Abstract
Background
In the outpatient setting, the majority of antibiotic prescriptions are for acute respiratory infections (ARIs), but most of these infections are viral and antibiotics are unnecessary. We analyzed provider-specific antibiotic prescribing in a group of outpatient clinics affiliated with an academic medical center to inform future interventions to minimize unnecessary antibiotic use.
Methods
We conducted a cross-sectional study of patients who presented with an ARI to any of 15 The Emory Clinic (TEC) primary care clinic sites between October 2015 and September 2017. We performed multivariable logistic regression analysis to examine the impact of patient, provider, and clinic characteristics on antibiotic prescribing. We also compared provider-specific prescribing rates within and between clinic sites.
Results
A total of 53.4% of the 9600 patient encounters with a diagnosis of ARI resulted in an antibiotic prescription. The odds of an encounter resulting in an antibiotic prescription were independently associated with patient characteristics of white race (adjusted odds ratio [aOR] = 1.59; 95% confidence interval [CI], 1.47–1.73), older age (aOR = 1.32, 95% CI = 1.20–1.46 for patients 51 to 64 years; aOR = 1.32, 95% CI = 1.20–1.46 for patients ≥65 years), and comorbid condition presence (aOR = 1.19; 95% CI, 1.09–1.30). Of the 109 providers, 13 (12%) had a rate significantly higher than predicted by modeling.
Conclusions
Antibiotic prescribing for ARIs within TEC outpatient settings is higher than expected based on prescribing guidelines, with substantial variation in prescribing rates by site and provider. These data lay the foundation for quality improvement interventions to reduce unnecessary antibiotic prescribing.
Keywords: epidemiology, primary care, quality improvement
There are an estimated 2 million illnesses and 23 000 deaths annually in the United States that are caused by antibiotic-resistant bacteria [1]. Unnecessary antibiotic prescribing contributes to antibiotic resistance through selective pressure on bacteria [2–4]. Within the outpatient setting, the majority of antibiotic prescriptions are for acute respiratory infections (ARIs), which are one of the most common visit diagnoses [5–9]. Most ARIs are viral and treatment with antibiotics provides no benefit to the patient [10–12] but does pose risk, not only of acquisition of antibiotic-resistant bacteria but also of adverse outcomes (eg, Clostridiodes difficile infections, allergic reactions) and increased healthcare costs [7, 9, 11, 13–16]. According to Shehab et al [15], approximately 80% of emergency department visits for antibiotic-associated adverse events were attributable to an allergic reaction. Furthermore, antibiotics are the most important cause of C difficile-associated diarrhea, and eliminating unnecessary antibiotic prescribing is a key prevention step [11, 16, 17].
Factors that contribute to unnecessary antibiotic prescribing have been identified in prior studies, including characteristics of the patient, provider, and setting such as patient race, provider type, and clinic location [6, 18–20]. Analyzing patterns and sources of variation in antibiotic prescribing for patients with respiratory infections could help with design of interventions to change provider prescribing [6]. The objective of this study was to identify predictors of unnecessary antibiotic prescribing for ARIs in outpatient primary care clinics within The Emory Clinic (TEC) of Emory Healthcare. Obtaining this information should serve as a critical first step to develop a quality improvement initiative utilizing provider feedback and behavior change interventions to reduce unnecessary antibiotic prescribing.
METHODS
Study Design, Data Source, and Study Population
A cross-sectional study was conducted including eligible patient encounters with a presenting diagnosis of ARI in 15 primary care clinics between October 2015 and September 2017. Deidentified data for eligible encounters were obtained from the Emory Healthcare Clinical Data Warehouse. Eligible encounters were defined as all patient visits to one of the primary care clinics within TEC with an International Classification of Diseases, Tenth Revision (ICD-10) primary diagnosis code consistent with an ARI. These ICD-10 codes were selected based on a prior study that examined ARI prescribing [6, 13] and included any encounter with a primary diagnostic code for acute nasopharyngitis (J00), acute sinusitis (J01.x), acute pharyngitis (J02.x), acute tonsillitis (J03.x), acute laryngitis and tracheitis (J04.x), acute obstructive laryngitis (J05), acute upper respiratory infections (J06.x), acute bronchitis (J20.x), acute bronchiolitis (J21.x), and unspecified acute lower respiratory infection (J22). We chose to use a prescribing metric that incorporated both antibiotic-inappropriate ARI encounters (ie, acute nasopharyngitis) and potentially antibiotic-appropriate ARI encounters (ie, acute sinusitis or pharyngitis) for several reasons [6]. First, we were concerned that the subjectivity of coding these syndromes could bias the results if clinics or providers preferentially code antibiotic-appropriate syndromes when prescribing antibiotics. For example, providers might select acute sinusitis as the diagnosis in patients for whom they are prescribing antibiotics and acute nasopharyngitis in patients without an antibiotic prescription, despite similarity in patient presentation. Second, although a small percentage of sinusitis or pharyngitis cases are antibiotic-appropriate, most patients who present with related symptoms have a viral infection, and so routine antibiotic use is not recommended unless patients meet specific criteria [21, 22]. Given that the majority of patients presenting with a potentially antibiotic-appropriate ARI diagnosis do not have a bacterial infection, inclusion of these diagnoses may be beneficial when evaluating and comparing provider prescribing rates.
Additional variables were abstracted from administrative data including the following: secondary diagnostic codes; patient demographic information such as age, gender, and race; patient comorbid conditions; the presence of coinfection (other infection documented during index encounter); provider type; prescribing provider study-designated identification (ID) number; and whether an antibiotic was prescribed. Eligible visits with missing patient race or provider ID were excluded (accounting for <1% of encounters). This protocol was reviewed by Emory University Institutional Review Board (Atlanta, GA) with a determination that it did not constitute human subjects research.
Covariate Definitions
Patient race was defined as white, black, or other for encounter-level analysis and recategorized as white or nonwhite for provider- and clinic-level analysis. Patient age was categorized into 3 groups: 0 to 50 years, 50 to 64 years, and ≥65 years. Patients were considered to have a comorbidity if they had documentation of any of the following conditions within the 12 months before the eligible encounter: congestive heart failure, chronic lung disease, chronic kidney disease, diabetes, cancer, or human immunodeficiency virus/acquired immune deficiency syndrome. Patient coinfection was defined as the presence of an infection other than ARI at the time of eligible encounter based on ICD-10 codes. The ICD-10 codes for comorbid conditions and coinfections were based on prior work by Meeker et al [13]. Because patient coinfections were present in less than 2% (n = 179) of the eligible encounters, this variable was not included in further analysis. Providers were classified by professional training as Staff Physicians, Advanced Practice Providers ([APPs] including Nurse Practitioners and Physician Assistants), or Resident Physicians, and further categorized as Staff Physician or Other Staff (APPs or Residents) for provider- and clinic-level analysis. Fifteen distinct TEC locations were included in the study, but because 5 clinics had 200 or fewer encounters, they were aggregated into 1 group to avoid imprecise frequency values. Furthermore, 2 other clinics were grouped together because they have the same providers, for a final count of 10 TEC locations. Clinics were labeled Clinic A through Clinic J, where Clinic B is the composite group of 5 clinics and Clinic G the composite of 2 clinics.
Statistical Analysis
Patient, provider, and clinic characteristics were compared between ARI encounters with and without an antibiotic prescription. Variables that were associated with antibiotic prescribing based on a χ2 test (P < .10) were eligible for inclusion in multivariable analysis. Multivariable logistic regression at the encounter level was performed to identify significant predictors for clinic- and provider-specific antibiotic prescribing. In addition, unadjusted prescribing rates were calculated by TEC location.
For provider-level analysis, the study population was restricted to providers with 10 or more encounters (n = 9435) to avoid imprecise frequency values. Unadjusted provider-specific prescribing rates were computed, along with descriptive statistics for the mean age, racial distribution, and presence of comorbidities among each provider’s patients. Given the potential for providers’ workload to affect their ARI prescribing rate, each provider’s annual clinic during the study period was also abstracted using billing data. A linear regression analysis was then used to evaluate for correlation between overall encounter numbers and ARI encounter numbers for each provider, as well as between overall encounter numbers and prescribing rate. Finally, crude prescribing rates were calculated using only antibiotic-inappropriate diagnostic codes (excluding sinusitis, pharyngitis, and tonsillitis as potentially antibiotic-appropriate [6]) to determine whether these rates were significantly different than those calculated for all ARI visits.
Standardized Prescribing Ratios.
Using results of multivariable analysis, encounter-level data were used to estimate the probability of an encounter resulting in an antibiotic prescription. The best-fitting model was selected based on both an assessment of the clinical relevance of predictors and evaluation of the results of 3 goodness-of-fit tests (Hosmer-Lemeshow, Pearson, and Deviance). This model was then used to predict provider- and clinic-specific prescribing rates, which were designated as the “expected” prescribing rates. These rates were then compared with the observed prescribing rates, and the provider- and clinic-specific observed to expected (O/E) ratios and corresponding 95% confidence intervals ([CIs] using Byar approximation and the Mid-P exact test for observed numbers of antibiotics prescribed that are less than or equal to 5 [23]) were subsequently calculated. Statistical analyses were done using SAS, version 9.4 (SAS Institute).
RESULTS
Study Population and Encounter-Level Analysis
Of 10 362 eligible visits with the primary diagnosis of an ARI, 9600 met inclusion criteria (Table 1). Patients were seen by 152 providers at 10 different TEC locations, and the largest proportion of encounters occurred at Clinic J (29.3%), Clinic I (13.6%), and Clinic H (12.8%). The majority of patients were female (70.8%), white (55.7%), and older than 50 (59.0%). One quarter of encounters involved patients with comorbidities (25.5%), and less than 2% of encounters involved patients with coinfections. More than half of encounters (53.4%) resulted in antibiotics being prescribed.
Table 1.
Characteristics of Eligible Encounters With Acute Respiratory Infections From the Emory Clinic Primary Care Locations, October 2015 to September 2017 (N = 9600)
| Variables | Frequency | % |
|---|---|---|
| Patient Sex | ||
| Male | 2808 | 29.3 |
| Female | 6792 | 70.8 |
| Patient Age | ||
| 0 to 50 years | 3957 | 41.2 |
| 51 to 64 years | 2518 | 26.2 |
| 65+ years | 3125 | 32.6 |
| Patient Race | ||
| White | 5344 | 55.7 |
| Black | 3758 | 39.2 |
| Othera | 498 | 5.2 |
| Patient Comorbidities Present | ||
| Yes | 3405 | 35.5 |
| No | 6195 | 64.5 |
| Provider Types | ||
| Staff Physician | 7233 | 75.3 |
| APP | 1427 | 14.9 |
| Resident | 940 | 9.8 |
| Clinic Locations | ||
| Clinic A | 500 | 5.2 |
| Clinic B | 116 | 1.2 |
| Clinic C | 339 | 3.5 |
| Clinic D | 589 | 6.1 |
| Clinic E | 702 | 7.3 |
| Clinic F | 938 | 9.8 |
| Clinic G | 1075 | 11.2 |
| Clinic H | 1304 | 13.6 |
| Clinic I | 1228 | 12.8 |
| Clinic J | 2809 | 29.3 |
Abbreviations: APP, Advanced Practice Provider (Nurse Practitioners and Physicians Assistants).
aThe other category for race includes Asian, American Indian or Alaskan native, Native Hawaiian or Other Pacific, and multiple.
Correlation of provider workload (overall encounters) with ARI encounters was fairly high and significant (r = 0.78, P < .001), whereas provider workload and prescribing frequency were poorly correlated (r = 0.18, P < .01). Finally, when prescribing rates were modified to only include encounters coded with antibiotic-inappropriate categories, both the prescribing rate and variability among prescribers remained high, with a clinic-specific median prescribing rate of 43% (range, 15%–74%).
There were significant differences in the antibiotic prescription rates based on patient age, patient race, the presence of comorbidities, provider type, and clinic locations (Table 2). Multivariable logistic regression analysis identified several characteristics predictive of prescribing antibiotics. Encounters with white patients are more likely to result in an antibiotic prescription compared with encounters with black patients (adjusted odds ratio [aOR] = 0.71, 95% CI = 0.64–0.78, white patients as reference) or other races (aOR = 0.65, 95% CI = 0.53–0.79, white patients as reference). Encounters with patients who were older than 50 years (51 to 64 years, aOR = 1.34, 95% CI = 1.20–1.50; 65+ years, aOR = 1.31, 95% CI = 1.17–1.47) or who had comorbid conditions (aOR = 1.23; 95% CI, 1.12–1.35) were more likely to result in antibiotic prescriptions. There was a difference in prescribing patterns between Staff Physicians and Residents, but there was no difference between Staff Physicians and APPs (Table 3).
Table 2.
Antibiotic Prescribing During Outpatient Visits for Acute Respiratory Infections, The Emory Clinic Primary Care, October 2015 and September 2017 (N = 9600)
| Antibiotics Prescribed | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | n | Yes | % | No | % | RRa | 95% CI | P Valueb | |
| Patient Sex | |||||||||
| Femalec | 6792 | 3614 | 53 | 3178 | 47 | - | - | - | - |
| Male | 2808 | 1540 | 55 | 1268 | 45 | 1.03 | 0.99 | 1.07 | .14 |
| Patient Race | |||||||||
| Whitec | 5344 | 3140 | 59 | 2204 | 41 | - | - | - | |
| Black | 3758 | 1760 | 47 | 1998 | 53 | 0.80 | 0.77 | 0.83 | <.001 |
| Otherd | 498 | 228 | 46 | 270 | 54 | 0.78 | 0.71 | 0.86 | <.001 |
| Patient Age | |||||||||
| 0–50 yearsc | 3957 | 1896 | 48 | 2061 | 52 | - | - | - | |
| 51–64 years | 2518 | 1409 | 56 | 1109 | 44 | 1.17 | 1.11 | 1.22 | <.001 |
| 65+ years | 3125 | 1823 | 58 | 1302 | 42 | 1.22 | 1.17 | 1.27 | <.001 |
| Patient Comorbidities Present | |||||||||
| Absentc | 6195 | 3201 | 52 | 2994 | 48 | - | - | - | |
| Present | 3405 | 1927 | 57 | 1478 | 43 | 1.10 | 1.05 | 1.14 | <.001 |
| Provider Type | |||||||||
| Staff Physicianc | 7233 | 4186 | 58 | 3047 | 42 | - | - | - | |
| APP | 1427 | 612 | 43 | 815 | 57 | 0.74 | 0.70 | 0.79 | <.001 |
| Resident | 940 | 330 | 35 | 610 | 65 | 0.61 | 0.56 | 0.66 | <.001 |
| Clinic Locations | |||||||||
| Clinic A | 500 | 115 | 23 | 385 | 77 | - | - | - | |
| Clinic B | 116 | 27 | 23 | 89 | 77 | 1.10 | 0.70 | 1.46 | .95 |
| Clinic C | 339 | 207 | 61 | 132 | 39 | 2.65 | 2.21 | 3.18 | <.001 |
| Clinic D | 589 | 439 | 75 | 150 | 25 | 3.24 | 2.74 | 3.83 | <.001 |
| Clinic E | 702 | 283 | 40 | 419 | 60 | 1.75 | 1.46 | 2.11 | <.001 |
| Clinic F | 938 | 467 | 50 | 471 | 50 | 2.16 | 1.82 | 2.57 | <.001 |
| Clinic G | 1075 | 571 | 53 | 504 | 47 | 2.31 | 1.95 | 2.74 | <.001 |
| Clinic H | 1304 | 509 | 39 | 795 | 61 | 1.70 | 1.43 | 2.02 | <.001 |
| Clinic I | 1228 | 491 | 40 | 737 | 60 | 1.74 | 1.46 | 2.07 | <.001 |
| Clinic J | 2809 | 2019 | 72 | 790 | 28 | 3.13 | 2.66 | 3.67 | <.001 |
Abbreviations: APP, Advanced Practice Provider (Nurse Practitioners and Physicians Assistants); CI, confidence interval; RR, risk ratios.
aUnadjusted RRs.
b P value from χ2 test.
cReference group.
dThe other category of race includes Asian, American Indian or Alaskan native, Native Hawaiian or Other Pacific, and multiple.
Table 3.
Multivariable Model of Antibiotic Prescribing Among the Emory Clinic Primary Care Encounters With Acute Respiratory Infections (N = 9600)
| Variables | aORa | 0.95 CI | P Value | |
|---|---|---|---|---|
| Patient Raceb | ||||
| Black | 0.71 | 0.64 | 0.78 | .04 |
| Otherc | 0.65 | 0.53 | 0.79 | <.01 |
| Patient Ageb | ||||
| 51–64 years | 1.34 | 1.20 | 1.50 | <.01 |
| 65+ years | 1.31 | 1.17 | 1.47 | .02 |
| Patient comorbidityb | 1.23 | 1.12 | 1.35 | <.001 |
| Provider Typeb | ||||
| APP | 0.98 | 0.85 | 1.12 | .17 |
| Resident | 0.77 | 0.63 | 0.94 | .02 |
| Clinic Locationsb | ||||
| Clinic B | 1.26 | 0.75 | 2.07 | <.001 |
| Clinic C | 5.09 | 3.71 | 7.04 | <.001 |
| Clinic D | 10.43 | 7.80 | 14.04 | <.001 |
| Clinic E | 1.98 | 1.52 | 2.59 | <.001 |
| Clinic F | 3.74 | 2.90 | 4.84 | .03 |
| Clinic G | 4.39 | 3.40 | 5.70 | <.001 |
| Clinic H | 2.29 | 1.80 | 2.94 | <.001 |
| Clinic I | 2.81 | 2.14 | 3.70 | .01 |
| Clinic J | 8.33 | 6.58 | 10.62 | <.001 |
Abbreviations: aOR, adjusted odds ratio; APP, Advanced Practice Provider (nonphysician members including Nurse Practitioners, Physicians Assistants, Certified Nurse Midwives, Clinical Psychologists, Nonclinical Psychologists, Clinical Nurse Specialists); CI, confidence interval; TEC, The Emory Clinic.
aAdjusted for all other covariates.
bReference groups for the nominal variables are as follows: not white race for patient race, absence of any comorbidities for patient comorbidities, ages 0 to 50 for patient age, not Staff Physician for provider type, and Clinic A for TEC location.
cThe other subcategory of race includes Asian, American Indian or Alaskan native, Native Hawaiian or Other Pacific, and multiple.
When summarizing data at the clinic location level, unadjusted antibiotic prescribing rates varied greatly. Clinic D (75%) and Clinic J (72%) prescribed antibiotics more frequently compared with the other clinics (P < .001; Supplementary Figure 1). This variability persists even after adjusting for the influence of patient age, comorbidities, and provider type between the clinic locations; the adjusted odds of a patient receiving an antibiotic significantly varied by clinic locations in multivariable analysis (Table 3).
Provider-Level Analysis
After excluding providers with less than 10 encounters for ARI, there were 109 providers with 9435 eligible encounters. Unadjusted provider-specific prescribing rates showed variability in prescribing patterns (median, 43%; interquartile range, 27%–60%) (Supplementary Figure 1). In multivariable analysis, the patient characteristics of white race (aOR = 1.59; 95% CI, 1.47–1.73), age (51 to 64 years, aOR = 1.32, 95% CI = 1.20–1.46; 65+ years, aOR = 1.32, 95% CI = 1.20–1.46), and comorbid condition presence (aOR = 1.19; 95% CI, 1.09–1.30) remained in the model as significant predictors of provider prescribing (Table 4).
Table 4.
Best Fit Multivariable Logistic Regression Model of Antibiotic Prescribing for Acute Respiratory Infections Among Providers With ≥10 Encounters at The Emory Clinic Primary Care for Use With Provider-Specific Prediction (N = 9435)
| Variables | aOR | 95% CI | |
|---|---|---|---|
| Patient white racea | 1.59 | 1.47 | 1.73 |
| Patient comorbiditya | 1.19 | 1.09 | 1.30 |
| Patient Agea | |||
| 51–64 years | 1.32 | 1.20 | 1.46 |
| 65+ years | 1.32 | 1.20 | 1.46 |
| Goodness-of-Fitb | |||
| Pearson (P value) | .238 | ||
| Deviance (P value) | .238 | ||
| Hosmer-Lemeshow (P value) | .313 | ||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
aReference groups for the nominal variables are as follows: nonwhite race for patient race, absence of any comorbidities, and ages 0 to 50.
bThree separate statistical measures indicating whether the model is a good fit to the data when the null hypothesis (the model does not lack fit) is true.
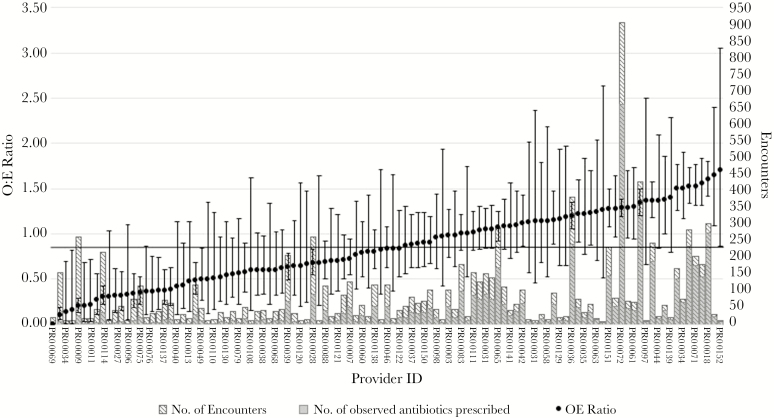
There were significant differences in O/E ratios for antibiotic prescribing between providers, but these ratios were not clearly correlated with the number of patient encounters each provider had (Figure 1). Of the 109 providers, 65 (60%) had an O/E ratio less than 1.0 with 28 of these (26%) statistically significant, and 41 (38%) had an O/E ratio greater than 1.0 with 13 (12%) statistically significant.
Figure 1.
Stacked bar graph of total number of encounters and observed antibiotics prescribed overlaid by caterpillar plot of observed/expected (O:E) ratio of antibiotics prescribed by provider at The Emory Clinic Primary care from October 2015 to September 2017 (N = 9435). The O/E ratios were calculated using predicted probabilities from a multivariable logistic model adjusting for patient characteristics of age, race, and presence of comorbid conditions. Error bars denote 95% confidence interval.
DISCUSSION
We found that more than 50% of patients with a diagnosis of an ARI received an antibiotic during our 2-year study of outpatient visits. A 20% prescribing rate has previously been identified as a reasonable target for good practice in this setting [6, 13] given that the majority of these infections are viral, and so the data suggest that ≥30% of patients may be receiving unnecessary antibiotics.
Similar to previous studies [5, 20, 24, 25], we found that the patient characteristics of age, race, and comorbid condition(s) presence were significant predictors of antibiotic use within multivariable logistic regression analyses. However, these factors did not explain the entirety of the variation seen in antibiotic prescribing rates between different prescribers or different clinics, because significant differences persisted even when controlling for these variables. For example, when controlling for patient characteristics, the odds of receiving an antibiotic prescription were approximately 10 times higher for patients who visited Clinic D compared with those who visited Clinic A. We considered whether encounter volume and time pressure within a clinic might influence prescribing decisions [26]; however, provider workload was not correlated with the decision to prescribe an antibiotic. Studies have noted that cultural factors (such as patient attitudes) and other external forces (such as insurance type, accessibility and price of antibiotics, or public opinion) may influence the prescribing of antibiotics [7, 26–29]. Prior studies have also suggested that provider beliefs stemming from the number of years of experience and method of training factor into the decision to prescribe antibiotics [6, 7, 26], and that the providers have individual treatment styles regardless of patient characteristics [6]. We found that resident physicians had lower rates of prescribing than attending physicians, which may reflect an impact of trainee education regarding antimicrobial stewardship. All internal medicine residents within our system are required to rotate on an infectious disease consult service during their training, and they interact with a multidisciplinary stewardship team at every hospital in which they work.
Other local factors, such as the culture within each provider’s practice, may contribute to the decision-making process in prescribing an antibiotic [18, 20]. Providers within a clinic may coalesce around prescribing practices, especially if they share patients who have expectations about receiving antibiotics in particular situations. In addition, our clinics utilize a system where patients can leave messages with clinic staff about concerns or symptoms that are conveyed electronically to providers. If these messages are framed and handled in a similar way as a result of practice culture (eg, if patients are told they need to make an appointment versus if a provider who receives a message about ARI symptoms calls in an antibiotic prescription), they may result in increased or decreased numbers of antibiotic prescriptions. The etiology of clinic-specific differences in prescribing behavior merits further investigation.
These differences in prescribing behavior among providers and clinics may offer targets for future interventions. We postulate that the provider-specific O/E ratios could be used in potential interventions to illustrate the degree of overprescribing and to offer providers targets for improvement, similar to what has been proposed among inpatient stewardship programs [30]. At the clinic level, prescribing targets could be created using the expected frequency of encounters with prescriptions accounting for patient-mix at the clinic to serve as a baseline, from which improvements could be tracked. The impact of providing such feedback and metrics could then be assessed.
We acknowledge several limitations in this study. Classification of visit diagnosis was based on ICD-10-CM (Clinical Modification) codes, and it is possible that misclassifications occurred. We attempted to limit the impact of misclassifications by including both diagnoses for which antibiotics should not be prescribed (eg, acute upper respiratory infection) and those for which antibiotics are sometimes indicated (eg, acute pharyngitis and sinusitis) because there was concern for potential bias due to diagnostic uncertainty and potential provider inclination to support antibiotic prescription. An analysis of crude prescribing rates for only antibiotic-inappropriate diagnoses showed a similar degree of variability to the results of this study. In addition, information regarding provider and clinic diagnostic coding utilization would be lost if antibiotic-inappropriate diagnoses were the main focus. In addition, interactions between the patient, provider, and setting predictors were not considered, and some providers and clinics were excluded. Some older pediatric patients who see a family medicine practitioner were included in the study population but only accounted for 2% of all ARI encounters. Provider analysis was restricted to providers with 10 or more encounters, and clinic analysis was restricted to clinics with 100 or more encounters during the 2-year study period.
CONCLUSIONS
Despite the limitations of this study, important conclusions can be drawn from the results. Similar to previous studies, antibiotic prescription rates for ARIs are higher within TEC adult primary care clinics than what should be appropriate [31, 32]. In addition, there is significant variation in the prescribing behavior between TEC providers and clinics even when controlling for the key patient characteristics that influence whether antibiotics are prescribed. These data lay the foundation for quality improvement interventions to reduce antibiotic prescribing rates. Our team is using these data to define the context of peer-to-peer interactions within the outlier clinics as a first step to change prescriber practice.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We thank Dr. Zachary Binney and Michael Garber (Emory University) for their assistance.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: Emory Quality Conference, Emory University School of Medicine, April 2, 2018, Atlanta, GA.
References
- 1. Centers for Disease Control and Prevention. . About Antimicrobial Resistance. 2018. Available at: https://www.cdc.gov/drugresistance/about.html. Accessed 9 April 2018. [Google Scholar]
- 2. Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Infect Control 2006; 34:S3–10; discussion S64–73. [DOI] [PubMed] [Google Scholar]
- 3. Lieberman JM. Appropriate antibiotic use and why it is important: the challenges of bacterial resistance. Pediatr Infect Dis J 2003; 22:1143–51. [DOI] [PubMed] [Google Scholar]
- 4. Spellberg B, Guidos R, Gilbert D, et al. . The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis 2008; 46:155–64. [DOI] [PubMed] [Google Scholar]
- 5. Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother 2014; 69:234–40. [DOI] [PubMed] [Google Scholar]
- 6. Jones BE, Sauer B, Jones MM, et al. . Variation in outpatient antibiotic prescribing for acute respiratory infections in the Veteran population: a cross-sectional study. Ann Intern Med 2015; 163:73–80. [DOI] [PubMed] [Google Scholar]
- 7. Steinman MA, Gonzales R, Linder JA, Landefeld CS. Changing use of antibiotics in community-based outpatient practice, 1991-1999. Ann Intern Med 2003; 138:525–33. [DOI] [PubMed] [Google Scholar]
- 8. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 2009; 302:758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roumie CL, Halasa NB, Grijalva CG, et al. . Trends in antibiotic prescribing for adults in the United States–1995 to 2002. J Gen Intern Med 2005; 20:697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA 1997; 278:901–4. [PubMed] [Google Scholar]
- 11. Fiore DC, Fettic LP, Wright SD, Ferrara BR. Antibiotic overprescribing: still a major concern. J Fam Pract 2017; 66:730–6. [PubMed] [Google Scholar]
- 12. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician 2016; 94:560–5. [PubMed] [Google Scholar]
- 13. Meeker D, Linder JA, Fox CR, et al. . Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA 2016; 315:562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tackling A Crisis for Future Health and Wealth of Nations. Review on Antimicrobial Resistance. 2014. https://amr-review.org/Publications.html Accessed 9 April 2018. [Google Scholar]
- 15. Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis 2008; 47:735–43. [DOI] [PubMed] [Google Scholar]
- 16. Thomas C, Stevenson M, Riley TV. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review. J Antimicrob Chemother 2003; 51:1339–50. [DOI] [PubMed] [Google Scholar]
- 17. Srigley JA, Brooks A, Sung M, et al. . Inappropriate use of antibiotics and Clostridium difficile infection. Am J Infect Control 2013; 41:1116–8. [DOI] [PubMed] [Google Scholar]
- 18. Aspinall SL, Berlin JA, Zhang Y, Metlay JP. Facility-level variation in antibiotic prescriptions for veterans with upper respiratory infections. Clin Ther 2005; 27:258–62. [DOI] [PubMed] [Google Scholar]
- 19. McCullough AR, Pollack AJ, Plejdrup Hansen M, et al. . Antibiotics for acute respiratory infections in general practice: comparison of prescribing rates with guideline recommendations. Med J Aust 2017; 207:65–9. [DOI] [PubMed] [Google Scholar]
- 20. Steinman MA, Landefeld CS, Gonzales R. Predictors of broad-spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA 2003; 289:719–25. [DOI] [PubMed] [Google Scholar]
- 21. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. . Clinical practice guideline (updated): adult sinusitis. Otolaryngol Head Neck Surg 2015; 152(2 Suppl):S1–39. [DOI] [PubMed] [Google Scholar]
- 22. Chow AW, Benninger MS, Brook I, et al. . IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis 2012; 54:e72–e112. [DOI] [PubMed] [Google Scholar]
- 23. Soe MM, Sullivan KM, Dean AG, et al.. Standardized mortality ratio and confidence interval. In: Open EPI. 2006. Available at: http://www.openepi.com/SMR/SMR.htm. Accessed 13 April 2018. [Google Scholar]
- 24. Donnelly JP, Baddley JW, Wang HE. Antibiotic utilization for acute respiratory tract infections in U.S. emergency departments. Antimicrob Agents Chemother 2014; 58:1451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goyal MK, Johnson TJ, Chamberlain JM, et al. . Racial and ethnic differences in antibiotic use for viral illness in emergency departments. Pediatrics 2017; 140. doi: 10.1542/peds.2017-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gidengil CA, Mehrotra A, Beach S, et al. . What drives variation in antibiotic prescribing for acute respiratory infections? J Gen Intern Med 2016; 31:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ackerman S, Gonzales R. The context of antibiotic overuse. Ann Intern Med 2012; 157:211–2. [DOI] [PubMed] [Google Scholar]
- 28. Hicks LA, Bartoces MG, Roberts RM, et al. . US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60:1308–16. [DOI] [PubMed] [Google Scholar]
- 29. Ladd E. The use of antibiotics for viral upper respiratory tract infections: an analysis of nurse practitioner and physician prescribing practices in ambulatory care, 1997–2001. J Am Acad Nurse Pract 2005; 17:416–24. [DOI] [PubMed] [Google Scholar]
- 30. Fridkin SK, Srinivasan A. Implementing a strategy for monitoring inpatient antimicrobial use among hospitals in the United States. Clin Infect Dis 2014; 58:401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Centers for Disease Control and Prevention. Antibiotic Use in the United States, 2017: Progress and Opportunities. 2017. Available at: https://www.cdc.gov/antibiotic-use/stewardship-report/outpatient.html. Accessed 9 April 2018. [Google Scholar]
- 32. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. . Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315:1864–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.