Abstract
Background: Family-based weight loss treatment (FBT) for childhood obesity, the current “gold standard,” is typically provided in weekly groups for 6 months. Although this program is considered effective, it poses limitations to treatment engagement, due to time commitment and lack of widespread availability. A guided self-help version of FBT (gshFBT; eleven 20-minute sessions and one 1-hour over 5 months) was developed to circumvent such limitations. The current study examined the comparative efficacy of a 5-month FBT and gshFBT program.
Methods: Participants included 50 parent–child dyads enrolled in FBT between 2011 and 2013 and 50 parent–child dyads enrolled in gshFBT between 2009 and 2010. Data were collected at baseline, posttreatment, and 6-month follow-up. Noninferiority analyses were conducted to assess comparative efficacy of changes in parent and child weight status, child nutrition, child physical activity, and drop-out.
Results: Results indicated that gshFBT was noninferior to FBT in changes in child BMI z-score, overweight parent BMI, child nutritional intake, child vigorous physical activity, and drop-out. Results did not support noninferiority for changes in moderate to vigorous physical activity.
Conclusions: gshFBT is less intensive, more flexible, and may be similarly effective to FBT and could reach a greater proportion of the pediatric overweight population. Further research, including a randomized clinical trial, is needed to confirm these results.
Keywords: child, family-based treatment, guided self-help, obesity, parent
Introduction
Childhood obesity is a major public health concern in the United States with over 30% of children having overweight or obesity.1 Children with overweight or obesity are at an increased risk for many medical comorbidities,2–4 bullying by peers, lower self-esteem, decreased quality of life, and higher rates of body dissatisfaction.5–7 They also have significantly higher health care costs relative to children with healthy weight.8 These factors underscore the need for interventions to treat a greater majority of children with overweight and obesity.
The current “gold standard” treatment for childhood obesity is family-based weight loss treatment (FBT). FBT provides nutrition and physical activity advice, along with behavior therapy and parenting skills.9 Treatment includes weekly child and parent group sessions as well as individual behavioral coaching to target personalized family goals and barriers.10–12 Overall, families receive over 36 hours of treatment over 6 months. In addition, when treatment is provided in groups, the schedule is fixed (i.e., offered on one night a week), which prevents some families from participating. Although shown to be effective,13 FBT is time and cost intensive and not feasible for all families. When FBT was adapted for delivery in primary care, only 28% of the families who were offered the treatment enrolled, and only 63% of enrolled families completed the intervention.14 Research is needed to develop interventions for families for whom FBT is not feasible.
A guided self-help model of FBT (gshFBT) could provide the major components of FBT while being more appropriate for dissemination.15 In gshFBT, families are given manuals that cover the major topics provided in group-based FBT, and attend bimonthly 20-minute one-on-one meetings to track weight, assist in adherence to the program, and problem-solve barriers to treatment success. The gshFBT model can be provided by someone with little training, requires less frequent and intense visits than FBT, and resembles the Medicare coverage for adults with obesity (www.cms.gov). Initial data on this model showed that gshFBT, relative to a waitlist control group, resulted in significant decreases in child BMIz.15 A gsh treatment was also as effective as more intensive treatment for adolescents with overweight and obesity.16 Based on previous research showing that the increased number of contacts is associated with better outcomes in adult weight loss programs,17 the outcomes of FBT should exceed those in gshFBT (FBT, twenty 90-minute sessions over 6 months; gshFBT, twelve, 20-minute sessions over 5 months). Yet, if gshFBT produces similar weight losses to FBT, this could increase access to care and potentially allow earlier intervention in the disease process.
Little is known about the comparative outcomes of FBT and gshFBT for childhood obesity. Thus, we conducted a quasi-experimental study to get an initial indication of how these two treatments compare. This study examined whether gshFBT was noninferior to FBT on changes in child weight, nutrition, physical activity, parent weight status, and treatment completion rates.
Methods
Overall Study Design
Participants came from two independent studies conducted at the University of California San Diego (UCSD).15,18 The gshFBT study was conducted between 2009 and 2011 and the FBT study was conducted between 2011 and 2014. Outcome measures were assessed at baseline (month 0), posttreatment (month 5), and 6 months follow-up (month 11). Both studies were approved by the UCSD Institutional Review Board.
Participants
All participants were recruited through print advertisements, email advertisements, pediatrician referrals, and direct mailing. Inclusion criteria for both studies included the following: (1) participating parent who could read English ≥6th grade level, and (2) parent and child willing to commit to treatment and assessment attendance. Exclusion criteria included the following: (1) current psychiatric or eating disorder, (2) current serious physical illness, (3) medication regiment that may impact weight, (4) dietary restrictions, or (5) physical difficulties that would prevent participants from engaging in physical activity. Unique to the gshFBT study, enrolled children were limited on the upper end for weight status (BMI percentile ≤98th%) and parents were not required to be overweight to participate.
For the original gshFBT study,15 50 children (BMI 85th% to ≤98th%) and their parents were recruited and randomized to either intervention (gshFBT) or delayed treatment control. Children randomized to delayed treatment control participated in treatment following the completion of treatment for the immediate group. For the original FBT study,18 150 children (BMI percentile: 85th% to 99th%) and their overweight parents were recruited and randomized to a parent-only or a parent and child treatment.
Because this was a nonrandomized study, systematic matching procedures were used to reduce the number of variables that could influence outcomes. Children enrolled in the parent and child arm of the FBT study (n = 75) were reviewed and matched to the 50 gshFBT children. Considering the weight difference in recruitment, we first selected children in the FBT study who were 98th% BMI or lower (n = 55) before matching them to the gshFBT children by gender and age (see Table 1 for baseline demographics of the resulting samples included in this study).
Table 1.
Baseline Demographic Characteristics in gshFBT and FBT (Means and Standard Deviations)
| gshFBT (N = 50) | FBT (N = 50) | |
|---|---|---|
| Child | ||
| Gender | ||
| % Female | 64.0 | 66.0 |
| Age (mean) | 10.38 (1.32) | 9.46 (1.10)* |
| BMIz (mean) | 1.71 (0.28) | 1.84 (0.29) |
| Ethnicity, % | ||
| Caucasian | 56.0 | 54.0 |
| Latino | 12.0 | 12.0 |
| African American | 2.0 | 10.0 |
| Other | 30.0 | 24.0 |
| Parent | ||
| Gender | ||
| % Female | 80.0 | 88.0 |
| Age (mean) | 42.76 (5.61) | 43.10 (6.63) |
| BMI (mean) | 27.77 (6.12) | 31.78 (6.36)* |
| Ethnicity, % | ||
| Caucasian | 64.0 | 60.0 |
| Latino | 12.0 | 12.0 |
| African American | 4.0 | 8.0 |
| Other | 20.0 | 20.0 |
| Income, % | ||
| <$20,000 | 2.0 | 6.0 |
| $20,001–$40,000 | 12.0 | 8.0 |
| $40,001–$60,000 | 4.0 | 12.0 |
| >$60,000 | 72.0 | 72.0 |
| Not reported | 10.0 | 2.0 |
p < 0.01.
FBT, family-based weight loss treatment; gshFBT, guided self-help version of FBT.
Treatment Arms
Guided self-help version of FBT
gshFBT families attended eleven 20-minute sessions and one 1-hour session (session 2) to allow for time to discuss dietary recommendations over 5 months.15 Families received three manuals (i.e., parent, child, and activities) covering the major components of FBT. Individual gshFBT sessions assisted the family in understanding and implementing the topics and strategies outlined in their manuals. Perfect attendance in gshFBT equated to 4.67 hours of treatment. Mean treatment attendance for the gshFBT group was 4.19 ± 1.07 hours (two families did not complete the 1-hour session).
FBT
FBT families attended twenty 90-minute sessions over 6 months.18 Sessions included 60-minute child and parent separate group sessions, and a 30-minute parent and child individual session with a behavioral coach. The parent group sessions covered topics on nutrition, physical activity, behavior therapy skills (e.g., stimulus control), and parenting skills. The child group sessions provided the same information (without the parenting skills) in a developmentally appropriate manner. Perfect attendance in FBT equated to 28.5 hours of direct contact. Mean attendance for the FBT group was 20.25 ± 6.54 hours at the 5-month time point and 22.32 ± 7.37 hours at the 6-month time point (end of treatment).
Measures
Child measurements
Child BMI standardized
Child height and weight were obtained at baseline, posttreatment, and follow-up. Height and weight were taken in duplicate using a portable Schorr height board (Schorr, Inc., Olney, MD) and Tanita Digital Scale (model WB-110A). The average of the two values was used in analyses. BMI was calculated (kg/m2) and translated to BMIz score.19 In FBT, since the treatment was longer, child BMIz was calculated using weight at month 5.
Child nutrition
Child nutrition was measured at baseline and posttreatment using three 24-hour dietary recalls conducted by trained dietary assessors on three nonconsecutive days using a multiple pass method. This method of dietary assessment is valid for children.20–22 Recalls were scored using the Nutrition Data Systems for Research (NDS-R) nutrient calculation software (www.ncc.umn.edu/products/ndsr.html). Dietary information was analyzed for average daily caloric intake from baseline to mid-treatment, percent of the child's estimated energy requirements consumed each day,23 and mean daily caloric intake at posttreatment. A low activity level was used to calculate estimated energy requirements consumed each day.
Child physical activity
Child physical activity was assessed at baseline and posttreatment using ActiGraph Accelerometers (model GT1M, www.theactigraphy.com), which are small (3.8 × 3.7 × 1.8 cm), lightweight (27 g), uniaxial accelerometers worn on a belt around the waist. The ActiGraph technology is valid for quantifying activity levels in laboratory and field settings.24 Moderate to vigorous physical activity (MVPA) intensities were determined from the Freedson age adjusted equation.25,26 Activity categories were summed to calculate minutes of valid days (>10 hours). We report MVPA and vigorous physical activity as a percent of total ActiGraph wear-time to adjust for differences in the amount of time the children wore the accelerometers.
Parent measurements
Parent BMI
Parent height and weight were measured in the same manner of the child and translated to BMI.
Treatment completion
Treatment completion was defined as attending posttreatment and 6-month follow-up assessments.
Demographics
Demographics included parent and child age, parent and child gender, parent marital status, ethnicity, and income.
Statistical Analyses
Statistical analyses were performed using SPSS version 22.27 Descriptive analyses were calculated to examine demographic characteristics of the families (see Table 1). Increasingly complex unconditional means regression models were created to determine the most appropriate model to use for analysis. For each variable, the simplest model, which included a fixed effect, was chosen. Noninferiority was determined by examining effect sizes, 95% confidence intervals (CIs) for effect sizes, and means.28–30 These analyses were chosen to reduce type I error. Effect sizes were calculated for each treatment and for each time point (baseline to posttreatment and baseline to follow-up). Effect sizes, CIs, and predetermined bounds were compared between FBT and FBTgsh. Noninferiority was determined if FBTgsh fell within the lower bound of the 95% CI for FBT. Due to lack of published effect sizes of treatment on other outcome variables (physical activity, nutrition intake, self-monitoring, and parenting), means between FBT and FBTgsh were compared to determine if means fell within preset bounds.31 Preset bounds were set according to a prior noninferiority publication. Linear regressions were conducted for variables with significantly different baseline values. Analyses were considered significant at the p < 0.05 level (see Table 2 for means of outcome variables in both groups).
Table 2.
Outcomes across Time Points for gshFBT and FBT (Means and Standard Deviations)
| gshFBT | FBT | |||||
|---|---|---|---|---|---|---|
| Month 0 | Month 5 | Month 11 | Month 0 | Month 5 | Month 11 | |
| Child | ||||||
| Weight | ||||||
| BMIz | 1.71 (0.28) | 1.52 (0.3) | 1.51 (0.38) | 1.84 (0.29) | 1.61 (0.42) | 1.63 (0.43) |
| Nutrition (kcal) | 1572 (395) | 1502 (381) | — | 1669 (338) | 1449 (298) | — |
| Physical activity (% wear time) | ||||||
| MVPA | 11.73 (4.6) | 11.64 (4.7) | — | 20.36 (5.5) | 20.42 (5.7) | — |
| Vigorous | 1.37 (1.1) | 1.57 (1.2) | — | 0.82 (0.82) | 0.84 (0.96) | — |
| Parent | ||||||
| BMI | 27.7 (6.1) | 27.1 (6.1) | 27.7 (6.2) | 31.8 (6.4) | 30.3 (6.1) | 30.2 (5.9) |
| Drop out | 10% | 14% | ||||
MVPA, moderate to vigorous physical activity.
Missing Data
Missing data ranged from 3% to 20% of the values per variable. Higher frequency of missing values was a result of assessment drop-out and a “prefer not to answer” option in the surveys. Little's Missing Completely at Random (MCAR) Test confirmed that the data were missing at random, p = 0.458. Variables missing more than 5% of the data (N = 5) were corrected using maximum likelihood estimation as this is an inherent process in unconditional means regression models. Mean imputation was implemented for the Eating in the Absence of Hunger (EAH) posttreatment variables.
Results
Child Outcomes
Child BMIz
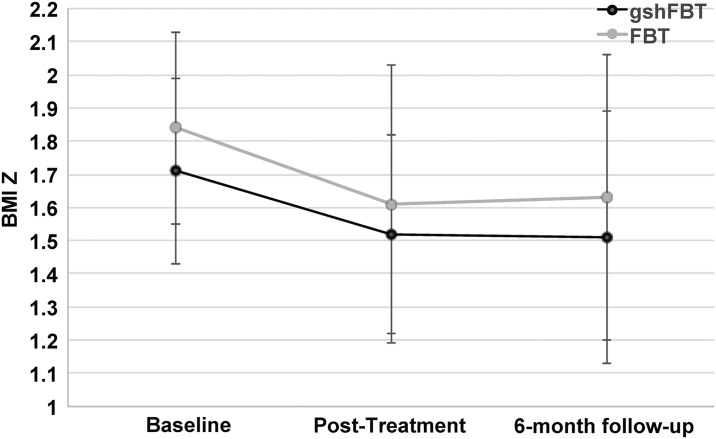
For child BMIz score, both unconditional means regression models for FBT and gshFBT were significant [F(1, 49.22) = 1170.52, p < 0.001; F(1, 50.35) = 1374.61, p < 0.001]. Treatment effect sizes on child BMIz score for FBT and gshFBT were moderate from baseline to posttreatment (FBT d = 0.65, 95% CI = 0.33–0.98; gshFBT d = 0.62, 95% CI = 0.31–0.93) and posttreatment to 6-month follow-up (FBT d = 0.57, 95% CI = 0.28–0.85; gshFBT d = 0.58, 95% CI = 0.29–0.88), and similar to previously published studies on FBT.9 A linear regression controlling for baseline child BMIz score was also conducted, since baseline child BMIz score was found to be significantly different between FBT and gshFBT (p = 0.02). Results of the regression indicated that treatment group did not significantly predict child BMIz score at posttreatment (b = 0.079, t = 1.546, p = 0.126) and 6-month follow-up (b = 0.009, t = 0.144, p = 0.886; see Fig. 1). Thus, noninferiority for overall treatment outcome was assumed.
Figure 1.
Child weight changes in gshFBT and FBT at baseline, posttreatment and 6-month follow-up.
Child nutrition
When comparing average daily caloric intake from baseline to posttreatment, the unconditional means models were significant for FBT [F(1, 47.56) = 1980.14, p < 0.001] and gshFBT [F(1, 49.89) = 1087.11, p < 0.001]. Moreover, despite differences in effect sizes between groups for average daily caloric intake from baseline to posttreatment (FBT: d = 0.69, 95% CI = 0.34–1.03, gshFBT: d = 0.18, 95% CI = 0.09–0.27) and percent of the child's estimated energy requirements consumed each day (FBT: d = 0.66, 95% CI = 0.33–0.99, gshFBT: d = 0.20, 95% CI = 0.10–0.30), posttreatment means for both treatments fell within the preset bound of 300 calories. Thus, gshFBT seemed to have a comparable effect on caloric intake relative to FBT. Thus, noninferiority was assumed.
Child physical activity
Unconditional means models were significant for FBT and gshFBT for MVPA [F(1, 48.43) = 1040.67, p < 0.000; F(1, 48.58) = 353.61, p < 0.001], and vigorous activity [F(1, 49.36) = 70.19, p < 0.001; F(1, 49.06) = 98.55, p < 0.001]. Due to significantly different baseline values, linear regression analyses were used to determine noninferiority for MVPA and vigorous activity. Outcomes were found to be significantly different for MVPA (b = −3.655, t = −2.650, p = 0.010). Yet, child vigorous physical activity was not significantly different between groups (b = 0.359, t = 1.372, p = 0.175). Thus, noninferiority was assumed. Although the researchers set initial bound of 90 minutes per physical activity category, data from each category were compared based on percentage of time spent in each category due to a large variability in total wear time. Thus, the initial bound could not be utilized.
Parent Outcomes
Parent BMI
Due to a significant difference between treatment groups on parent BMI at baseline, a linear regression was conducted. When examining whether treatment group could predict parent BMI, results showed a trend toward significance at posttreatment (b = 0.512, t = 1.754, p = 0.083) and were statistically significant at follow-up (b = 0.873, t = 1.99, p = 0.05). Since not all parents in gshFBT met criteria for overweight at baseline, we also compared the weight change of the overweight and obese parents in gshFBT (N = 25) to those in FBT (N = 50). Although these two groups were unequal in participant size, parent BMI means at baseline were no longer significantly different, and parent weight loss in FBT and gshFBT was comparable across time points. With only overweight parents included in the gshFBT sample, noninferiority, as determined by a bound of 1 BMI point, was assumed at posttreatment and follow-up time points.
Family Drop Out
The bound for noninferiority for treatment compliance was set at 6% or 3 participants. Treatment drop out was similar between FBT (N = 5) and gshFBT (N = 7).
Discussion
This study provided an initial comparison of two models for the treatment of childhood obesity; FBT and a less intensive program, gshFBT. Results showed that gshFBT was noninferior to FBT on child BMIz, parent BMI, child dietary intake, child vigorous physical activity, and treatment completion (10% dropout in gshFBT vs 14% in FBT). Noninferiority was not found for changes in MVPA.
In this study, gshFBT resulted in child weight loss that was noninferior to FBT over the 11 months of the study. Although weight maintenance is also seen as a success in child weight-loss treatments as children are still growing, both treatments resulted in significant weight loss. These results are similar to published outcomes comparing a gsh version of a lifestyle modification program (six 45-minute individual sessions) and a group lifestyle modification program (six 45-minute individual sessions + 17 group sessions) for adolescents with overweight or obesity.16 Despite initial differences on sample age and race/ethnicity, both studies suggest that gsh may be a promising model for treatment of youth with overweight and obesity. Relative to FBT, the gshFBT model is more efficient and simpler to implement, while maintaining the focus on parenting skills and key behavioral strategies necessary for lifestyle change. Furthermore, compared to FBT, gshFBT can more easily accommodate the family's busy schedule (i.e., does not require group attendance at a specific time), is less time intensive for the family, and requires less interventionist time (15 families in gshFBT require 65 hours of interventionist time vs 190 hours of interventionist time in FBT [includes group and individual coaching time]). Thus, there would be a greater opportunity to disseminate the gshFBT model. Our preliminary findings support the need for future randomized control studies to examine the efficacy of gshFBT in child weight loss.
When comparing parents with overweight or obesity from each group (FBT and gshFBT), our results also found that gshFBT was noninferior to FBT on parent weight loss at posttreatment and 6-month follow-up. Although preliminary due to differences in group size, if repeated, these findings could have great implications for family health and could impact more than just the target child and participating parent.
Furthermore, this study provided preliminary evidence of the noninferiority of gshFBT on child daily caloric intake, relative to FBT. Twenty-four hour dietary recalls are currently the most widely used self-report method to assess nutrition and caloric consumption as participants are randomly polled and caloric intake is calculated using standardized data bases.32 Yet, this measure also is limited by the potential for biased reporting of dietary intake33–35 due to participant forgetfulness, estimation of portion sizes, and reliance on child report of food consumed during school hours. Thus, future studies should replicate and further test this hypothesis.
Results were less conclusive for child physical activity. Our study showed that gshFBT was noninferior to FBT on changes in child vigorous physical but not MVPA. However, although accelerometers are currently the most accurate way to assess physical activity, there are some indications that this instrument can be unreliable due to lack of sufficient wear time in children.36,37 Wear time in this study was variable, which is why we used percent of time spent in each physical activity category. Future studies, including accelerometer data, should use additional incentives to increase child wear time and reduce measure variability.
To our knowledge, this is the first study to compare gshFBT to FBT among 8–12-year-old children with overweight and obesity and their parents. Despite the nonrandomized nature of this pilot, both treatments were conducted in the same research laboratory with the same recruitment procedures, and numerous steps were taken to reduce sources of bias. Limitations include the nonrandomized design, relatively small sample size, lack of inclusion of children with higher BMIz, differences in inclusion criteria (parent BMI), baseline differences in child MVPA between groups, and potential differences in parent–child interactions depending on parental weight status. Also, there was a difference in interventionist credentials between treatment group; gshFBT treatment sessions were led by graduate students in clinical psychology while FBT group leaders were licensed psychologists or postdocs. However, all gshFBT interventionists attended a 4-hour training regarding behavioral intervention for the study and were supervised by Kerri Boutelle (last author) weekly during treatment. Despite differences in credentials, gshFBT proved to be noninferior to FBT in most of the study main outcomes, further speaking to the use of gshFBT in other settings and by other professionals besides psychologist. Thus, despite these limitations, this study suggests that gshFBT was noninferior to FBT on measures of child and parental weight loss, child dietary intake, and child physical activity except MVPA. These preliminary results could have significant implications for the dissemination of effective treatments of childhood obesity in numerous settings (i.e., community centers and primary care clinics) and support the need for future research on gshFBT. Although the comparative effects and the cost-effectiveness of FBT and gshFBT should be further explored in randomized controlled trials with longer follow-ups, these data suggest that gshFBT is a promising model for future research.
Acknowledgment
R21DK080266; R01DK075861 to KB; F31DK117556; K23114480. The funding did not influence reported results. The views discussed may not reflect views of NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Ogden C, Carroll M, Kit B, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311:806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guh D, Zhang W, Bansbak N, et al. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Key T, Schatzkin A, Willett W, et al. Diet, nutrition and the prevention of cancer. Pub Health Nutr 2004;7:187–200 [DOI] [PubMed] [Google Scholar]
- 4. Pulgarón E. Childhood obesity: A review of increased risk for physical and psychological comorbidities. Clin Ther 2013;35:A18–A32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gray W, Kahhan N, Janicke D. Peer victimization and pediatric obesity: A review of the literature. Psychol Schools 2008;46:720–727 [Google Scholar]
- 6. Strauss R, Pollack H. Social marginalization of overweight children. Arch Pediatr Adolesc Med 2003;157:746–752 [DOI] [PubMed] [Google Scholar]
- 7. Thompson J, Shroff H, Herbozo S, et al. Relations among multiple peer influences, body dissatisfaction, eating disturbance, and self-esteem: A comparison of average weight, at risk of overweight, and overweight adolescent girls. J Pediatr Psychol 2007;32:24–29 [DOI] [PubMed] [Google Scholar]
- 8. Janicke D, Harman J, Jamoom E, et al. The relationship among child weight status, psychosocial functioning, and pediatric health care expenditures in a medicaid population. J Pediatr Psychol 2010;35:883–891 [DOI] [PubMed] [Google Scholar]
- 9. Young K, Northern J, Lister K, et al. A meta-analysis of family-behavioral weight-loss treatments for children. Clin Psychol Rev 2007;27:240–249 [DOI] [PubMed] [Google Scholar]
- 10. Epstein L. Family-based behavioural intervention for obese children. Int J Obes Relat Metab Disord 1996;20 Suppl 1:S14–S21 [PubMed] [Google Scholar]
- 11. US Preventive Services Task Force, Grossman D, Bibbins-Domingo K, et al. Screening for obesity in children and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2017;317:2417–2426 [DOI] [PubMed] [Google Scholar]
- 12. Whitlock E, O'Connor E, Williams S, et al. Effectiveness of weight management interventions in children: A targeted systematic review for the USPSTF. Pediatrics 2010;125:e396–e418 [DOI] [PubMed] [Google Scholar]
- 13. Epstein L, Valoski A, Wing R, et al. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol 1994;13:373–383 [DOI] [PubMed] [Google Scholar]
- 14. Riggs K, Lozano P, Mohelnitzky A, et al. An adaptation of family-based behavioral pediatric obesity treatment for a primary care setting: Group health family wellness program pilot. Perm J 2014;18:4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boutelle K, Norman G, Rock C, et al. Guided self-help for the treatment of pediatric obesity. Pediatrics 2013;131:e1435–e1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berkowitz R, Rukstalis M, Bishop-Gilyard C, et al. Treatment of adolescent obesity comparing self-guided and group lifestyle modification programs: A potential model for primary care. J Pediatr Psychol 2013;38:978–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perri M, Sears S, Jr., Clark J. Strategies for improving maintenance of weight loss. Toward a continuous care model of obesity management. Diabetes Care 1993;16:200–209 [DOI] [PubMed] [Google Scholar]
- 18. Boutelle K, Rhee K, Liang J, et al. Effect of attendance of the child on body weight, energy intake, and physical activity in childhood obesity treatment: A randomized clinical trial. JAMA Pediatr 2017;171:622–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuczmarski R, Ogden C, Grummer-Strawn L, et al. CDC growth charts: United States. Adv Data 2000;314:1–27 [PubMed] [Google Scholar]
- 20. Johnson R, Driscoll P, Goran M. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc 1996;96:1140–1144 [DOI] [PubMed] [Google Scholar]
- 21. Lytle L, Murray D, Perry C, et al. Validating fourth-grade students' self-report of dietary intake: Results from the 5 A Day Power Plus program. J Am Diet Assoc 1998;98:570–572 [DOI] [PubMed] [Google Scholar]
- 22. Lytle L, Nichaman M, Obarzanek E, et al. Validation of 24-hour recalls assisted by food records in third-grade children. The CATCH Collaborative Group. J Am Diet Assoc 1993;93:1431–1436 [DOI] [PubMed] [Google Scholar]
- 23. Trumbo P, Schlicker S, Yates A, et al. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc 2002;102:1621–1630 [DOI] [PubMed] [Google Scholar]
- 24. Nichols J, Morgan C, Chabot L, et al. Assessment of physical activity with the Computer Science and Applications, Inc., accelerometer: Laboratory versus field validation. Res Q Exerc Sport 2000;71:36–43 [DOI] [PubMed] [Google Scholar]
- 25. Freedson P, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30:777–781 [DOI] [PubMed] [Google Scholar]
- 26. Trost SG, Pate RR, Sallis JF, et al. Age and gender differences in objectively measured physical activity in youth. Med Sci Sports Exerc 2002;34:350–355 [DOI] [PubMed] [Google Scholar]
- 27. Corp I. IBM Statistics for Macintosh, Version 22.0. Armonk NY: IBM Corp., 2013 [Google Scholar]
- 28. D'Agostino R, Massaro JM, Sullivan LM. Non-inferiority trials: Design concepts and issues—The encounters of academic consultants in statistics. Stat Med 2003;22:169–186 [DOI] [PubMed] [Google Scholar]
- 29. Rothmann M, Li N, Chen G, et al. Design and analysis of non-inferiority mortality trials in oncology. Stat Med 2003;22:239–264 [DOI] [PubMed] [Google Scholar]
- 30. Walker E, Nowacki A. Understanding equivalence and non-inferiority testing. J Gen Int Med 2011;26:192–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boutelle K, Cafri G, Crow S. Parent-only treatment for childhood obesity: A randomized controlled trial. Obesity (Silver Spring) 2011;19:574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Subar A, Thompson F, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: The Eating at America's Table Study. Am J Epidemiol 2001;154:1089–1099 [DOI] [PubMed] [Google Scholar]
- 33. Burrows T, Martin R, Collins C. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J Am Diet Assoc 2010;110:1501–1510 [DOI] [PubMed] [Google Scholar]
- 34. Lichtman S, Pisarska K, Berman E, et al. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med 1992;327:1893–1898 [DOI] [PubMed] [Google Scholar]
- 35. Livingstone M, Robson P, Wallace J. Issues in dietary intake assessment of children and adolescents. Br J Nutr 2004;92:S214–S222 [DOI] [PubMed] [Google Scholar]
- 36. Barreira T, Schuna J, Tudor-Locke C, et al. Reliability of accelerometer-determined physical activity and sedentary behavior in school-aged children: A 12-country study. Int J Obes 2015;5:S29–S35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Herrmann S, Barreira T, Kang M, et al. Impact of accelerometer wear time on physical activity data: A NHANES semisimulation data approach. Br J Sports Med 2014;48:278–282 [DOI] [PubMed] [Google Scholar]