Abstract
Background
Regular use of aspirin has been associated with a reduced risk of cancer at several sites but the data for endometrial cancer are conflicting. Evidence regarding use of other analgesics is limited.
Patients and methods
We pooled individual-level data from seven cohort and five case–control studies participating in the Epidemiology of Endometrial Cancer Consortium including 7120 women with endometrial cancer and 16 069 controls. For overall analyses, study-specific odds ratios (ORs) and 95% confidence intervals (CI) were estimated using logistic regression and combined using random-effects meta-analysis; for stratified analyses, we used mixed-effects logistic regression with study as a random effect.
Results
At least weekly use of aspirin and non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) was associated with an approximately 15% reduced risk of endometrial cancer among both overweight and obese women (OR = 0.86 [95% CI 0.76–0.98] and 0.86 [95% CI 0.76–0.97], respectively, for aspirin; 0.87 [95% CI 0.76–1.00] and 0.84 [0.74–0.96], respectively, for non-aspirin NSAIDs). There was no association among women of normal weight (body mass index < 25 kg/m2, Pheterogeneity = 0.04 for aspirin, Pheterogeneity = 0.003 for NSAIDs). Among overweight and obese women, the inverse association with aspirin was stronger for use 2–6 times/week (OR = 0.81, 95% CI 0.68–0.96) than for daily use (0.91, 0.80–1.03), possibly because a high proportion of daily users use low-dose formulations. There was no clear association with use of acetaminophen.
Conclusion
Our pooled analysis provides further evidence that use of standard-dose aspirin or other NSAIDs may reduce risk of endometrial cancer among overweight and obese women.
Keywords: endometrial cancer, aspirin, nonsteroidal anti-inflammatory drugs, acetaminophen
Key Message
In our pooled analysis of data from seven cohort and five case–control studies in the Epidemiology of Endometrial Cancer Consortium, at least weekly use of aspirin and nonsteroidal anti-inflammatory drugs was associated with a 15% reduced risk of endometrial cancer among overweight and obese women. Use of these medications may reduce risk of endometrial cancer among overweight and obese women.
Introduction
Endometrial cancer, the fourth most common cancer among women in high-income countries, affects more than 380 000 women worldwide each year [1], including 63 000 in the United States [2], and age-standardized incidence rates are increasing. A major risk factor is exposure to estrogen in the absence of a progestogen [3]; the main source of estrogen in post-menopausal women is adipose tissue, where aromatase converts androgens to estrogens. Estimates suggest one in three endometrial cancers are attributable to overweight and obesity [4].
While regular use of aspirin reduces risk of colorectal and possibly other cancers [5], data for endometrial cancer are less clear. Meta-analyses suggest an inverse association that is stronger among obese women [6–9], but they are susceptible to publication bias and the included studies varied in their categorization of medication use and adjustment for confounders. They were also unable to separate standard from low-dose aspirin, yet individual studies have reported weaker associations for low-dose aspirin [6, 10]. It is plausible that anti-inflammatory medications might be more protective among obese women because obesity is associated with chronic low-grade inflammation [11]. Furthermore, aromatase-mediated conversion of androgens in fat cells is the primary source of estrogen in post-menopausal women and nonsteroidal anti-inflammatory drugs (NSAIDs) have been shown to down-regulate aromatase activity in cell lines [12]. By suppressing inflammation and aromatase, NSAIDs may mitigate some of the excess endometrial cancer risk associated with obesity.
A recent review called for studies pooling data from multiple sources to clarify the relation between aspirin and endometrial cancer [13]. To this end, we pooled individual-level data from 12 studies in the Epidemiology of Endometrial Cancer Consortium (E2C2) to evaluate associations between analgesic use and endometrial cancer risk. Our a priori hypothesis was that use of aspirin (standard-dose) and other NSAIDs, but not low-dose aspirin or acetaminophen, would be associated with reduced risk, particularly among obese women.
Methods
We included five case–control and seven cohort studies that provided data regarding use of aspirin, non-aspirin (NA-) NSAIDs and/or acetaminophen (supplementary Table S1, available at Annals of Oncology online). All studies were approved by the relevant institutional review board(s) and participants provided informed consent.
The E2C2 data harmonization process has been described [14]. In brief, cohort studies are analyzed as nested case–control studies with up to four controls per case, matched on year-of-birth, cohort entry date and other study-specific criteria as appropriate, randomly selected from cohort members who had not had a hysterectomy or endometrial cancer by the case diagnosis date. Studies provided information on demographic, anthropometric, reproductive, medical and lifestyle factors (e.g. height, weight [see supplementary Table S2, available at Annals of Oncology online], parity, oral contraceptive (OC) and menopausal hormone therapy (MHT) use, diabetes, smoking) according to specified definitions. We excluded cases (and their matched controls) with non-epithelial tumors or tumors of unknown histology (196 cases/754 controls) and women missing data for aspirin, NA-NSAIDs and acetaminophen (814 cases/4977 controls, including controls individually-matched to cases without data). With the exception of the Breast Cancer Detection Demonstration Project (BCDDP) where women reported past and current medication use, cases (and matched controls) diagnosed before collection of medication data in the cohort studies were also excluded (344 cases/502 controls). The final study group comprised 7120 cases and 16 069 controls.
Supplementary Table S1, available at Annals of Oncology online shows the questions used to ascertain medication use in each study. The Australian National Endometrial Cancer Study (ANECS) asked about use in the five years before enrolment while the other case–control studies asked about ever use. For the Iowa Women’s Health Study (IOWA), Multiethnic Cohort Study (MEC), NIH-AARP Diet and Health Study (NIH) and Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO), data were collected at baseline and not updated during follow-up. For the Black Women’s Health Study (BWHS), data were updated from the most recent questionnaire before a participant became a case/was selected as a control. Data for the Swedish Women's Lifestyle and Health Study (SWLHS) came from the national pharmacy prescription database. ‘Regular’ medication use was defined as use at least once/week, but this definition differed slightly depending on the questions used to ascertain medication use, for example, BWHS only asked women to report use of at least 3 days/week. In studies with information about frequency of use, we further classified women as using the medications less than once/week, once/week, 2–6 times/week or daily. These cut points were selected for pragmatic reasons based on categories used in the original studies and in order to look separately at women who reported daily aspirin use as this was considered more likely to be low dose.
Statistical analyses
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) and Stata version 13 (StataCorp LP, College Station, TX, USA). For the overall models, pooled odds ratios (pORs) were calculated using a two-stage method. First, study-specific ORs and 95% confidence intervals (CI) were estimated for the associations between regular use of medications (yes/no) and risk of endometrial cancer using multivariable logistic regression (conditional regression for the matched studies). Models were adjusted for age (continuous), parity, body mass index (BMI) (kg/m2, continuous) and OC use (ever/never; further adjustment for OC duration in studies with this information made little difference), highest level of education (high-school/college/university) and smoking (never/former/current). See supplementary Methods, available at Annals of Oncology online, for further details regarding models and handling of missing data. Study-specific estimates were pooled using random-effects models and heterogeneity was assessed using I2 and Q statistics.
To address our primary hypothesis that any inverse association with medication use would be more pronounced among obese women, we stratified by BMI (normal <25, overweight 25–29.9, obese ≥30 kg/m2). We also assessed whether associations differed by study design, race, parity, OC use and, among post-menopausal women, use of MHT, or between type 1 and type 2 cancers (see supplementary Methods, available at Annals of Oncology online). For stratified analyses, we used generalized mixed regression models allowing the exposure effect to vary across studies [15]. Models were constructed to allow for the individual-level case–control matching in cohort studies with each unmatched case–control study treated as a single set (this gave identical estimates to standard unconditional models for these studies).
To estimate the potential impact of changing aspirin use if observed associations were causal, we used the age-standardized incidence rate for endometrial cancer in the United States [16], the BMI distribution in the USA female population (33% normal weight, 27% overweight, 40% obese) [17], and relative risks for overweight and obesity in the study population (overweight = 1.5; obese = 3.5) to estimate incidence rates by BMI. We then used the relative risks for overweight and obese women who used aspirin (versus all normal weight women, assuming no aspirin effect in this group) to estimate the potential reduction in incidence and thus the proportion and number of cancers potentially preventable if all overweight/obese women took aspirin at least once a week.
Results
The proportion of controls classified as regular users of aspirin ranged from 9% to 43% across the studies, NA-NSAIDs from 9% to 36% and acetaminophen from 15% to 36% (supplementary Table S1, available at Annals of Oncology online). The prevalence was lowest in Estrogen, Diet and Genetics of Endometrial Cancer Study (EDGE) where women were asked to report medications used continuously for at least 6 months and, for aspirin, the Swedish study, which only recorded prescription medications.
Aspirin
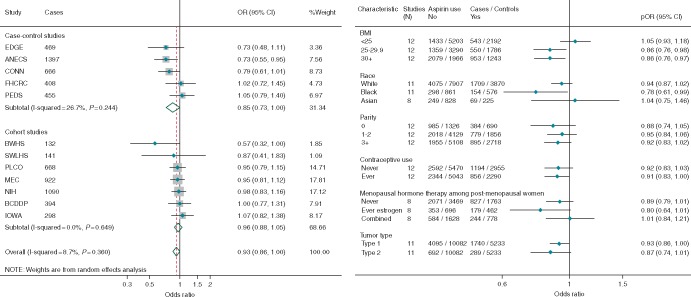
Overall, there was a borderline significant inverse association between regular use of aspirin and endometrial cancer risk (Figure 1A; pOR = 0.93, 95% CI 0.86–1.00). There was no significant heterogeneity between the studies, but the inverse association was stronger for case–control (pOR = 0.85, 95% CI 0.72–1.01) than for cohort studies (pOR = 0.96, 95% CI 0.88–1.05). Figure 1B shows no association among women of normal weight, but regular use of aspirin was associated with a 14% risk reduction among both overweight (OR = 0.86, 95% CI 0.76–0.98) and obese (OR = 0.86, 95% CI 0.76–0.97) women (Pheterogenity = 0.04). This pattern was also seen when we excluded studies that had previously published results stratified by BMI (OR [95% CI] for BMI < 25 and ≥25: 1.09 [0.93–1.26] and 0.87 [0.78–0.97]) and when we stratified by study design (case–control studies 0.95 [0.76–1.19] and 0.81 [0.68–0.95]; cohort studies 1.10 [0.95–1.26] and 0.89 [0.81–0.99]. Figure 1B also shows that the association was strongest for black women, likely because of a higher prevalence of overweight/obesity (77% among controls), than for white (51%) and Asian (29%) women. The association did not differ significantly by parity, OC or MHT use, or between type 1 and type 2 cancers.
Figure 1.
Forest plots showing adjusted estimates and 95% confidence intervals (CIs) for the association between regular use of aspirin and risk of endometrial cancer (A) overall, by study design with estimates ordered from smallest to largest and (B) stratified by participant characteristics and tumor type. The size of the box indicates the weight of the study, the line represents the 95% CI and the diamonds represent the pooled estimates. OR, odds ratio; EDGE, Estrogen, Diet, Genetics and Endometrial Cancer Study; ANECS, The Australian National Endometrial Cancer Study; FHCRC, Fred Hutchinson Cancer Research Center Study; CONN, Connecticut Endometrial Cancer Study; PEDS, Patient Epidemiologic Data System; BWHS, Black Women’s Health Study; NIH, NIH AARP Diet and Health Study; IOWA, Iowa Women’s Health Study; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; MEC, Multiethnic Cohort Study; SWLHS, Swedish Women's Lifestyle and Health Study; BCDDP, Breast Cancer Detection Demonstration Project.
Table 1 shows that in studies with information about frequency of use, there was no association between endometrial cancer and use of aspirin once/week (OR = 0.98, 95% CI 0.80–1.20) or daily (OR = 0.96, 95% CI 0.86–1.07), and only a suggestive inverse association with use two to six times/week (OR = 0.89, 95% CI 0.78–1.02). However, among overweight and obese women there was a significant 19% reduction in risk of endometrial cancer for use 2–6 times/week and a non-significant 9% reduction for daily use. Only two studies [ANECS and Fred Hutchinson Cancer Research Center Study (FHCRC)] provided data regarding aspirin dose; both showed an inverse association with use of standard aspirin two or more times/week and, combined, the estimates were 0.62 (0.47–0.82) for standard and 1.14 (0.82–1.58) for low-dose aspirin.
Table 1.
Odds ratios (OR) and 95% confidence intervals (CIs) for the association between frequency of aspirin and nonsteroidal anti-inflammatory drugs (NSAID) use and endometrial cancer risk, overall and by body mass index (BMI)
| Aspirina |
Non-aspirin NSAIDsa |
|||||
|---|---|---|---|---|---|---|
| Frequency of use | Cases | Controls | Cases | Controls | ||
| N (%) | N (%) | ORb (95% CI) | N (%) | N (%) | ORb (95% CI) | |
| Overall | ||||||
| <1/week | 3472 (70) | 6130 (65) | 1.00 (Ref) | 3443 (76) | 6878 (77) | 1.00 (Ref) |
| 1/week | 179 (4) | 428 (4) | 0.98 (0.80–1.20) | 124 (3) | 319 (3) | 0.80 (0.62–1.02) |
| 2–6/week | 450 (9) | 1114 (12) | 0.89 (0.78–1.02) | 446 (10) | 771 (9) | 0.93 (0.81–1.08) |
| Daily | 877 (17) | 1790 (19) | 0.96 (0.86–1.07) | 492 (11) | 989 (11) | 0.94 (0.82–1.08) |
| BMI < 25.0 kg/m2 | ||||||
| <1/week | 919 (71) | 2925 (68) | 1.00 (Ref) | 954 (81) | 3277 (80) | 1.00 (Ref) |
| 1/week | 52 (4) | 196 (5) | 1.04 (0.74–1.46) | 28 (2) | 159 (4) | 0.65 (0.42–1.01) |
| 2–6/week | 128 (10) | 495 (11) | 1.05 (0.84–1.32) | 90 (8) | 284 (7) | 1.10 (0.84–1.44) |
| Daily | 199 (15) | 710 (16) | 1.09 (0.90–1.31) | 100 (9) | 359 (9) | 1.19 (0.93–1.53) |
| BMI ≥ 25.0 kg/m2 | ||||||
| <1/week | 2466 (69) | 3108 (62) | 1.00 (Ref) | 2421 (75) | 3493 (74) | 1.00 (Ref) |
| 1/week | 126 (3) | 224 (4) | 0.93 (0.72–1.20) | 92 (3) | 153 (3) | 0.88 (0.65–1.19) |
| 2–6/week | 312 (9) | 600 (12) | 0.81 (0.68–0.96) | 342 (11) | 477 (10) | 0.87 (0.73–1.04) |
| Daily | 672 (19) | 1066 (21) | 0.91 (0.80–1.03) | 384 (12) | 621 (13) | 0.85 (0.73–1.00) |
| P-trend 0.02 | ||||||
Includes the Australian National Endometrial Cancer Study (ANECS), Connecticut Endometrial Cancer Study (CONN), FHCRC, PEDS (aspirin only), Iowa Women’s Health Study (IOWA), NIH and Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO).
Adjusted for age at diagnosis/interview (continuous), parity (continuous), BMI (kg/m2, continuous) and oral contraceptive use (ever/never), highest level of education and smoking.
Compared with all normal-weight women (assuming no association with aspirin use in this group), obese women who did not use aspirin were 3.6 times as likely to develop endometrial cancer (pOR = 3.63, 95% CI 3.32–3.96), but this was reduced to 3.2 times for obese women who used aspirin (pOR = 3.20, 95% CI 2.88–3.57). For overweight women the risks were 1.54 (1.41–1.68) for non-users versus 1.35 (1.20–1.51) for users of aspirin. If the association between aspirin use and endometrial cancer is causal, and all overweight and obese women took aspirin at least once a week, we estimate that this could translate to a reduction in incidence of up to 7.5% equivalent to 4600 fewer cases/year in the United States.
Non-aspirin NSAIDs
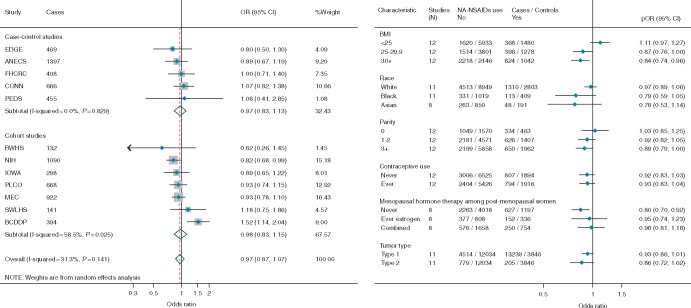
There was no overall association between regular use of non-aspirin NSAIDs and risk of endometrial cancer and little difference between case–control and cohort studies, although the results from cohort studies were very heterogeneous (Figure 2A). However, similar to aspirin, NA-NSAID use was associated with a 13% reduction in risk among overweight women and a statistically significant 16% reduction in risk among obese women (Figure 2B, Pheterogenity = 0.003). The association did not differ significantly by race, parity, OC or MHT use or for type 1 and type 2 cancers.
Figure 2.
Forest plots showing adjusted estimates and 95% confidence intervals (CIs) for the association between regular use of non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) and risk of endometrial cancer (A) overall, by study design with estimates ordered from smallest to largest and (B) stratified by participant characteristics and tumor type. The size of the box indicates the weight of the study, the line represents the 95% CI and the diamonds represent the pooled estimates. BMI, body mass index; pOR, pooled odds ratios; EDGE, Estrogen, Diet, Genetics and Endometrial Cancer Study; ANECS, The Australian National Endometrial Cancer Study; FHCRC, Fred Hutchinson Cancer Research Center Study; CONN, Connecticut Endometrial Cancer Study; PEDS, Patient Epidemiologic Data System; BWHS, Black Women’s Health Study; NIH, NIH AARP Diet and Health Study; IOWA, Iowa Women’s Health Study; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; MEC, Multiethnic Cohort Study; SWLHS, Swedish Women's Lifestyle and Health Study; BCDDP, Breast Cancer Detection Demonstration Project.
Table 1 shows that in studies with information about frequency of use, there was no trend with increasing frequency of use overall or among women with BMI <25, but a suggestive trend toward lower risk with increasing frequency among women with BMI of 25 kg/m2 or higher (Ptrend = 0.02).
Acetaminophen
There was no association between regular use of acetaminophen and endometrial cancer risk in the seven studies with information available (supplementary Figure S1A, available at Annals of Oncology online). Stratification by BMI suggested an inverse association among overweight women (OR = 0.79, 95% CI 0.64–0.96) but no association among normal weight (1.10, 95% CI 0.91–1.33) or obese women (1.04, 95% CI 0.86–1.24) (supplementary Figure S1B, available at Annals of Oncology online). Estimates did not differ appreciably by the other variables considered. Too few studies had information about frequency of use to assess this.
Discussion
Our a priori hypothesis was that use of standard-dose aspirin and other NSAIDs would be associated with a reduced risk of endometrial cancer, particularly among obese women, but that there would be no association with low-dose aspirin or acetaminophen. Overall, our results largely support this hypothesis. Use of aspirin 2–6 times/week was associated with significantly reduced risk of endometrial cancer among overweight/obese women, but not among normal-weight women. Furthermore, the association with daily aspirin use, which likely includes most low-dose use [18], was weaker and in the two studies with dose information, the inverse association was restricted to standard-dose formulations. We also saw reductions in risk for regular use of non-aspirin NSAIDs among overweight/obese women, but no clear pattern with acetaminophen use. The results did not differ significantly between type 1 and type 2 cancers, although the associations with type 2 cancers were slightly stronger. Although the potential risk reduction with aspirin is modest (10%–20%), if this association is causal and all overweight/obese women used standard-dose aspirin at least once a week, this could translate into up to 4600 fewer endometrial cancers per year in the United States.
Our results for aspirin are consistent with two meta-analyses (including seven studies in the current analysis) which reported modest inverse associations between regular aspirin use and endometrial cancer among obese women although they could not distinguish between standard and low-dose use preparations [7, 9]. One meta-analysis also reported a non-significant risk reduction for NSAIDs but did not consider whether this might vary by BMI [7]. Limited randomized trial evidence is also consistent with a modest beneficial effect of standard-dose aspirin. A pooled analysis of data from trials of aspirin to prevent vascular events, reported no uterine cancers among women randomized to aspirin (versus 9 in the placebo group, P = 0.003) [19]. Similarly, in a trial of aspirin among patients with Lynch syndrome, only five endometrial cancers were diagnosed among 427 women randomized to 600 mg aspirin/day (versus 13 among 434 in the placebo group) [20]. This study also reported that aspirin reduced the adverse effects of obesity on colorectal cancer risk [21]. The weaker association with daily (presumed to be largely low-dose) aspirin use in our analysis is consistent with the Women’s Health Study, which did not show any reduction in endometrial cancer risk among those randomized to 100 mg aspirin every second day [22]. Seven studies (ANECS, FHCRC, Patient Epidemiologic Data System, MEC and three others [23–25]) have previously reported no clear evidence for an association between acetaminophen use and endometrial cancer.
Strengths of our analysis include the large sample size, inclusion of published and unpublished data, and greater ability to standardize exposure levels and adjust consistently for confounders. Although previous meta-analyses reported inverse associations between aspirin use and endometrial cancer among obese women [6, 7, 9], these may be subject to publication bias if studies that saw no association had not published their data. Our analysis includes five studies that had not previously published data evaluating aspirin use in relation to endometrial cancer (Connecticut Endometrial Cancer Study, BCDDP, BWHS, PLCO, SWLHS) and we included 40%–50% more cases for two previously published studies (MEC and NIH). Although only two studies provided information about aspirin dose, we were able to assess this indirectly by looking separately at daily users who are most likely to use low-dose preparations.
Limitations of our study include the self-reported nature of the data for all studies except SWLHS (which used linkage to prescriptions data but could not capture over-the-counter use), and the possibilities of bias in individual studies. Also, despite the large sample, numbers were still limited for some sub-group analyses. Overall, the associations we observed with aspirin use were stronger among the case–control studies than the cohort studies although this difference disappeared when we stratified by BMI. Although case–control studies might overestimate the strength of association because of selection or recall bias, changing medication use over time in cohort studies would lead to misclassification which could attenuate associations. A systematic comparison of studies evaluating aspirin and cancer incidence concluded that results from case–control studies were highly correlated with those from randomized trials; in contrast, estimates from cohort studies were weaker if aspirin use was not updated during follow-up [26]. The fact that several cohort studies in this analysis did not update medication use after baseline (IOWA, MEC, NIH, PLCO), the difference between normal-weight and overweight/obese women was seen in both case–control and cohort studies, and no association was seen for acetaminophen, suggests that our results are not an artefact due to bias in the case-control studies.
An inverse association between use of anti-inflammatory medications and endometrial cancer risk among overweight/obese women is biologically plausible [27]. Several risk factors for endometrial cancer, including obesity [11], are associated with systemic chronic low-grade inflammation. Prospective studies have reported higher endometrial cancer risks among women with higher concentrations of inflammatory markers [28–30] with one suggesting the risk was greatest for women who were both obese and had high levels of inflammatory markers [30]. Both aspirin and NSAIDs inhibit cyclooxygenase (COX), leading to a reduction in prostaglandin levels and, in breast cancer cell lines, COX inhibitors also down-regulate aromatase activity [12]. Cross-sectional studies suggest post-menopausal women who regularly use NSAIDs have lower estradiol levels than nonusers [31, 32]. In vitro studies suggest aspirin and NSAIDs also have antiproliferative and antineoplastic effects that are independent of COX inhibition [33, 34] and can inhibit the proliferation of endometrial cancer cells [35, 36].
In conclusion, our analysis provides further evidence that use of standard-dose aspirin or other NSAIDs might reduce the risk of endometrial cancer among overweight and obese women. Future studies should clarify the relationship with low-dose aspirin and should include regularly updated measures of medication use (dose, frequency), ideally in a well-powered randomized trial to minimize bias and confounding. If confirmed, clinicians could consider aspirin or NSAIDs as an option to reduce the greatly increased risk of endometrial cancer among obese women who have an intact uterus.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the contributions of all study participants and staff. Data on endometrial cancer pathology for BWHS were obtained from several state cancer registries (AZ, CA, CO, CT, DE, DC, FL, GA, IL, IN, KY, LA, MD, MA, MI, NJ, NY, NC, OK, PA, SC, TN, TX, VA) and results reported do not necessarily represent their views.
Funding
This work was supported by the National Health and Medical Research Council of Australia (APP339435, APP1073898, APP1061341, APP1061779 to ANECS); Cancer Council Tasmania (403031, 457636, to ANECS); the Intramural Research Programs of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, United States (BCDDP and PLCO); the National Cancer Institute/National Institutes of Health (R01-CA058420, UMI-CA164974, R03-CA169888 to BWHS; R35 CA39779, R01 CA47749, R01 CA75977, N01 HD 2 3166, K05 CA92002, R01 CA105212, R01 CA87538 to FHCRC); the National Institutes of Health (R01CA098346 to CONN; R01 CA38918, P30 CA0008748 to EDGE; R01 CA39742 to IOWA; U01CA54281 to MEC); and the Swedish Research Council (521-2011-2955 to SWLHS).
Disclosure
The authors have declared no conflicts of interest.
References
- 1. Ferlay J, Soerjomataram I, Ervik M. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer 2013; http://globocan.iarc.fr (31 December 2018, date last accessed).
- 2. Noone A, Howlader N, Krapcho M. et al. SEER Cancer Statistics Review, 1975–2015. Bethesda, MD: National Cancer Institute; https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018.
- 3. Weiderpass E, Adami HO, Baron JA. et al. Risk of endometrial cancer following estrogen replacement with and without progestins. J Natl Cancer Inst 1999; 91(13): 1131–1137. [DOI] [PubMed] [Google Scholar]
- 4. Whiteman DC, Webb P, Green AC. et al. Cancers in Australia in 2010 attributable to modifiable factors: summary and conclusions. Aust N Z J Public Health 2015; 39(5): 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao Y, Nishihara R, Wu K. et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol 2016; 2(6): 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neill AS, Nagle CM, Protani MM. et al. Aspirin, nonsteroidal anti-inflammatory drugs, paracetamol and risk of endometrial cancer: a case-control study, systematic review and meta-analysis. Int J Cancer 2013; 132(5): 1146–1155. [DOI] [PubMed] [Google Scholar]
- 7. Verdoodt F, Friis S, Dehlendorff C. et al. Non-steroidal anti-inflammatory drug use and risk of endometrial cancer: a systematic review and meta-analysis of observational studies. Gynecol Oncol 2016; 140(2): 352–358. [DOI] [PubMed] [Google Scholar]
- 8. Brasky TM, Cohn DE, Bernardo BM.. Aspirin and endometrial cancer risk. Gynecol Oncol Rep 2016; 17: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang D, Bai B, Xi Y, Zhao Y.. Can aspirin reduce the risk of endometrial cancer? A systematic review and meta-analysis of observational studies. Int J Gynecol Cancer 2016; 26(6): 1111–1120. [DOI] [PubMed] [Google Scholar]
- 10. Brasky TM, Moysich KB, Cohn DE, White E.. Non-steroidal anti-inflammatory drugs and endometrial cancer risk in the VITamins And Lifestyle (VITAL) cohort. Gynecol Oncol 2013; 128(1): 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gregor M, Hotamisligil G.. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011; 29: 415–445. [DOI] [PubMed] [Google Scholar]
- 12. Diaz-Cruz ES, Shapiro CL, Brueggemeier RW.. Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells. J Clin Endocrinol Metab 2005; 90: 2563–2570. [DOI] [PubMed] [Google Scholar]
- 13. Verdoodt F, Kjaer SK, Friis S.. Influence of aspirin and non-aspirin NSAID use on ovarian and endometrial cancer: summary of epidemiologic evidence of cancer risk and prognosis. Maturitas 2017; 100: 1–7. [DOI] [PubMed] [Google Scholar]
- 14. Setiawan VW, Yang HP, Pike MC. et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol 2013; 31: 2607–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stewart GB, Altman DG, Askie LM. et al. Statistical analysis of individual participant data meta-analyses: a comparison of methods and recommendations for practice. PLoS One 2012; 7(10): e46042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute SEER Program. Cancer Stat Facts: Uterine Cancer; https://seer.cancer.gov/statfacts/html/corp.html (11 December 2017, date last accessed).
- 17. Flegal KM, Kruszon-Moran D, Carroll MD. et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016; 315(21): 2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gu Q, Dillon CF, Eberhardt MS. et al. Preventive aspirin and other antiplatelet medication use among U.S. adults aged ≥40 years: data from the National Health and Nutrition Examination Survey, 2011-2012. Public Health Rep 2015; 130(6): 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rothwell PM, Price JF, Fowkes FG. et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet 2012; 379(9826): 1602–1612. [DOI] [PubMed] [Google Scholar]
- 20. Burn J, Gerdes AM, Macrae F. et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011; 378(9809): 2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Movahedi M, Bishop DT, Macrae F. et al. Obesity, aspirin, and risk of colorectal cancer in carriers of hereditary colorectal cancer: a prospective investigation in the CAPP2 Study. J Clin Oncol 2015; 33(31): 3591–3597. [DOI] [PubMed] [Google Scholar]
- 22. Cook NR, Lee IM, Zhang SM. et al. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med 2013; 159(2): 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Friis S, Nielsen G, Mellemkjaer L. et al. Cancer risk in persons receiving prescriptions for paracetamol: a Danish cohort study. Int J Cancer 2002; 97(1): 96–101. [DOI] [PubMed] [Google Scholar]
- 24. Viswanathan AN, Feskanich D, Schernhammer ES, Hankinson SE.. Aspirin, NSAID, and acetaminophen use and the risk of endometrial cancer. Cancer Res 2008; 68(7): 2507–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walter RB, Brasky TM, White E.. Cancer risk associated with long-term use of acetaminophen in the prospective VITamins and lifestyle (VITAL) study. Cancer Epidemiol Biomarkers Prev 2011; 20(12): 2637–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Algra AM, Rothwell PM.. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol 2012; 13(5): 518–527. [DOI] [PubMed] [Google Scholar]
- 27. Modugno F, Ness RB, Chen C, Weiss NS.. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol Biomarkers Prev 2005; 14(12): 2840–2847. [DOI] [PubMed] [Google Scholar]
- 28. Dossus L, Becker S, Rinaldi S. et al. Tumor necrosis factor (TNF)-alpha, soluble TNF receptors and endometrial cancer risk: the EPIC study. Int J Cancer 2011; 129(8): 2032–2037. [DOI] [PubMed] [Google Scholar]
- 29. Dossus L, Lukanova A, Rinaldi S. et al. Hormonal, metabolic, and inflammatory profiles and endometrial cancer risk within the EPIC cohort—a factor analysis. Am J Epidemiol 2013; 177(8): 787–799. [DOI] [PubMed] [Google Scholar]
- 30. Trabert B, Eldridge RC, Pfeiffer RM. et al. Prediagnostic circulating inflammation markers and endometrial cancer risk in the prostate, lung, colorectal and ovarian cancer (PLCO) screening trial. Int J Cancer 2017; 140(3): 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hudson AG, Gierach GL, Modugno F. et al. Nonsteroidal anti-inflammatory drug use and serum total estradiol in postmenopausal women. Cancer Epidemiol Biomarkers Prev 2008; 17(3): 680–687. [DOI] [PubMed] [Google Scholar]
- 32. Gates MA, Tworoger SS, Eliassen AH. et al. Analgesic use and sex steroid hormone concentrations in postmenopausal women. Cancer Epidemiol Biomarkers Prev 2010; 19(4): 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang X, Morham SG, Langenbach R, Young DA.. Malignant transformation and antineoplastic actions of nonsteroidal antiinflammatory drugs (NSAIDs) on cyclooxygenase-null embryo fibroblasts. J Exp Med 1999; 190(4): 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Langley RE, Burdett S, Tierney JF. et al. Aspirin and cancer: has aspirin been overlooked as an adjuvant therapy? Br J Cancer 2011; 105(8): 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arango HA, Icely S, Roberts WS. et al. Aspirin effects on endometrial cancer cell growth. Obstet Gynecol 2001; 97(3): 423–427. [DOI] [PubMed] [Google Scholar]
- 36. Gao J, Niwa K, Sun W. et al. Non-steroidal anti-inflammatory drugs inhibit cellular proliferation and upregulate cyclooxygenase-2 protein expression in endometrial cancer cells. Cancer Sci 2004; 95(11): 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.