Abstract
Background
Nivolumab is approved as an option for third- or later-line treatment of advanced gastric/gastroesophageal junction (G/GEJ) cancer in several countries after ATTRACTION-2. To further improve the therapeutic efficacy of first-line therapy, exploration of a nivolumab-chemotherapy combination is warranted. In part 1 (phase II) of ATTRACTION-4, the safety and efficacy of nivolumab combined with S-1 plus oxaliplatin (SOX) or capecitabine plus oxaliplatin (CapeOX) as first-line therapy for unresectable advanced or recurrent human epidermal growth factor receptor 2 (HER2)-negative G/GEJ cancer were evaluated.
Patients and methods
Patients were randomized (1 : 1) to receive nivolumab (360 mg intravenously every 3 weeks) plus SOX (S-1, 40 mg/m2 orally twice daily for 14 days followed by 7 days off; oxaliplatin, 130 mg/m2 intravenously on day 1 every 3 weeks) or CapeOX (capecitabine, 1000 mg/m2 orally twice daily for 14 days followed by 7 days off; oxaliplatin, 130 mg/m2 intravenously on day 1 every 3 weeks) until disease progression, unacceptable toxicity, or consent withdrawal.
Results
Of 40 randomized patients, 39 (nivolumab plus SOX, 21; nivolumab plus CapeOX, 18) and 38 (21 and 17, respectively) comprised the safety and efficacy populations, respectively. Most frequent (>10%) grade 3/4 treatment-related adverse events were neutropenia (14.3%) in the nivolumab plus SOX group, and neutropenia (16.7%), anemia, peripheral sensory neuropathy, decreased appetite, type 1 diabetes mellitus, and nausea (11.1% each) in the nivolumab plus CapeOX group. No treatment-related death occurred. Objective response rate was 57.1% (95% confidence interval 34.0–78.2) with nivolumab plus SOX and 76.5% (50.1–93.2) with nivolumab plus CapeOX. Median overall survival was not reached (NR) in both groups. Median progression-free survival was 9.7 months (5.8–NR) and 10.6 months (5.6–12.5), respectively.
Conclusion
Nivolumab combined with SOX/CapeOX was well tolerated and demonstrated encouraging efficacy for unresectable advanced or recurrent HER2-negative G/GEJ cancer. ATTRACTION-4 has proceeded to part 2 (phase III) to compare nivolumab plus SOX/CapeOX versus placebo plus SOX/CapeOX.
Clinicaltrials.gov ID
Keywords: nivolumab, gastric/gastroesophageal cancer, capecitabine, S-1, oxaliplatin, programmed death-1
Key Message
In the randomized, phase II (part 1) ATTRACTION-4 trial, nivolumab combined with chemotherapy [S-1 plus oxaliplatin (SOX) or capecitabine plus oxaliplatin (CapeOX)] was evaluated in patients with chemonaïve, unresectable advanced or recurrent gastric/gastroesophageal junction cancer. Nivolumab plus SOX/CapeOX was well tolerated and demonstrated encouraging efficacy in this population.
Introduction
Gastric/gastroesophageal junction (G/GEJ) cancer is the fifth most common cancer and the third leading cause of cancer deaths worldwide. In 2012, almost 1 000 000 new cases and 723 000 deaths were estimated to have occurred [1]. Incidence and mortality rates of gastric cancer are highest in Eastern Asia. Half of the total cases in the world occur here; the age-standardized incidence rate per 100 000 in men (35.4) is more than twice that in women (13.8). Furthermore, a mortality rate of ∼24 per 100 000 in men and 9.8 per 100 000 in women is reported here [1].
The standard of care for first-line treatment of unresectable advanced or metastatic G/GEJ cancer is fluoropyrimidine- and platinum-based therapy [trastuzumab is added for human epidermal growth factor receptor 2 (HER2)-positive patients] [2, 3]. Oral fluoropyrimidines (e.g. capecitabine or S-1) and oxaliplatin are replacing infusions of 5-fluorouracil and cisplatin, respectively, because of noninferior efficacy, convenience, and better tolerance [4–7]. In Asia, the standard of care for unresectable or metastatic G/GEJ cancer currently includes a doublet regimen of S-1 or capecitabine plus cisplatin or oxaliplatin [8–10]. In the West, three-drug combination regimens including docetaxel or epirubicin with the fluoropyrimidine-platinum combination have become options after the V325 and REAL-2 studies [7, 11].
Although several clinical trials have investigated the efficacy of molecular agents for G/GEJ cancer, only trastuzumab and ramucirumab achieved favorable survival times. For HER2-positive advanced G/GEJ cancer, first-line trastuzumab plus fluoropyrimidine- and platinum-based therapy can achieve an overall survival (OS) of up to 13.8 months [12]. Ramucirumab monotherapy and combined with paclitaxel showed survival benefits as second-line chemotherapy over best supportive care and paclitaxel alone, respectively [13, 14]. Despite these advances, median survival time for patients with this disease stage is poor (∼6–14 months) [5, 15]. In fact, all global phase III studies with molecular agents (including the RAINFALL study [16] that investigated ramucirumab in first-line therapy), except those mentioned earlier, provided negative results. There is clearly an unmet need for any potential novel agent that will improve survival in these patients, especially in first-line treatment.
Immuno-oncology agents targeting programmed death-1 (PD-1) and PD-ligand 1 (PD-L1) have shown promising activity in several malignant diseases. Tumors expressing PD-L1 bind to PD-1, an immunoinhibitory receptor expressed on T cells, and inhibit T-cell-mediated immune responses [17]. PD-L1 was detected in ∼12%–65% of gastric cancer tissues; importantly, the prognosis was poorer in patients with PD-L1 expression in tumors than in those without [15, 18]. Nivolumab, a fully human IgG4 monoclonal antibody targeting PD-1, has shown activity and improved survival as monotherapy for several advanced tumor types [19–22] or when combined with other immunotherapy for melanoma [23]. In ATTRACTION-2, a double-blind, placebo-controlled, randomized phase III study in patients with unresectable advanced or recurrent G/GEJ cancer refractory to or intolerant of ≥2 prior chemotherapy regimens, nivolumab monotherapy resulted in a significantly longer OS versus placebo [5.3 versus 4.1 months; hazard ratio (HR) 0.63; 95% confidence interval (CI) 0.51–0.78; P < 0.0001]. Furthermore, nivolumab increased the 12-month OS rate (26.2% versus 10.9%), progression-free survival (PFS) rate (7.6% versus 1.5%), and objective response rate (ORR) (11.2% versus 0.0%) versus placebo [15]. In the phase I/II CheckMate 032 study in patients with chemotherapy-refractory G/GEJ/esophageal cancer, nivolumab monotherapy resulted in an ORR of 12%, median OS of 6.2 months, and 12-month OS rate of 39%. Clinical activity was observed irrespective of tumor PD-L1 expression [24].
In addition to the well-known effects of chemotherapy against tumor replication, it has been suggested that antitumor effects of chemotherapy may occur through modulation of the immune system [25, 26]. It is reported that oxaliplatin can induce immunologic death of cancer cells and thereby enhance the efficacy of immuno-oncology agents. This phenomenon, coupled with encouraging clinical activity and safety with nivolumab-chemotherapy combination as first-line therapy for advanced non-small-cell lung cancer [27], provides a strong rationale for ATTRACTION-4, a two-part study designed to evaluate nivolumab-chemotherapy combination. Part 1 aimed to explore the safety and efficacy of nivolumab with chemotherapy, whereas part 2, a double-blind study, will compare nivolumab plus chemotherapy versus placebo plus chemotherapy in terms of OS and PFS. Here, we report the results for the safety and efficacy of nivolumab plus chemotherapy as first-line therapy in unresectable advanced or recurrent G/GEJ cancer in part 1 of ATTRACTION-4.
Patients and methods
Study design
ATTRACTION-4 is a randomized, phase II/III, two-part study. Part 1 was conducted at 13 centers in Japan and South Korea from March 2016 (data cut-off date 31 July 2017). Part 2 is currently ongoing at 138 sites in Japan, South Korea, and Taiwan. In part 1 (NCT02746796), an open-label study, patients were randomized 1 : 1 using an interactive web response system to receive nivolumab with S-1 (tegafur–gimeracil–oteracil potassium) plus oxaliplatin (SOX) or nivolumab with capecitabine plus oxaliplatin (CapeOX) (supplementary Figure S1, available at Annals of Oncology online). Treatment was continued until disease progression, unacceptable toxicity, or consent withdrawal. All patients were examined at discontinuation of the protocol treatment and 28 days post-treatment, and were followed up. Criteria for starting part 2 are provided as supplementary material, available at Annals of Oncology online. The study was approved by the institutional review boards at all sites and conformed to the Declaration of Helsinki guidelines. All patients provided written informed consent.
Patients
Briefly, patients with unresectable advanced or recurrent HER2-negative G/GEJ cancer, Eastern Cooperative Oncology Group performance status of 0 or 1, and no prior chemotherapy except neoadjuvant or adjuvant chemotherapy completed ≥180 days before randomization were included. Additional details are provided as supplementary material, available at Annals of Oncology online.
Treatment
Patients received nivolumab (360 mg intravenously once in 3 weeks) plus SOX (S-1, 40 mg/m2 orally twice daily for 14 days followed by 7 days off; oxaliplatin, 130 mg/m2 intravenously on day 1 every 3 weeks) or CapeOX (capecitabine, 1000 mg/m2 orally twice daily for 14 days followed by 7 days off; oxaliplatin, 130 mg/m2 intravenously on day 1 every 3 weeks) (supplementary Figure S1, available at Annals of Oncology online). Additional details are provided as supplementary material, available at Annals of Oncology online.
End points and assessments
Primary end point of part 1
Safety was assessed by recording adverse events (AEs), which were coded using the Medical Dictionary for Regulatory Activities version 20.1 and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 [28].
Secondary end points of part 1
These included ORR, OS, PFS, duration of response (DOR), best overall response (BOR), disease control rate (DCR), time to response (TTR), and change in tumor burden (percent change in the sum of diameters of target lesions) over time assessed by the site investigator and centrally by the Independent Review Committee according to Response Evaluation Criteria In Solid Tumors, version 1.1 [29]. For patients with available tumor samples, PD-L1 tumor expression was determined by immunohistochemistry carried out at a central laboratory (28-8 pharmDx assay; Dako, Carpinteria, CA). PD-L1 positivity was defined as staining in ≥1% of tumor cells. Additional details are provided as supplementary material, available at Annals of Oncology online.
Statistical analysis
In part 1, for the safety evaluation alone, based on the previously reported incidences of grade ≥3 AEs with SOX/CapeOX, the incidence of each grade ≥3 AE was assumed to be 10%. A sample size of 15 patients per cohort was required to detect a grade ≥3 AE in ≥1 patient with approximately 80% power. The intent-to-treat (ITT) population consisted of all randomized patients, the safety analysis set (SAS) consisted of patients given ≥1 dose of nivolumab/chemotherapy, and the full analysis set (FAS) consisted of patients from the SAS who had multiple cancers measurable lesions using computed tomography/magnetic resonance imaging within 14 days before randomization.
Results
Demographics and baseline characteristics
Of 49 screened patients, 40 were randomized to receive nivolumab plus SOX (n = 21) and nivolumab plus CapeOX (n = 19) [median age (range) 62.5 (37–80) years; male 67.5%]. One patient did not receive the protocol treatment (SAS, n = 39) and one patient in the nivolumab plus CapeOX group received nivolumab for another study (FAS, n = 38) (supplementary Figure S2, available at Annals of Oncology online). Demographics and baseline characteristics were comparable between the groups (Table 1). From the SAS, 14/21 patients (66.7%) in the nivolumab plus SOX group and 15/18 patients (83.3%) in the nivolumab plus CapeOX group discontinued nivolumab treatment. Median (range) duration of treatment was 6.8 (0–15) months with median (range) follow-up time of 13.2 (12.2–15.2) months.
Table 1.
Demographics and baseline characteristics (ITT population)
| Total | Nivolumab plus SOX | Nivolumab plus CapeOX | |
|---|---|---|---|
| N = 40 | n = 21 | n = 19 | |
| Median age (min–max), years | 62.5 (37–80) | 61.0 (37–77) | 65.0 (39–80) |
| Males, n (%) | 27 (67.5) | 12 (57.1) | 15 (78.9) |
| BMI, mean (SD), kg/m² | 21.9 (4.11) | 21.5 (4.21) | 22.3 (4.07) |
| Country, n (%) | |||
| Japan | 20 (50.0) | 10 (47.6) | 10 (52.6) |
| South Korea | 20 (50.0) | 11 (52.4) | 9 (47.4) |
| ECOG PS, n (%) | |||
| 0 | 20 (50.0) | 10 (47.6) | 10 (52.6) |
| 1 | 20 (50.0) | 11 (52.4) | 9 (47.4) |
| G/GEJ cancer, n (%) | |||
| Advanced | 24 (60.0) | 15 (71.4) | 9 (47.4) |
| Recurrent | 16 (40.0) | 6 (28.6) | 10 (52.6) |
| Prior surgery, n (%) | 17 (42.5) | 7 (33.3) | 10 (52.6) |
| Organs with metastases (≥2), n (%) | 29 (72.5) | 15 (71.4) | 14 (73.7) |
| Tumor PD-L1 quantifiable, n (%) | 37 (92.5) | 19 (90.5) | 18 (94.7) |
| <1% expression status | 31 (83.8) | 15 (78.9) | 16 (88.9) |
| ≥1% expression status | 6 (16.2) | 4 (21.1) | 2 (11.1) |
BMI, body mass index; CapeOX, capecitabine plus oxaliplatin; ECOG PS, Eastern Cooperative Oncology Group performance status; G/GEJ, gastric/gastroesophageal junction; ITT, intent-to-treat; PD-L1, programmed death-ligand 1; SD, standard deviation; SOX, S-1 (tegafur–gimeracil–oteracil potassium) plus oxaliplatin.
Safety
Mean (standard deviation [SD]) relative dose intensity of nivolumab was comparable between groups [nivolumab plus SOX, 90.7% (9.8); nivolumab plus CapeOX, 91.9% (7.2)]. All patients in the SAS in both arms experienced AEs and treatment-related AEs (TRAEs) (grade ≥3 TRAEs, 24 [61.5%]) (Table 2). Grade 3/4 TRAEs occurring in >10% of patients were neutropenia (14.3%) in the nivolumab plus SOX group, and neutropenia (16.7%), anemia, peripheral sensory neuropathy, decreased appetite, type 1 diabetes mellitus, and nausea (11.1% each) in the nivolumab plus CapeOX group. Four serious TRAEs (diarrhea, lung infection, prostatitis, and intracranial hemorrhage) occurred in four (19.0%) patients in the nivolumab plus SOX group and nine serious TRAEs (decreased appetite, type 1 diabetes mellitus, diarrhea, colitis, lung abscess, infusion-related reaction, and adrenocorticotropic hormone deficiency) occurred in six (33.3%) patients in the nivolumab plus CapeOX group. Four TRAEs (increased alanine aminotransferase, increased aspartate aminotransferase, peripheral sensory neuropathy, and intracranial hemorrhage) in three (14.3%) patients in the nivolumab plus SOX group and two TRAEs (peripheral sensory neuropathy and adrenocorticotropic hormone deficiency) in two (11.1%) patients in the nivolumab plus CapeOX group led to discontinuation of the protocol treatment. Almost all patients [nivolumab plus SOX, 20 (95.2%); nivolumab plus CapeOX, 17 (94.4%)] had TRAEs leading to reduced or delayed dosing of chemotherapy; the most frequent (>10%) ones included thrombocytopenia (57.1%), neutropenia (47.6%), nausea (19.0%), diarrhea, vomiting, abdominal pain, peripheral sensory neuropathy, and fatigue (14.3% each) in the nivolumab plus SOX group, and neutropenia (44.4%), decreased appetite (27.8%), palmar-plantar erythrodysesthesia syndrome (22.2%), nausea, diarrhea, vomiting, peripheral sensory neuropathy (16.7% each), and peripheral neuropathy and type 1 diabetes mellitus (11.1% each) in the nivolumab plus CapeOX group. No treatment-related deaths occurred.
Table 2.
Adverse events (SAS population)
| Total | Nivolumab plus SOX | Nivolumab plus CapeOX | ||||
|---|---|---|---|---|---|---|
|
N = 39 |
n = 21 |
n = 18 |
||||
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Any TRAE | 39 (100.0) | 24 (61.5) | 21 (100.0) | 12 (57.1) | 18 (100.0) | 12 (66.7) |
| Treatment-related SAEs | 10 (25.6) | 6 (15.4) | 4 (19.0) | 3 (14.3) | 6 (33.3) | 3 (16.7) |
| TRAEs leading to discontinuationa | 5 (12.8) | 2 (5.1) | 3 (14.3) | 1 (4.8) | 2 (11.1) | 1 (5.6) |
| TRAEs leading to dose delay or reduction | 37 (94.9) | 18 (46.2) | 20 (95.2) | 10 (47.6) | 17 (94.4) | 8 (44.4) |
| TRAEs (≥20%) | ||||||
| Neutropeniab | 25 (64.1) | 6 (15.4) | 13 (61.9) | 3 (14.3) | 12 (66.7) | 3 (16.7) |
| Peripheral sensory neuropathy | 24 (61.5) | 3 (7.7) | 12 (57.1) | 1 (4.8) | 12 (66.7) | 2 (11.1) |
| Decreased appetite | 23 (59.0) | 2 (5.1) | 12 (57.1) | 0 | 11 (61.1) | 2 (11.1) |
| Diarrhea | 22 (56.4) | 3 (7.7) | 14 (66.7) | 2 (9.5) | 8 (44.4) | 1 (5.6) |
| Nausea | 20 (51.3) | 2 (5.1) | 11 (52.4) | 0 | 9 (50.0) | 2 (11.1) |
| Thrombocytopeniac | 18 (46.2) | 1 (2.6) | 14 (66.7) | 0 | 4 (22.2) | 1 (5.6) |
| Fatigue | 13 (33.3) | 1 (2.6) | 7 (33.3) | 0 | 6 (33.3) | 1 (5.6) |
| Vomiting | 11 (28.2) | 0 | 5 (23.8) | 0 | 6 (33.3) | 0 |
| Constipation | 10 (25.6) | 0 | 5 (23.8) | 0 | 5 (27.8) | 0 |
| Abdominal pain | 8 (20.5) | 3 (7.7) | 4 (19.0) | 2 (9.5) | 4 (22.2) | 1 (5.6) |
| Dysgeusia | 8 (20.5) | 0 | 3 (14.3) | 0 | 5 (27.8) | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 8 (20.5) | 0 | 0 | 0 | 8 (44.4) | 0 |
| Peripheral neuropathy | 8 (20.5) | 1 (2.6) | 6 (28.6) | 1 (4.8) | 2 (11.1) | 0 |
| Pyrexia | 8 (20.5) | 0 | 4 (19.0) | 0 | 4 (22.2) | 0 |
| Peripheral edema | 7 (17.9) | 0 | 6 (28.6) | 0 | 1 (5.6) | 0 |
| Stomatitis | 7 (17.9) | 0 | 3 (14.3) | 0 | 4 (22.2) | 0 |
| Anemia | 6 (15.4) | 2 (5.1) | 2 (9.5) | 0 | 4 (22.2) | 2 (11.1) |
| Decreased white blood cell count | 6 (15.4) | 0 | 2 (9.5) | 0 | 4 (22.2) | 0 |
All values presented as n (%).
Discontinuation may be caused due to one or more product (nivolumab/oxaliplatin/S-1/capecitabine).
Includes the MedDRA preferred term ‘decreased neutrophil count’.
Includes the MedDRA preferred term ‘decreased platelet count’.
AE, adverse event; CapeOX, capecitabine plus oxaliplatin; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event; SAS, safety analysis set; SOX, S-1 (tegafur–gimeracil–oteracil potassium) plus oxaliplatin; TRAE, treatment-related adverse event.
Efficacy
Objective response rate
The ORRs by site investigator assessment were comparable in both groups [nivolumab plus SOX, 14/21 (66.7%, 95% CI 43.0–85.4); nivolumab plus CapeOX, 12/17 (70.6%, 44.0–89.7)] (Table 3); ORRs were 12/21 (57.1%, 34.0–78.2) and 13/17 (76.5%, 50.1–93.2), respectively, when assessed centrally. In patients with PD-L1-positive tumors (nivolumab plus SOX, 4; nivolumab plus CapeOX, 1), ORR was 2/4 (50.0%) and 1/1 (100.0%), respectively, whereas in patients with PD-L1-negative tumors, ORR was 10/17 (58.8%) and 12/16 (75.0%), respectively (central assessment).
Table 3.
Summary of response and survival data (FAS population)
| Total | Nivolumab plus SOX | Nivolumab plus CapeOX | |
|---|---|---|---|
| N = 38 | n = 21 | n = 17 | |
| OS, median (95% CI)a, months | NR (13.9, NR) | NR (11.9, NR) | NR (11.2, NR) |
| 6-month rate (95% CI)b | 94.6 (80.1, 98.6) | 100.0 (100.0, 100.0) | 88.2 (60.6, 96.9) |
| Site investigator assessment | |||
| ORR, n (%) (95% CI)c | 26 (68.4) (51.3, 82.5) | 14 (66.7) (43.0, 85.4) | 12 (70.6) (44.0, 89.7) |
| BOR, n (%) | |||
| CR, n (%) (95% CI)c | 1 (2.6) (0.1, 13.8) | 1 (4.8) (0.1, 23.8) | 0 (0.0) (0.0, 19.5) |
| PR, n (%) (95% CI)c | 25 (65.8) (48.6, 80.4) | 13 (61.9) (38.4, 81.9) | 12 (70.6) (44.0, 89.7) |
| SD, n (%) (95% CI)c | 7 (18.4) (7.7, 34.3) | 4 (19.0) (5.4, 41.9) | 3 (17.6) (3.8, 43.4) |
| PD | 4 (10.5) | 2 (9.5) | 2 (11.8) |
| Not evaluable | 1 (2.6) | 1 (4.8) | 0 |
| PFS, median (95% CI)a, months | 9.5 (6.9, 11.1) | 9.8 (6.8, NR) | 7.2 (4.3, 11.2) |
| 6-month rate (95% CI)b | 75.0 (57.4, 86.2) | 79.3 (53.7, 91.7) | 69.7 (41.7, 86.1) |
| DCR, n (%) (95% CI)c | 33 (86.8) (71.9, 95.6) | 18 (85.7) (63.7, 97.0) | 15 (88.2) (63.6, 98.5) |
| Central assessment | |||
| ORR, n (%) (95% CI)c | 25 (65.8) (48.6, 80.4) | 12 (57.1) (34.0, 78.2) | 13 (76.5) (50.1, 93.2) |
| BOR, n (%) | |||
| CR, n (%) (95% CI)c | 10 (26.3) (13.4, 43.1) | 7 (33.3) (14.6, 57.0) | 3 (17.6) (3.8, 43.4) |
| PR, n (%) (95% CI)c | 15 (39.5) (24.0, 56.6) | 5 (23.8) (8.2, 47.2) | 10 (58.8) (32.9, 81.6) |
| SD, n (%) (95% CI)c | 7 (18.4) (7.7, 34.3) | 5 (23.8) (8.2, 47.2) | 2 (11.8) (1.5, 36.4) |
| PD | 2 (5.3) | 1 (4.8) | 1 (5.9) |
| Not evaluable | 4 (10.5) | 3 (14.3) | 1 (5.9) |
| PFS, median (95% CI)a, months | 9.7 (6.8, 12.5) | 9.7 (5.8, NR) | 10.6 (5.6, 12.5) |
| 6-month rate (95% CI)b | 70.9 (52.5, 83.2) | 72.9 (46.4, 87.8) | 68.6 (40.0, 85.7) |
| DCR, n (%) (95% CI)c | 32 (84.2) (68.7, 94.0) | 17 (81.0) (58.1, 94.6) | 15 (88.2) (63.6, 98.5) |
| TTR, median (min–max), months | 1.3 (1.2–6.2) | 1.3 (1.2–3.0) | 1.3 (1.2–6.2) |
| DOR, median (95% CI)a, months | 9.9 (5.8, NR) | 9.9 (3.9, NR) | 9.7 (4.4, NR) |
Estimated using the Kaplan–Meier method and 95% CI of median was calculated using the Brookmeyer and Crowley method.
Estimated using the Kaplan–Meier method and 95% CI was estimated using the Greenwood formula for variance and double logarithmic transformation.
95% CI was calculated using the Clopper–Pearson method.
BOR, best overall response; CapeOX, capecitabine plus oxaliplatin; CI, confidence interval; CR, complete response; DCR, disease control rate; DOR, duration of response; FAS, full analysis set; NR, not reached; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; SOX, S-1 (tegafur–gimeracil–oteracil potassium) plus oxaliplatin; TTR, time to response.
Disease control rate
DCR was 18/21 (85.7%, 63.7–97.0) with nivolumab plus SOX and 15/17 (88.2%, 63.6–98.5) with nivolumab plus CapeOX by site investigator assessment and 17/21 (81.0%, 58.1–94.6) and 15/17 (88.2%, 63.6–98.5), respectively, by central assessment.
Time to response
Median TTR (min–max) (central assessment) was 1.3 months (1.2–3.0) with nivolumab plus SOX and 1.3 months (1.2–6.2) with nivolumab plus CapeOX.
Duration of response
Median DOR (central assessment) was 9.86 months [3.91–not reached (NR)] with nivolumab plus SOX and 9.69 months (4.37–NR) with nivolumab plus CapeOX.
Overall survival
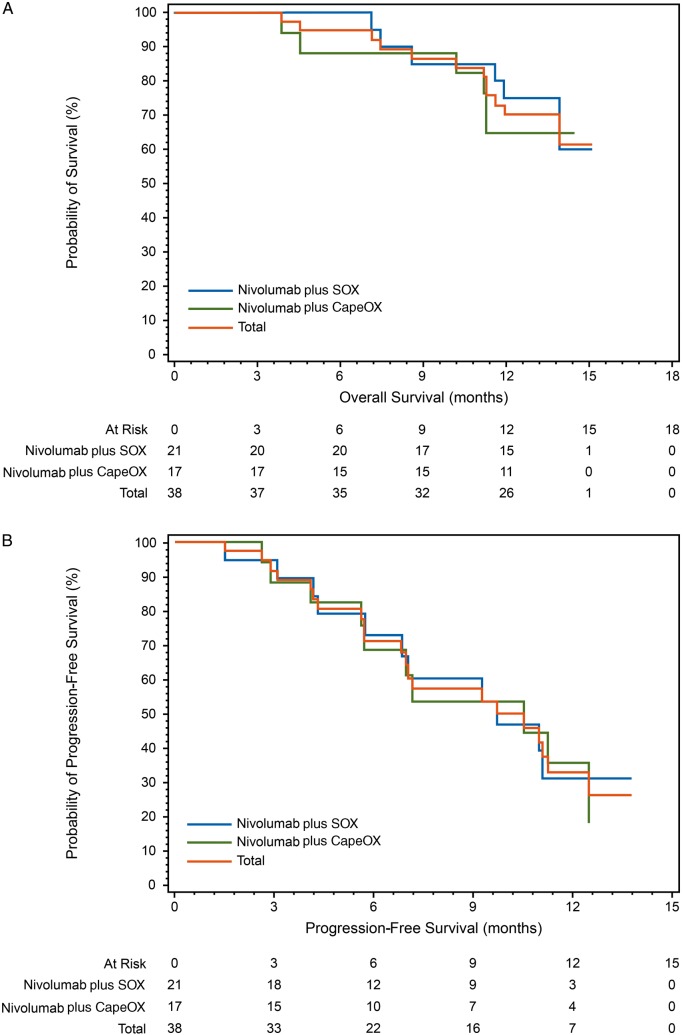
Median OS was NR (13.9 months–NR) for the total population, NR (11.9 months–NR) for the nivolumab plus SOX group, and NR (11.2 months–NR) for the nivolumab plus CapeOX group (Figure 1A).
Figure 1.
Kaplan–Meier curves for (A) overall survival and (B) progression-free survival (central assessment) (FAS population) CapeOX, capecitabine plus oxaliplatin; FAS, full analysis set; SOX, S-1 (tegafur–gimeracil–oteracil potassium) plus oxaliplatin.
Progression-free survival
Median PFS for the overall population was 9.7 months (6.8–12.5) and 9.5 months (6.9–11.1) by central and investigator assessment, respectively. Median PFS was 9.8 months (6.8–NR) for nivolumab plus SOX and 7.2 months (4.3–11.2) for nivolumab plus CapeOX by site investigator assessment and 9.7 months (5.8–NR) and 10.6 months (5.6–12.5), respectively, by central assessment (Figure 1B).
Change in tumor burden over time
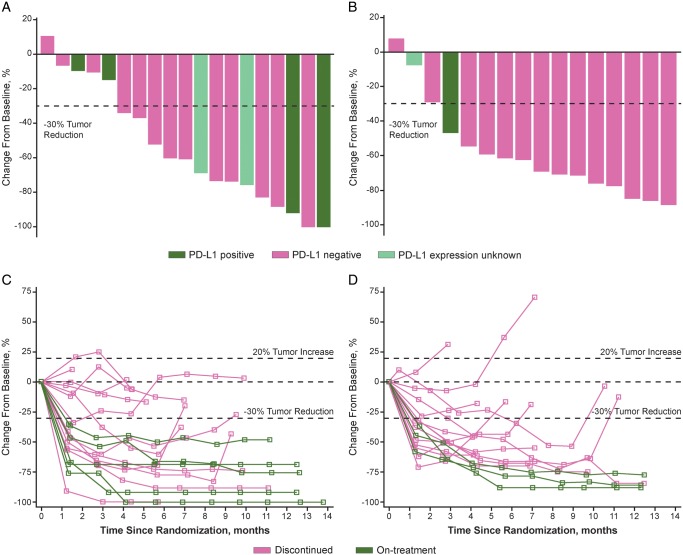
All patients except one each in both groups showed a tumor burden reduction as the best response (central assessment) (Figure 2A and B). Change in size of target lesion from baseline over time is shown in Figure 2C and D.
Figure 2.
(A) Best change from baseline in the sum of longest target lesion diameters in each patient receiving nivolumab plus SOX. (B) Best change from baseline in the sum of longest target lesion diameters in each patient receiving nivolumab plus CapeOX. (C) Percent change in sum of longest diameters of target lesion from baseline in each patient receiving nivolumab plus SOX. (D) Percent change in sum of longest diameters of target lesion from baseline in each patient receiving nivolumab plus CapeOX (central assessment) (FAS population). CapeOX, capecitabine plus oxaliplatin; FAS, full analysis set; PD-L1, programmed death-ligand 1; SOX, S-1 (tegafur–gimeracil–oteracil potassium) plus oxaliplatin.
Discussion
Part 1 of the ATTRACTION-4 study demonstrated that nivolumab plus chemotherapy has a manageable safety profile and clinically relevant antitumor activity.
In this study, the incidences and types of TRAEs were consistent with those known to be associated with chemotherapy and nivolumab [15, 19, 21–23]. While peripheral sensory neuropathy, decreased neutrophil count, and decreased platelet count were some of the most frequently reported TRAEs in both groups (Table 2), these are expected AEs associated with oxaliplatin and/or fluoropyrimidines [30–32] and were mostly grade 1/2 AEs. Discontinuations due to TRAEs were reported in five patients. No treatment-related deaths occurred. Immune-related toxicities were also comparable to those reported with other immunotherapies in similar patient populations [33].
An objective response (complete response or partial response) was observed in approximately two-thirds of patients regardless of the chemotherapy administered with nivolumab; this is numerically higher than that reported previously for SOX or CapeOX in patients with G/GEJ cancer [5, 7, 34]. Overall, DCR in both groups was comparable with that previously reported for SOX or CapeOX [34]. Similar to previous studies with immunotherapies, early and durable responses were achieved (Table 3) [15, 33]. Notably, antitumor response with nivolumab was independent of tumor PD-L1 status; this was in line with previous studies of nivolumab in G/GEJ cancer [15]. Responses were coupled with a clinically relevant PFS that was numerically longer compared with previous studies with SOX and CapeOX [5]. While median OS was not reached within a median follow-up time of 13.2 months, it appears that it would be longer than 15 months. Long durable survival benefit beyond median OS of immune checkpoint inhibitors has been observed in other cancer types. Survival benefits of adding nivolumab to standard doublet chemotherapy for first-line treatment of advanced G/GEJ cancer should be confirmed in part 2 of ATTRACTION-4.
Because no substantial differences in the safety and efficacy were observed between the two groups, it is considered that nivolumab could be combined with either chemotherapy.
Although these results from part 1 of ATTRACTION-4 present novel and clinically relevant findings, the patient population is relatively small and this part of the study lacks a standard of care comparator arm. Part 2 of ATTRACTION-4, being conducted in a larger population, will play an important role in validating these results.
In conclusion, these results suggest that nivolumab in combination with SOX or CapeOX in patients with untreated unresectable advanced or recurrent G/GEJ cancer may be a potential therapeutic option with a manageable safety profile and encouraging efficacy that warrants evaluation in a phase III study.
Supplementary Material
Acknowledgements
We would like to thank the patients and their families, as well as the investigators and participating study teams, for making this study possible. We thank Shunsuke Hagihara for providing statistical support and the project leader, Mitsunobu Tanimoto (Ono Pharmaceutical). Writing and editorial assistance was provided by Ruhi Ubale, PhD, of Cactus Communications, and was funded by Ono Pharmaceutical Co., Ltd., Osaka, Japan, and Bristol-Myers Squibb Inc., Princeton, NJ.
Funding
This study was funded by Ono Pharmaceutical Co., Ltd., Osaka, Japan, and Bristol-Myers Squibb Inc., Princeton, NJ (no grant number is applicable).
Disclosure
NB received honoraria from Ono Pharmaceutical, Bristol-Myers Squibb, AstraZeneca, and Chugai Pharmaceutical. MHR received research grants from Ono Pharmaceutical; honoraria from DAE HWA Pharmaceutical, Lilly Korea, Bristol-Myers Squibb, and Ono Pharmaceutical; consulting fees from Lilly Korea; and served on the advisory board for Bristol-Myers Squibb and Ono Pharmaceutical. KK received research grants from Ono Pharmaceutical, MSD, Shionogi, and Merck Serono. HCC received research grants from Ono Pharmaceutical, Eli Lilly, GSK, MSD, Merck Serono, Bristol-Myers Squibb, Ono Pharmaceutical, and Taiho; honoraria from Merck Serono, Eli Lilly, and Foundation Medicine/Roche; and consulting fees from Taiho, Celltrion, MSD, Eli Lilly, Quintiles, Bristol-Myers Squibb, and Merck Serono. KM, KWL, HC, WKK, MT, SF, and MA received research grants from Ono Pharmaceutical. YK received research grants and speaker fees from Ono Pharmaceutical, MSD, Eli Lilly, Merck, AstraZeneca, Daiichi Sankyo, Taiho, Chugai Pharmaceutical, NCC, Kyowa Hakko Kirin, Takeda, Sanofi, Yakult Pharmaceuticals, Bristol-Myers Squibb, Boehringer Ingelheim, Bayer, Pfizer, and Novartis, and research grants from Linical and TCOG. KY received research grants from Ono Pharmaceutical and served on the speakers bureau for Ono Pharmaceutical and Bristol-Myers Squibb. HH received research grants from Ono Pharmaceutical, AstraZeneca, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eli Lilly, Merck Serono, MSD, Taiho, Chugai, Boehringer Ingelheim, Eisai, LSK BioPharma, Incyte, and Pfizer. LTC received research grants from Ono Pharmaceutical, Ministry of Science and Technology (Taiwan), Ministry of Health and Welfare (Taiwan), Novartis, Pfizer, GSK, Merck Serono, TTY Biopharm, OBI Pharma, Polaris Pharma, SynCore Biotechnology, and Celgene, and honoraria from Novartis, Eli Lilly, TTY Biopharm, PharmaEngine, Shire, MSD, Bristol-Myers Squibb, Ono Pharmaceutical, SynCore Biotechnology, Five Prime Therapeutics, and Merrimack. YKK received research grants from DAE HWA Pharmaceutical and consulting fees from Ono Pharmaceutical, Bristol-Myers Squibb, DAE HWA Pharmaceutical, and Blueprint Medicines.
References
- 1. Ferlay J, Soerjomataram I, Ervik M. et al. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012 v1.0. IARC CancerBase No. 11. International Agency for Research on Cancer; http://globocan.iarc.fr (16 November 2018, date last accessed).
- 2. Ajani JA, D'Amico TA, Almhanna K. et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14(10): 1286–1312. [DOI] [PubMed] [Google Scholar]
- 3. Smyth EC, Verheij M, Allum W. et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27(Suppl 5): v38–v49. [DOI] [PubMed] [Google Scholar]
- 4. Okines AF, Norman AR, McCloud P. et al. Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol 2009; 20(9): 1529–1534. [DOI] [PubMed] [Google Scholar]
- 5. Yamada Y, Higuchi K, Nishikawa K. et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol 2015; 26(1): 141–148. [DOI] [PubMed] [Google Scholar]
- 6. Kang YK, Kang WK, Shin DB. et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 2009; 20(4): 666–673. [DOI] [PubMed] [Google Scholar]
- 7. Cunningham D, Starling N, Rao S. et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008; 358(1): 36–46. [DOI] [PubMed] [Google Scholar]
- 8. Irino T, Takeuchi H, Terashima M. et al. Gastric cancer in Asia: unique features and management. Am Soc Clin Oncol Educ Book 2017; 37: 279–291. [DOI] [PubMed] [Google Scholar]
- 9. Kuo YC, Liu HT, Lin YL. et al. Modified biweekly oxaliplatin and capecitabine for advanced gastric cancer: a retrospective analysis from a medical center. Biomed J 2014; 37: 141–146. [DOI] [PubMed] [Google Scholar]
- 10. Shen L, Shan YS, Hu HM. et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol 2013; 14(12): e535–e547. [DOI] [PubMed] [Google Scholar]
- 11. Van Cutsem E, Moiseyenko VM, Tjulandin S. et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006; 24(31): 4991–4997. [DOI] [PubMed] [Google Scholar]
- 12. Bang YJ, Van Cutsem E, Feyereislova A. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376(9742): 687–697. [DOI] [PubMed] [Google Scholar]
- 13. Fuchs CS, Tomasek J, Yong CJ. et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014; 383(9911): 31–39. [DOI] [PubMed] [Google Scholar]
- 14. Wilke H, Muro K, Van Cutsem E. et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014; 15(11): 1224–1235. [DOI] [PubMed] [Google Scholar]
- 15. Kang YK, Boku N, Satoh T. et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390(10111): 2461–2471. [DOI] [PubMed] [Google Scholar]
- 16. Fuchs CS, Shitara K, Di Bartolomeo M. et al. RAINFALL: a randomized, double-blind, placebo-controlled phase III study of cisplatin (Cis) plus capecitabine (Cape) or 5FU with or without ramucirumab (RAM) as first-line therapy in patients with metastatic gastric or gastroesophageal junction (G-GEJ) adenocarcinoma. J Clin Oncol 2018; 36(Suppl 4): 5. [Google Scholar]
- 17. Zou W, Wolchok JD, Chen L.. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 2016; 8(328): 328rv4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geng Y, Wang H, Lu C. et al. Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3+ Tregs in gastric cancer and its clinical significance. Int J Clin Oncol 2015; 20(2): 273–281. [DOI] [PubMed] [Google Scholar]
- 19. Borghaei H, Paz-Ares L, Horn L. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373(17): 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brahmer J, Reckamp KL, Baas P. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373(2): 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferris RL, Blumenschein G Jr, Fayette J. et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016; 375(19): 1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Motzer RJ, Escudier B, McDermott DF. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373(19): 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larkin J, Chiarion-Sileni V, Gonzalez R. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373(1): 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janjigian YY, Bendell J, Calvo E. et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol 2018; 36(28): 2836–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hato SV, Khong A, de Vries IJ, Lesterhuis WJ.. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Can Res 2014; 20(11): 2831–2837. [DOI] [PubMed] [Google Scholar]
- 26. Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G.. Immunological aspects of cancer chemotherapy. Nat Rev Immunol 2008; 8(1): 59–73. [DOI] [PubMed] [Google Scholar]
- 27. Rizvi NA, Hellmann MD, Brahmer JR. et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016; 34(25): 2969–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NIH NCI Division of Cancer Treatment and Diagnosis. Common Terminology Criteria for Adverse Events (CTCAE) Version 5, 2017; https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (16 November 2018, date last accessed).
- 29. Eisenhauer EA, Therasse P, Bogaerts J. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45(2): 228–247. [DOI] [PubMed] [Google Scholar]
- 30.Teysuno (S-1) Summary of Product Characteristics; http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001242/WC500104415.pdf (16 November 2018, date last accessed).
- 31.ELOXATIN (oxaliplatin) Prescribing Information; https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021759s012lbl.pdf (16 November 2018, date last accessed).
- 32.XELODA (capecitabine) Prescribing Information; https://www.gene.com/download/pdf/xeloda_prescribing.pdf (16 November 2018, date last accessed).
- 33. Fuchs CS, Doi T, Jang RW. et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018; 4(5): e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim GM, Jeung HC, Rha SY. et al. A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer 2012; 48(4): 518–526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.