | 0.716 |
| Diarrhea | 0.001 | −0.304 | 0.305 | 0.997 |
Adjusted for baseline symptom score (for the respective symptom) and patient age.
IMPROVED, Improving Management of Patient-Reported Outcomes Via Electronic Data.
Intervention effect on patients’ health care utilization
We found no significant intervention effects on patients’ hospital length of stay (B = 0.16, 95% CI: −1.67 to 1.99; P = 0.862). Using a liberal α of 0.25, patients assigned to IMPROVED had a lower risk of readmissions (hazard ratio = 0.68, 95% CI: 0.37–1.26; P = 0.224).
Discussion
In this pilot randomized trial, we investigated the feasibility and preliminary efficacy of IMPROVED for improving symptom burden and health care utilization among hospitalized patients with advanced cancer. We enrolled over 80% of patients approached and participants completed nearly all the daily symptom assessments. The inpatient team discussed most of the symptom reports at the time of presentation during morning rounds and made an immediate plan to address the symptoms reported over one-fifth of the time. Notably, patients assigned to IMPROVED experienced a greater proportion of days with lower psychological distress. Collectively, these data demonstrate that IMPROVED is feasible, with encouraging preliminary efficacy for addressing patients’ symptoms.
To our knowledge, the current study is the first to investigate a global symptom monitoring intervention for hospitalized patients with cancer. Although prior studies in the outpatient setting have demonstrated the efficacy of symptom monitoring interventions for addressing patients’ symptom burden [11–13], this is the first study to demonstrate that a symptom monitoring intervention can be feasibly integrated into the inpatient care for hospitalized patients with cancer. Importantly, barriers from the outpatient setting, such as difficulties with timing, frequency, and actionability of symptom assessments, are less problematic in the inpatient setting, as clinicians can track and address their patients’ symptoms more frequently. Notably, in the current study, the rates of the inpatient team discussing and addressing patients’ symptom reports have room for improvement. In future work, we will strive to improve clinician engagement, which could result in additional downstream effects for enhancing the efficacy of IMPROVED.
Notably, we found that patients assigned to IMPROVED experienced improvements in their symptom burden throughout their hospital stay. This may have resulted from increased attention to patients’ depression and anxiety symptoms but could also be related to the fact that patients received more attention to their physical symptoms as well. These findings have salient clinical implications, as patients in our sample reported a remarkably high baseline symptom burden, and prior work has demonstrated that patients’ physical and psychological symptoms are associated with poor QOL, prolonged hospital admissions, and greater risk for readmissions [2–4, 7, 8]. Future work is needed to confirm these results and help elucidate the mechanisms underlying why certain symptoms changed more readily than others in response to IMPROVED.
We also investigated the preliminary efficacy for IMPROVED to enhance health care utilization outcomes. We found no significant effects on hospital length of stay, yet the hazard ratio for IMPROVED to decrease patients’ risk of unplanned hospital readmissions demonstrates promising potential meriting further investigation. Moreover, patients’ symptoms represent just one of the many factors affecting hospital length of stay and readmissions [1, 2]. Issues such as functional impairment, inadequate social support, and comorbid conditions likely place certain patients at greater risk for prolonged hospitalizations and unplanned readmissions [7, 21]. Thus, additional work is needed to better understand how symptom monitoring interventions may help to decrease patients’ health care utilization, while also seeking to identify patients for whom additional services may be needed.
Our study has several important limitations. First, we conducted this study at a single, academic medical center, limiting the generalizability of our results to other care settings and populations. Second, we lack information about factors that could influence the impact of IMPROVED, such as patients’ functional status and social supports. Third, we may be underestimating readmission risk for the minority of patients who are hospitalized outside of our health system. Most patients receiving cancer care at our institution are admitted within our health system, and we tracked all hospital readmissions to any hospital within our health system. Fourth, we lack information about changes in prescribing patterns because of IMPROVED, and our assessment of whether clinicians discussed and/or developed a plan to address patients’ symptoms, which we lack for participants in the usual care arm, is inherently subjective. Additionally, based on the nature of this intervention, clinicians cannot be blinded. Future efforts to understand the efficacy of symptom monitoring interventions should investigate the use of certain supportive care medications and additional supportive care services, such as palliative care, pain management, and social work.
Conclusion
Our study demonstrates that a symptom monitoring intervention for hospitalized patients with advanced cancer is feasible, with promising preliminary efficacy to address patients’ symptoms throughout their hospital stay. Importantly, we demonstrated that daily symptom monitoring can be integrated into routine inpatient oncologic care. In addition, our findings highlight the potential for inpatient symptom monitoring interventions to enhance symptom management for hospitalized patients with cancer who present with a remarkably high symptom burden. A larger randomized controlled trial (N = 320) to investigate the efficacy of this care model to enhance symptom management (primary outcome) and improve health care utilization outcomes (secondary outcomes) for hospitalized patients with cancer is currently underway.
Funding
National Cancer Institute K24 (CA181253 to JT); MGH Cancer Center Funds [to JT (no grant number applies)]; and the Scullen Center for Cancer Data Analysis [to EH (no grant number applies)].
Disclosure
The authors have declared no conflicts of interest.
Footnotes
Note: This study was previously presented as an Oral Presentation at the 2018 ASCO Annual Meeting in Chicago, IL.
References
- 1. Lage DE, Nipp RD, D'Arpino SM. et al. Predictors of posthospital transitions of care in patients with advanced cancer. J Clin Oncol 2018; 36(1): 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nipp RD, El-Jawahri A, Moran SM. et al. The relationship between physical and psychological symptoms and health care utilization in hospitalized patients with advanced cancer. Cancer 2017; 123(23): 4720–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cooley ME, Short TH, Moriarty HJ.. Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Psychooncology 2003; 12(7): 694–708. [DOI] [PubMed] [Google Scholar]
- 4. Chang VT, Hwang SS, Feuerman M, Kasimis BS.. Symptom and quality of life survey of medical oncology patients at a veterans affairs medical center: a role for symptom assessment. Cancer 2000; 88(5): 1175–1183. [DOI] [PubMed] [Google Scholar]
- 5. Di Maio M, Gallo C, Leighl NB. et al. Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol 2015; 33: 910–915. [DOI] [PubMed] [Google Scholar]
- 6. Portenoy RK, Thaler HT, Kornblith AB. et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res 1994; 3(3): 183–189. [DOI] [PubMed] [Google Scholar]
- 7. Prieto JM, Blanch J, Atala J. et al. Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem-cell transplantation. J Clin Oncol 2002; 20(7): 1907–1917. [DOI] [PubMed] [Google Scholar]
- 8. Brooks GA, Abrams TA, Meyerhardt JA. et al. Identification of potentially avoidable hospitalizations in patients with GI cancer. J Clin Oncol 2014; 32(6): 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Numico G, Cristofano A, Mozzicafreddo A. et al. Hospital admission of cancer patients: avoidable practice or necessary care? PLoS One 2015; 10(3): e0120827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeurkar N, Farrington S, Craig TR. et al. Which hospice patients with cancer are able to die in the setting of their choice? Results of a retrospective cohort study. J Clin Oncol 2012; 30(22): 2783–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Basch E, Deal AM, Kris MG. et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 2016; 34(6): 557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berry DL, Hong F, Halpenny B. et al. Electronic self-report assessment for cancer and self-care support: results of a multicenter randomized trial. J Clin Oncol 2014; 32(3): 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Strasser F, Blum D, von Moos R. et al. The effect of real-time electronic monitoring of patient-reported symptoms and clinical syndromes in outpatient workflow of medical oncologists: e -MOSAIC, a multicenter cluster-randomized phase III study (SAKK 95/06). Ann Oncol 2016; 27(2): 324–332. [DOI] [PubMed] [Google Scholar]
- 14. Fallon M, Walker J, Colvin L. et al. Pain management in cancer center inpatients: a cluster randomized trial to evaluate a systematic integrated approach-The Edinburgh pain assessment and management tool. J Clin Oncol 2018; 36(13): 1284–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hui D, Bruera E.. The Edmonton symptom assessment system 25 years later: past, present, and future developments. J Pain Symptom Manage 2017; 53(3): 630–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rhondali W, Nguyen L, Palmer L. et al. Self-reported constipation in patients with advanced cancer: a preliminary report. J Pain Symptom Manage 2013; 45(1): 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hui D, Shamieh O, Paiva CE. et al. Minimal clinically important differences in the Edmonton Symptom Assessment Scale in cancer patients: a prospective, multicenter study. Cancer 2015; 121(17): 3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kroenke K, Spitzer RL, Williams JBW, Löwe B.. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics 2009; 50(6): 613–621. [DOI] [PubMed] [Google Scholar]
- 19. Lee EC, Whitehead AL, Jacques RM, Julious SA.. The statistical interpretation of pilot trials: should significance thresholds be reconsidered? BMC Med Res Methodol 2014; 14: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schoenfeld D. Statistical considerations for pilot studies. Int J Radiat Oncol Biol Phys 1980; 6(3): 371–374. [DOI] [PubMed] [Google Scholar]
- 21. Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE.. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med 2015; 175(4): 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]