Abstract
Inflammatory Bowel Diseases (IBD), represented by Crohn’s Disease (CD) and Ulcerative Colitis (UC), are associated with significant morbidity in western countries and with increasing incidence in the developing world. While analysis of the genome of IBD patients, especially through genome-wide association studies, has unraveled multiple pathways involved in IBD pathogenesis only part of IBD heritability has been explained by genetic studies. This has demonstrated that environmental factors also play a significant role in promoting intestinal inflammation, mostly through their effects in the composition of the microbiome. However, in order for microbial dysbiosis to result in uncontrolled intestinal inflammation, the intestinal barrier formed by intestinal epithelial cells and the innate immune system should also be compromised. Finally, activation of the immune system depends on the working balance between effector and regulatory cells present in the intestinal mucosa, which have also been shown to be dysregulated in this patient population. Therefore, IBD pathogenesis is a result of the interplay of genetic susceptibility, environmental impact on the microbiome that through a weakened intestinal barrier will lead to inappropriate intestinal immune activation. In this review, we will approach the mechanisms proposed to cause IBD from the genetic, environmental, intestinal barrier and the immunologic perspectives.
Keywords: Crohn’s Disease, Environment, Etiology, Genetics, Inflammatory Bowel Diseases, Immunology, Microbiome, Ulcerative Colitis
Introduction
Inflammatory Bowel Diseases (IBD), represented by Crohn’s Disease (CD) and Ulcerative Colitis (UC), are chronic and remitting disorders responsible for causing inflammation of the gastrointestinal tract. These conditions are associated with significant morbidity and mortality, resulting substantial patient burden and costs to health-care systems . Approximately 1.6 million United States residents are affected with IBD, 785,000 with CD and 910,000 with UC (1). Prevalence is higher in developed western countries, with up to 2 million people suffering of these conditions in Europe (2). Interestingly, at the turn of the 21st century newer epidemiologic studies have shown that the incidence of IBD has been rising in developing countries in South America, Asia, Africa and Eastern Europe (1-3). The change in epidemiologic patterns in populations where IBD were not previously common raises again the question of important environmental factors that are involved in the development of these chronic inflammatory disorders.
The increased prevalence in specific populations around the world suggests a significant genetic component to the development of IBD, the new epidemiologic trends also demonstrate that environmental factors play a critical role in the pathogenesis of CD and UC. This may be the result of industrialization of developing countries (2, 3). Most environmental triggers could mediate IBD pathogenesis through their impact on the microbiome (3). However, in order for microbiome changes to result in inappropriate and continuing inflammation, the integrity of the intestinal barrier separating the lumen and the mucosa should also be compromised (4, 5). Finally, activation of the immune system, which would result in the phenotypes seen in clinical practice, depends on the working balance between effector and regulatory cells present in the intestinal mucosa, which have also been shown to be dysregulated in this patient population (6).
Therefore, it is the interplay of genetic susceptibility, environmental impact in the microbiome that through a weakened intestinal barrier will lead to inappropriate immune activation responsible for the clinical and endoscopic findings observed in IBD patients (Figures 1 and 2). In this review, we will approach the mechanisms proposed to cause IBD from the genetic, environmental, intestinal barrier and the immunologic perspectives.
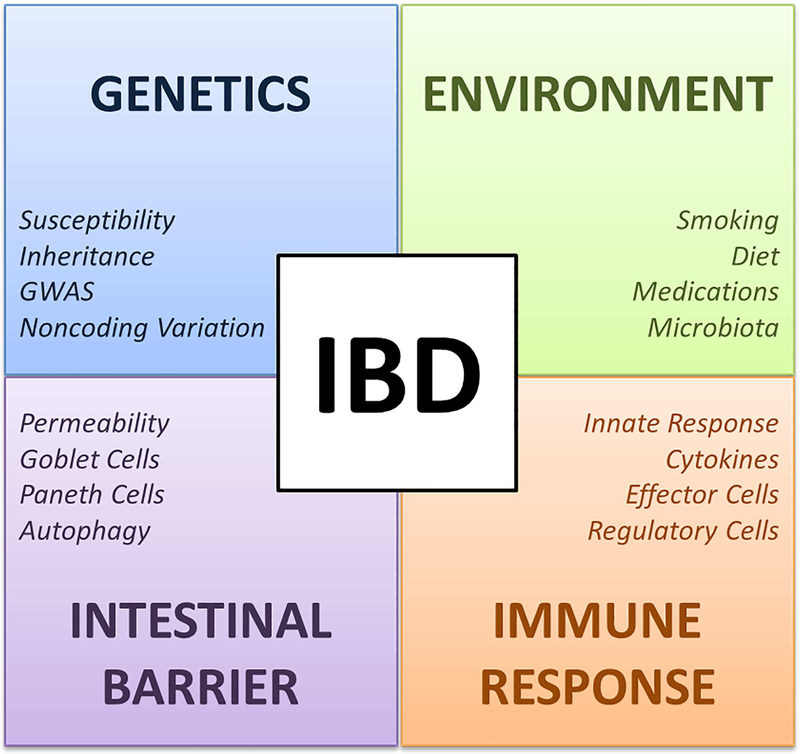
Figure 1. Mechanisms involved in the pathogenesis of Inflammatory Bowel Disease.
IBD pathogenesis is a result of the interplay between genetic, environmental, intestinal barrier and immune response factors. Abbreviations: GWAS, Genome-wide association studies; IBD, Inflammatory Bowel Diseases.
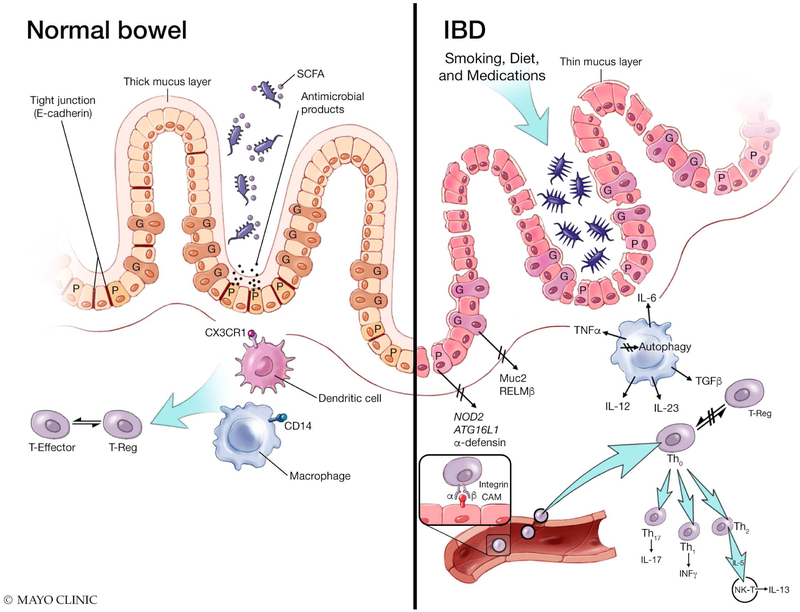
Figure 2. The intestinal mucosa in the normal bowel and IBD.
Upon exposure to environmental factors, patients with IBD develop microbial dysbiosis with decrease of short-chain fatty acids (SCFA) producing bacteria and increase in Proteobacteria. Mechanisms that maintain the intestinal barrier are also disrupted in the IBD mucosa, including: down regulation of E-cadherin in tight junctions; thickness of the mucus layer; abnormal goblet cell function, including Muc2 and RELMβ proteins; and dysfunctional Paneth cells associated mechanisms, including secretion of antimicrobial products, NOD2 and ATG16L1 gene associated functions. From the innate immune system perspective, the IBD mucosa has been shown to exhibit: a decrease in colonic macrophages expressing CD14, defective CX3CR1 antigen presentation by dendritic cells; and impaired Autophagy. Lastly, while leukocyte migration via integrin cellular adhesion molecules (CAMs) interactions also occurs in the normal mucosa, the balance between Effector and regulatory T cells (T-reg) appears to be disturbed in the IBD mucosa, resulting in uncontrolled activation of different T-cell lineages that migrate to the inflamed intestine.
Genetic Factors: Host Susceptibility
Family aggregation of IBD has long been recognized, but the inheritable component seems to be stronger in CD than in UC, with concordance rate in monozygotic twins 30-58% in the first compared to 10-15% in the latter (5, 7, 8). The relative risk of first degree relatives of IBD patients to develop the disease can be up to fivefold higher, when compared to unaffected individuals (9, 10). The study of patients with very early onset IBD, usually with positive family history and demonstrating severe manifestations of the disease, have helped identify rare genetic variants that may interfere with pathways leading to intestinal inflammation (11, 12). For example, a rare mutation affecting the regulatory function of the X-linked inhibitor of apoptosis (XIAP) gene was found to result in early onset IBD refractory to treatment in a 15-month old boy (13). XIAP is a positive regulator of NOD2 function, which is compromised in patients with IBD (14). Furthermore, mutations in the interleukin-10 receptor (IL10R) gene region, which can ultimately manifest as early onset IBD, have also been shown to be characterized by Medelian-like inheritance with highly penetrant variants (15). Nevertheless, childhood onset IBD represents only 10 to 25% of all IBD cases, and not all cases have been associated with these types of genetic abnormalities, suggesting that IBD is likely a complex polygenic disease (16).
The majority of information related to genetics of IBD has been obtained through genome-wide association studies (GWAS) (5, 8, 17). The conceptual basis of GWAS is that complex genetic disorders like IBD are polygenic, being driven by multiple, common genetic polymorphisms (17). Meta-analysis combining GWAS data identified 201 loci associated with IBD (18, 19). There are 41 CD and 30 UC specific loci that may indicate in some of the clinically, endoscopically and histologically differences seen in these patients (18, 19). However, approximately 137 out of 201 loci (68%) are associated with both CD and UC, indicating that these diseases share common inflammatory pathways (19). Interestingly, around seventy percent of IBD-loci are shared with other complex autoimmune and immunodeficiency diseases (19). Almost half of IBD-specific loci are associated with other immune-mediated diseases, with strong overlaps with ankylosing spondylitis and psoriasis, which can often present as extra-intestinal manifestations of CD and UC (19, 20). Genes implicated in the overlap between IBD and primary immunodeficiencies are associated with reduced levels of circulating T-cells or specific T-cell subsets, such as T-helper (Th) 17 cells or regulatory T-cells (Tregs), again supporting an imbalance between effector and regulatory cells in the intestinal mucosa (6, 19, 21).
The benefit of identifying genes and genetic loci implicated in IBD is to understand disease-relevant biological pathways that are crucial for the development of intestinal inflammation (8, 17, 22). Biological processes implicated by IBD specific loci include: barrier function, epithelial restitution, microbial defense, innate immune regulation, reactive oxygen species generation, autophagy, regulation of adaptive immunity, endoplasmic reticulum stress and metabolic pathways associated with cellular hemostasis (8, 22). For example, NOD2 the first gene to be associated with IBD, (23) recognizes the peptidoglycan product muramyl dipeptide (MDP), which modulates both innate and adaptive immune responses (23). Identification of this molecule unraveled the role of the epithelial barrier in the maintaining mucosal hemostasis. NOD2 mutations as well as others, such as polymorphisms within ATG16L1 gene, have also revealed autophagy as an important pathogenic process in IBD (24-26). Furthermore, multiple IBD risk alleles acting at the IL23R loci suggested a role of IL-23 signaling in CD and this pathway is currently targeted by commercially available drugs for the treatment of the disease (27, 28).
Up to 80-90% of GWAS-identified loci, however, are confined to noncoding variation that exerts its pathogenic effects through modulation of gene expression (17). Recent studies are focusing in small intra-nuclear molecules that can broadly regulate gene expression such as epigenetic markers, microRNAs and non-coding RNAs, which have all been implicated in the pathogenesis of IBD through different pathways.
Environmental Factors: The Role of the Microbiome
Despite the aforementioned advances, only about 25% of IBD heritability has been explained by genetic studies (3, 17). Furthermore, new epidemiologic trends of IBD in developing countries following industrialization suggest that environmental factors may play a significant role in promoting intestinal inflammation in genetic susceptible individuals (2, 3).
Smoking has been one of the most studied factors in the setting of IBD. Clinically, cigarette smoking has been shown to be harmful in patients with CD whereas protective to UC patients (8). Different pathways have been hypothesized to explain these findings, including: impairments of autophagy, direct toxicity to immune and mucus producing cells in addition to inducing changes in the microbiome (29-31). Moreover, exposure to diets rich in saturated fatty acids and processed meats has been reported to increase the risk of IBD (32-34). Conversely, a high fiber diet has been demonstrated to reduce the risk of CD by 40% (35). The metabolism of dietary fiber by colonic bacteria into light chain fatty acids that have anti-inflammatory properties could potentially explain this protective effect (36).
The use of medications, most notably antibiotics, has also been associated with increased risk of IBD (37, 38). This association usually results from changes in the intestinal microbiome after use of antibiotics during early stages of life, when the microbiota plays a critical role in shaping immune cell development (39). Non-steroidal anti-inflammatory drugs, anti-contraceptives and statins are other examples of medications that have been associated with up to two-fold-increased risk of CD and UC (40-42). Early life events such as mode of delivery, breast feeding, exposure to pets and infections (“hygiene hypothesis”) are also factors associated with significant risk of development of IBD, mostly given influences in the composition of the intestinal microbiota (43-46).
The intestinal microbiome has been long recognized to establish the connection between the outside environment and the intestinal mucosa. Microbial dysbiosis, as a result of decreased diversity of the microbiome, has been described in patients with IBD (47, 48). Whether this is the cause or consequence of the observed intestinal inflammation, or both, is yet to be determined (47). In IBD patients, there is a decrease of bacteria with anti-inflammatory capacities and an increase of bacteria with inflammatory capacities, when compared to healthy individuals (48, 49). The most frequently observed changes include a decrease of Firmicutes and increase in Proteobacteria and Bacterioidetes(4). The number of short-chain fatty acids producing bacteria (eg. (Faecalibacterium prausnitzzi) has been shown to be decreased in IBD patients, consequently affecting differentiation and expansion of Tregs as well as growth of epithelial cells (50). Interestingly, the lower number of F.praunitzii colonization has been shown to correlate with the risk of ileal CD after surgery and maintenance of clinical remission in UC (51-53). In contrast, the increase in Proteobacteria, most notably E. coli, with the ability to adhere to the intestinal epithelium affects the permeability of the intestine, alters the diversity and composition of the microbiota, and induces inflammatory responses by regulating the expression of inflammatory genes (54). Furthermore, the number of mucolytic bacteria seem to be increased in IBD, which consequently leads to higher presence of mucosa-associated bacteria in these patients (55, 56). Lastly, the number of sulfate-reducing bacteria, such as Desulfovibrio, is also increased in IBD resulting in the production of hydrogen-sulfate that damages the intestinal barrier and allows activation of mucosal inflammation (57).
The Intestinal Barrier: Epithelium and Innate Immunity
The intestinal mucosa exists in a functional equilibrium with the luminal contents, and disturbance of this equilibrium can lead to pathology such as IBD. The Intestinal Barrier composed by intestinal epithelial cells (IEC) and innate immune cells, maintains this balance between luminal contents and the mucosa. The importance of the epithelial barrier in IBD predisposition is supported by the finding of abnormal intestinal permeability in patients with CD and some of their first-degree relatives (58-61). Furthermore, analysis of intestinal biopsies from patients with CD has demonstrated down regulation of the junctional protein E-cadherin, which comprises the tight junctions of this physical barrier (62, 63). Other studies have also associated IBD with several transcription factors involved in epithelial regeneration, such as HNF4A and NKX2-3(64, 65).
Beyond just a promoting physical barrier between lumen and mucosa, IEC of the intestinal barrier consist of different cell types that maintain the equilibrium lumen-mucosa through different mechanisms, these include: enteroabsortive cells, goblet cells, neuroendocrine cells, Paneth cells and M cells (Figure 2) (66). Goblet-cells produce the mucus matrix covering the epithelium, essential to both mucosal defense and repair (67). Genetic deletion of Muc2, a major goblet-cell-derived secretory mucin, results in spontaneous colitis in murine models (68). RELMβ, another goblet cell specific protein, creates a bridge with the immune system by directing Th2 immunity and delivering luminal antigens to tolerogenic sets of dendritic cells (69, 70). Disturbance of the RELMβ gene reduces the severity of colitis in murine models (71).
While Goblet-cells seem to play a part in protecting from colitis developement, defects in Paneth Cell biology are associated with increased risk for CD (72, 73). Paneth cells reside at the base of small intestinal crypts and are responsible for crypt homeostasis, maintenance of intestinal stem-cell niche as well as secretion of antimicrobial effectors that control the equilibrium between microbiota and mucosa (73). The pathways of several key genetic risk factors of IBD impair Paneth cell function leading to colitis, most notably: NOD2 and autophagy. NOD2 is expressed by Paneth cells, dendritic Cells, macrophages and absorptive IEC. NOD2 risk variants are associated with lower levels of α-defensins in Paneth cells leading to impaired antimicrobial function (74). Not only through NOD2 but also via CD risk locus ATG16L1, Paneth cells function in autophagy is compromised in patients with CD (25, 75). Autophagy is an intracellular “self-cannibalism” that involves degradation and recycling of cytosolic contents and organelles, as well as resistance against infection and removal of intracellular microbes (76). Patients with ATG16L1and NOD2 mutations, as well equivalent murine models, demonstrate aberrancies in the secretory apparatus of Paneth cells resulting in defects in antibacterial autophagy (77). In dendritic cells, defects in these genes also result in an impaired ability to present exogenous antigens to T-cells (26).
Dendritic cells, macrophages, innate lymphoid cells and neutrophils complement the physical and functional barrier of IEC, as the first line of defense of a well-developed mucosal innate immune system. In the healthy human gut, intestinal macrophages, which are characterized by lack of CD14 expression, exist on a state of hyporesponsiveness, showing attenuated proliferation and chemotactic activity in response to either microbial ligands or host cytokines, while retaining the phagocytic and bactericidal function (78, 79). Furthermore, they also have the capacity of producing anti-inflammatory cytokines that promote regulatory T-cell differentiation and restrain Th1 and Th17 responses (80). Patients with CD have defective innate immune responses, including attenuated macrophage activity in vitro and impaired neutrophil recruitment, facilitating microbe passage through the mucosa (81). These patients also present with another inflammatory macrophage population that expresses dendritic cell markers, including CD14, and produce large amounts of pro-inflammatory cytokines, such as TNF-α and IL-6 (82). In contrast to phagocytic macrophages, dendritic cells constitute an interface for monitoring the environment and relaying signals to initiate appropriate adaptive responses (83). Sampling of bacteria by resident dendritic cells is mediated in part by a CX3CR1-dependent mechanism that permits direct dendritic-cell-microbe-contact (84). Genetic deletion of CX3CR1 results in decreased numbers of lamina propria macrophages and increased translocation of bacteria to mesenteric lymph nodes (85). Dendritic cells have also been shown to accumulate both in the mucosa of IBD patients and blockage of CD40/CD40L, between DC and effector T-cell populations, prevents experimental T cell-mediated colitis (86, 87).
Adaptive Immune Response: Effector and Regulatory Cells
Upon encounter with antigen, and microbial products that gain access through the intestinal barrier, dendritic cells and other antigen presenting cells initiate a cascade of pro- and anti-inflammatory signals to activate different subsets of local and circulating lymphocytes to migrate to effector sites where inflammation takes place. Migration of leukocytes to the inflamed intestine occurs via binding of integrin molecules located on leukocyte surface to cellular adhesion molecules (CAMs) expressed on the surface of endothelial cells (88). These endothelial cells, as a reflex of inflammatory signals in the mucosa, can also produce chemokines to attract leukocytes to sites where inflammation is taking place (89). The integrins αLβ2, α4β1, α4β7 and αEβ7 are heterodimeric receptors expressed on the surface of circulating leukocytes capable of interacting with different adhesions molecules for specific leukocyte trafficking to the intestine (88). Currently, multiple commercially available drugs are successfully used in clinical practice to target these molecules in order to prevent leukocyte migration to the intestine and control inflammation in patients with IBD (90, 91). New therapies that control inflammation in patients with CD and UC target endothelial cells CAMs or preventing lymphocyte egression from lymph nodes are currently under evaluation in clinical trials (89, 92, 93).
The disturbed balance between anti and pro-inflammatory signals with consequent migration of leukocytes to the intestinal mucosa results in and is perpetuated by an exaggerated T cell immune response, which is seen in both CD and UC (Figure 2). The T cells involved on immune responses in both diseases, however, seem to be different, what may explain phenotypic differences seen in clinical practice as well as response to new targeted therapies. Whereas the intercalated transmural inflammation of CD is resultant from an excessive Th1 and Th17 response, the uniform mucosal inflammation seen in UC patients is secondary to a Th2-type-like cytokine profile (22). In UC, increased secretion of the Th2-specific cytokine IL-5 is associated with more efficient activation of B cells and the induction of immune responses when compared to the Th1-response seen in CD patients (22, 94). Interestingly, UC-specific Th cells only show low levels of IL-4 production, which suggest they do not display all the features of classical Th2 cells (94, 95). Instead, UC is associated with the presence of CD1d-restricted non-classical natural killer T cells that produce IL-13, which is increased in the lamina of UC patients but not CD patients (22, 95).
Anti-IL13 inhibitors have shown conflicting results in treating patients with UC in clinical trials (96, 97).
In CD, the differentiation of Th type 1 and 17 occurs in response to the production of IL-12, IL-18, IL-23 and transforming growth factor beta (TGFβ) by antigen presenting cells (APC) and macrophages. In turn Th1 and Th17 cells secrete the pro-inflammatory cytokines IL-17, INF-γ and TNFa that feed into a self-sustaining amplification cycle whereby they stimulate APC, macrophages, fibroblasts and endothelial cells to produce TNFα, IL-1, IL-6, IL-8, IL-12 and IL-18 (5, 22, 98). Studies have shown that mice deficient in IL-12 p40 subunit and deficient in IL-23 are resistant to experimentally induced colitis suggesting that these cytokines are in the center of the CD inflammatory pathway (99, 100). Therapeutic agents targeting the shared p40 subunit between IL-12 and IL23 are currently used in clinical practice to control intestinal inflammation and new therapies targeting the IL-23 specific p19 subunit are currently under clinical evaluation (101, 102). Conversely, drugs directed to the Th17 specific cytokine IL-17A have demonstrated that they may worsen the inflammation in patients with CD (103). In addition to secretion of effector cytokines, Th17 cells also fulfill important homeostatic functions in the intestine, which may explain these conflicting results. In this scenario, IL-17A may also play a role in protecting barrier integrity and regulatory T-cell function, which are crucial to controlling immune activation and limiting inflammatory responses(104).
Treg cells and Th17 have opposing activities but arise from a common precursor upon TGF-β stimulation. The abundance of TGF-β in intestinal tissues contributes to normal homeostasis by promoting Treg-cell differentiation in naive lamina propria CD4+ T cells (105). In inflammatory states such as IBD, Th17-cell differentiation is promoted instead as a combination of mucosal TGF-β with other signals, including cytokines, metabolites and microbial signals (106). Therefore, the gut may use TGF-β pathways to promote T cells to carry out both pro- and anti-inflammatory programs depending on the local presence of cytokines and microbial products which may consequently affect initiation, persistence and relapses in human IBD. Many of the genes required for Treg- and TH17-cell differentiation have been implicated in IBD pathogenesis (8). In experimental models, inflammation driven by Th17 cells leads to colitis and can be suppressed by regulatory T cells. In this setting, Treg cells have not only an important function in restraining effector T cell populations, but also controlling innate inflammatory mechanisms(107). Loss-of-function mutations in the key Treg transcription factor FOXP3 results in IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked), which can be accompanied by intestinal inflammation (108). Treg cells are expanded in both inflamed and non-inflamed UC and could have a critical role in modulating the clinical spectrum of the disease (109). Therefore, the use of regulatory T-cells (Tregs) has been proposed as an alternative strategy to control excessive inflammation in IBD (110).
New immuno-therapies currently in in development and clinical trials, aim to reduce the inflammatory response of IBD by blocking downstream signaling pathways associated with different cytokines. Janus kinases (JAK) are a family of tyrosine kinases (JAK1, JAK2, JAK3 and TYK2) that working in paris, mediate intracellular communication between cytokine receptors and nuclear signals(111). These molecules act as a bridge between cytokine receptors and intra-nuclear proteins, most notably signal transducer and activator of transcription factors (STATs). The seven different members of the STAT family (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6), combine in pairs with different JAKs in order to mediate intracellular effects of specific cytokine pathways and are, therefore, potential therapeutic targets in the modulation of inflammation (112). In the case of IL-12 and IL-23, which are important cytokines in the pathogenesis of CD, JAK2 and TYK are activated by the membrane receptors and will consequently recruit STAT3 and STAT4 to deliver intra-nuclear signals associated with controlling Th1/Th17 responses seen in these patients (100, 111). Recent studies have also shown promising results of JAK-inhibitors in controlling inflammation in refractory UC (113).
Future Perspectives: Targeted Evaluation and Therapy
Understanding the mechanisms that lead to IBD pathogenesis is crucial in order to find new therapeutic strategies to improve patient therapies. Beyond identifying new pathways amendable for therapeutic intervention, new genomic studies should also aim to assist with risk stratification and possibly understanding different responses to available therapies and the development of more personalized medicine. Aiming for this targeted evaluation, from an environmental perspective, studies should attempt to characterize the composition of the microbiome, both quantitatively and functionally (metabolome), in order to assess how that interferes with outcomes of intestinal inflammation and response to therapy. Modulation of the microbiome has already shown promising results in small trials of fecal microbiota transplantation, mostly in patients with UC (114). Furthermore, the use of cell-based therapies, most notably stem cells, is in the horizon of individualized IBD therapeutic with the purpose of assisting with mucosal healing, recovery of the intestinal barrier and control aberrant immune responses (115). Lastly, with a variety of immunotherapies approaching clinical practice, future studies should consider an individualized treatment approach based on specific features of each individual’s inflammatory response, since inflammatory responses vary from patient to patient as well as over time in a single patient.
Acknowledgments:
KA Papadakis is supported by Grant R03AI131011-01A1 from NIAID
Abbreviations
- APC
Antigen presenting cells
- CAMs
Cellular adhesion molecules
- CD
Crohn’s Disease
- GWAS
Genome-wide association studies
- IBD
Inflammatory Bowel Diseases
- IEC
intestinal epithelial cells
- JAK
Janus kinases
- SCFA
Short-chain fatty acids
- STAT
Signal transducer and activator of transcription factors
- Th
T- helper
- T-regs
Regulatory T-cells
- TGFβ
Transforming growth factor beta
- UC
Ulcerative Colitis
- XIAP
X-linked inhibitor of apoptosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Aniwan S, Park SH, Loftus EV Jr.. Epidemiology, Natural History, and Risk Stratification of Crohn's Disease. Gastroenterol Clin North Am. 2017;46(3):463–80. [DOI] [PubMed] [Google Scholar]
- 2.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390(10114):2769–78. [DOI] [PubMed] [Google Scholar]
- 3.Shouval DS, Rufo PA. The Role of Environmental Factors in the Pathogenesis of Inflammatory Bowel Diseases: A Review. JAMA Pediatr. 2017;171(10):999–1005. [DOI] [PubMed] [Google Scholar]
- 4.Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11(1):1–10. [DOI] [PubMed] [Google Scholar]
- 5.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–34. [DOI] [PubMed] [Google Scholar]
- 6.Cader MZ, Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut. 2013;62(11):1653–64. [DOI] [PubMed] [Google Scholar]
- 7.Spehlmann ME, Begun AZ, Burghardt J, Lepage P, Raedler A, Schreiber S. Epidemiology of inflammatory bowel disease in a German twin cohort: results of a nationwide study. Inflamm Bowel Dis. 2008;14(7):968–76. [DOI] [PubMed] [Google Scholar]
- 8.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474(7351):307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orholm M, Munkholm P, Langholz E, Nielsen OH, Sorensen TI, Binder V. Familial occurrence of inflammatory bowel disease. N Engl J Med. 1991;324(2):84–8. [DOI] [PubMed] [Google Scholar]
- 10.Tysk C, Lindberg E, Jarnerot G, Floderus-Myrhed B. Ulcerative colitis and Crohn's disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking. Gut. 1988;29(7):990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruemmele FM, El Khoury MG, Talbotec C, Maurage C, Mougenot JF, Schmitz J, et al. Characteristics of inflammatory bowel disease with onset during the first year of life. J Pediatr Gastroenterol Nutr. 2006;43(5):603–9. [DOI] [PubMed] [Google Scholar]
- 12.Bianco AM, Zanin V, Girardelli M, Magnolato A, Martelossi S, Tommasini A, et al. A common genetic background could explain early-onset Crohn's disease. Med Hypotheses. 2012;78(4):520–2. [DOI] [PubMed] [Google Scholar]
- 13.Worthey EA, Mayer AN, Syverson GD, Helbling D, Bonacci BB, Decker B, et al. Making a definitive diagnosis: successful clinical application of whole exome sequencing in a child with intractable inflammatory bowel disease. Genet Med. 2011;13(3):255–62. [DOI] [PubMed] [Google Scholar]
- 14.Krieg A, Correa RG, Garrison JB, Le Negrate G, Welsh K, Huang Z, et al. XIAP mediates NOD signaling via interaction with RIP2. Proc Natl Acad Sci U S A. 2009;106(34):14524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361(21):2033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uhlig HH. Monogenic diseases associated with intestinal inflammation: implications for the understanding of inflammatory bowel disease. Gut. 2013;62(12):1795–805. [DOI] [PubMed] [Google Scholar]
- 17.McGovern DP, Kugathasan S, Cho JH. Genetics of Inflammatory Bowel Diseases. Gastroenterology. 2015;149(5):1163–76 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106(23):9362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Union of Immunological Societies Expert Committee on Primary I, Notarangelo LD, Fischer A, Geha RS, Casanova JL, Chapel H, et al. Primary immunodeficiencies: 2009 update. J Allergy Clin Immunol. 2009;124(6):1161–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3(7):521–33. [DOI] [PubMed] [Google Scholar]
- 23.Shaw MH, Kamada N, Warner N, Kim YG, Nunez G. The ever-expanding function of NOD2: autophagy, viral recognition, and T cell activation. Trends Immunol. 2011;32(2):73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuballa P, Huett A, Rioux JD, Daly MJ, Xavier RJ. Impaired autophagy of an intracellular pathogen induced by a Crohn's disease associated ATG16L1 variant. PLoS One. 2008;3(10):e3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Travassos LH, Carneiro LA, Ramjeet M, Hussey S, Kim YG, Magalhaes JG, et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11(1):55–62. [DOI] [PubMed] [Google Scholar]
- 26.Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16(1):90–7. [DOI] [PubMed] [Google Scholar]
- 27.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314(5804):1461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandborn WJ, Feagan BG, Fedorak RN, Scherl E, Fleisher MR, Katz S, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn's disease. Gastroenterology. 2008;135(4): 1130–41. [DOI] [PubMed] [Google Scholar]
- 29.Allais L, Kerckhof FM, Verschuere S, Bracke KR, De Smet R, Laukens D, et al. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ Microbiol. 2016;18(5):1352–63. [DOI] [PubMed] [Google Scholar]
- 30.Monick MM, Powers LS, Walters K, Lovan N, Zhang M, Gerke A, et al. Identification of an autophagy defect in smokers' alveolar macrophages. J Immunol. 2010;185(9):5425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sher ME, Bank S, Greenberg R, Sardinha TC, Weissman S, Bailey B, et al. The influence of cigarette smoking on cytokine levels in patients with inflammatory bowel disease. Inflamm Bowel Dis. 1999;5(2):73–8. [DOI] [PubMed] [Google Scholar]
- 32.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106(4):563–73. [DOI] [PubMed] [Google Scholar]
- 33.Jowett SL, Seal CJ, Pearce MS, Phillips E, Gregory W, Barton JR, et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut. 2004;53(10):1479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Fuchs CS, et al. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn's disease. Gut. 2014;63(5):776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, et al. A prospective study of long-term intake of dietary fiber and risk of Crohn's disease and ulcerative colitis. Gastroenterology. 2013;145(5):970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3(10):858–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am J Gastroenterol. 2010;105(12):2687–92. [DOI] [PubMed] [Google Scholar]
- 38.Selby W, Pavli P, Crotty B, Florin T, Radford-Smith G, Gibson P, et al. Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn's disease. Gastroenterology. 2007;132(7):2313–9. [DOI] [PubMed] [Google Scholar]
- 39.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khalili H, Higuchi LM, Ananthakrishnan AN, Richter JM, Feskanich D, Fuchs CS, et al. Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut. 2013;62(8):1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ananthakrishnan AN, Higuchi LM, Huang ES, Khalili H, Richter JM, Fuchs CS, et al. Aspirin, nonsteroidal anti-inflammatory drug use, and risk for Crohn disease and ulcerative colitis: a cohort study. Ann Intern Med. 2012;156(5):350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kvasnovsky CL, Aujla U, Bjarnason I. Nonsteroidal anti-inflammatory drugs and exacerbations of inflammatory bowel disease. Scand J Gastroenterol. 2015;50(3):255–63. [DOI] [PubMed] [Google Scholar]
- 43.Bager P, Simonsen J, Nielsen NM, Frisch M. Cesarean section and offspring's risk of inflammatory bowel disease: a national cohort study. Inflamm Bowel Dis. 2012;18(5):857–62. [DOI] [PubMed] [Google Scholar]
- 44.Ng SC, Tang W, Leong RW, Chen M, Ko Y, Studd C, et al. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut. 2015;64(7):1063–71. [DOI] [PubMed] [Google Scholar]
- 45.Chu KM, Watermeyer G, Shelly L, Janssen J, May TD, Brink K, et al. Childhood helminth exposure is protective against inflammatory bowel disease: a case control study in South Africa. Inflamm Bowel Dis. 2013;19(3):614–20. [DOI] [PubMed] [Google Scholar]
- 46.Cholapranee A, Ananthakrishnan AN. Environmental Hygiene and Risk of Inflammatory Bowel Diseases: A Systematic Review and Meta-analysis. Inflamm Bowel Dis. 2016;22(9):2191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sartor RB, Wu GD. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology. 2017;152(2):327–39 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe. 2008;3(6):417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–6. [DOI] [PubMed] [Google Scholar]
- 51.Varela E, Manichanh C, Gallart M, Torrejon A, Borruel N, Casellas F, et al. Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Aliment Pharmacol Ther. 2013;38(2):151–61. [DOI] [PubMed] [Google Scholar]
- 52.Fujimoto T, Imaeda H, Takahashi K, Kasumi E, Bamba S, Fujiyama Y, et al. Decreased abundance of Faecalibacterium prausnitzii in the gut microbiota of Crohn's disease. J Gastroenterol Hepatol. 2013;28(4):613–9. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi K, Nishida A, Fujimoto T, Fujii M, Shioya M, Imaeda H, et al. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn's Disease. Digestion. 2016;93(1):59–65. [DOI] [PubMed] [Google Scholar]
- 54.Ahmed I, Roy BC, Khan SA, Septer S, Umar S. Microbiome, Metabolome and Inflammatory Bowel Disease. Microorganisms. 2016;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Waaij LA, Harmsen HJ, Madjipour M, Kroese FG, Zwiers M, van Dullemen HM, et al. Bacterial population analysis of human colon and terminal ileum biopsies with 16S rRNA-based fluorescent probes: commensal bacteria live in suspension and have no direct contact with epithelial cells. Inflamm Bowel Dis. 2005;11(10):865–71. [DOI] [PubMed] [Google Scholar]
- 56.Schultsz C, Van Den Berg FM, Ten Kate FW, Tytgat GN, Dankert J. The intestinal mucus layer from patients with inflammatory bowel disease harbors high numbers of bacteria compared with controls. Gastroenterology. 1999;117(5):1089–97. [DOI] [PubMed] [Google Scholar]
- 57.Png CW, Linden SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105(11):2420–8. [DOI] [PubMed] [Google Scholar]
- 58.Soderholm JD, Olaison G, Peterson KH, Franzen LE, Lindmark T, Wiren M, et al. Augmented increase in tight junction permeability by luminal stimuli in the non-inflamed ileum of Crohn's disease. Gut. 2002;50(3):307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, et al. Genetic basis for increased intestinal permeability in families with Crohn's disease: role of CARD15 3020insC mutation? Gut. 2006;55(3):342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Irvine EJ, Marshall JK. Increased intestinal permeability precedes the onset of Crohn's disease in a subject with familial risk. Gastroenterology. 2000;119(6):1740–4. [DOI] [PubMed] [Google Scholar]
- 61.May GR, Sutherland LR, Meddings JB. Is small intestinal permeability really increased in relatives of patients with Crohn's disease? Gastroenterology. 1993; 104(6) :1627–32. [DOI] [PubMed] [Google Scholar]
- 62.Muise AM, Walters TD, Glowacka WK, Griffiths AM, Ngan BY, Lan H, et al. Polymorphisms in E-cadherin (CDH1) result in a mis-localised cytoplasmic protein that is associated with Crohn's disease. Gut. 2009;58(8):1121–7. [DOI] [PubMed] [Google Scholar]
- 63.Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermuller N, et al. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281(1):G216–28. [DOI] [PubMed] [Google Scholar]
- 64.Darsigny M, Babeu JP, Dupuis AA, Furth EE, Seidman EG, Levy E, et al. Loss of hepatocyte-nuclear-factor-4alpha affects colonic ion transport and causes chronic inflammation resembling inflammatory bowel disease in mice. PLoS One. 2009;4(10):e7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pabst O, Zweigerdt R, Arnold HH. Targeted disruption of the homeobox transcription factor Nkx2-3 in mice results in postnatal lethality and abnormal development of small intestine and spleen. Development. 1999;126(10):2215–25. [DOI] [PubMed] [Google Scholar]
- 66.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60. [DOI] [PubMed] [Google Scholar]
- 67.Johansson ME, Ambort D, Pelaseyed T, Schutte A, Gustafsson JK, Ermund A, et al. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci. 2011;68(22):3635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(1):117–29. [DOI] [PubMed] [Google Scholar]
- 69.Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206(13):2947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483(7389):345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McVay LD, Keilbaugh SA, Wong TM, Kierstein S, Shin ME, Lehrke M, et al. Absence of bacterially induced RELMbeta reduces injury in the dextran sodium sulfate model of colitis. J Clin Invest. 2006;116(11):2914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Itoh H, Beck PL, Inoue N, Xavier R, Podolsky DK. A paradoxical reduction in susceptibility to colonic injury upon targeted transgenic ablation of goblet cells. J Clin Invest. 1999;104(11):1539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol. 2007;19(2):70–83. [DOI] [PubMed] [Google Scholar]
- 74.Fritz T, Niederreiter L, Adolph T, Blumberg RS, Kaser A. Crohn's disease: NOD2, autophagy and ER stress converge. Gut. 2011;60(11):1580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39(2):207–11. [DOI] [PubMed] [Google Scholar]
- 76.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456(7219):259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206:149–59. [DOI] [PubMed] [Google Scholar]
- 80.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8(10):1086–94. [DOI] [PubMed] [Google Scholar]
- 81.Smith AM, Rahman FZ, Hayee B, Graham SJ, Marks DJ, Sewell GW, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn's disease. J Exp Med. 2009;206(9):1883–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118(6):2269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rescigno M Intestinal dendritic cells. Adv Immunol. 2010;107:109–38. [DOI] [PubMed] [Google Scholar]
- 84.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307(5707):254–8. [DOI] [PubMed] [Google Scholar]
- 85.Medina-Contreras O, Geem D, Laur O, Williams IR, Lira SA, Nusrat A, et al. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest. 2011;121(12):4787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hart AL, Al-Hassi HO, Rigby RJ, Bell SJ, Emmanuel AV, Knight SC, et al. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129(1):50–65. [DOI] [PubMed] [Google Scholar]
- 87.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25(2):309–18. [DOI] [PubMed] [Google Scholar]
- 88.Arseneau KO, Cominelli F. Targeting leukocyte trafficking for the treatment of inflammatory bowel disease. Clin Pharmacol Ther. 2015;97(1):22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Danese S, Panes J. Development of drugs to target interactions between leukocytes and endothelial cells and treatment algorithms for inflammatory bowel diseases. Gastroenterology. 2014;147(5):981–9. [DOI] [PubMed] [Google Scholar]
- 90.Jovani M, Danese S. Vedolizumab for the treatment of IBD: a selective therapeutic approach targeting pathogenic a4b7 cells. Curr Drug Targets. 2013;14(12):1433–43. [DOI] [PubMed] [Google Scholar]
- 91.Vermeire S, O'Byrne S, Keir M, Williams M, Lu TT, Mansfield JC, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384(9940):309–18. [DOI] [PubMed] [Google Scholar]
- 92.Vermeire S, Ghosh S, Panes J, Dahlerup JF, Luegering A, Sirotiakova J, et al. The mucosal addressin cell adhesion molecule antibody PF-00547,659 in ulcerative colitis: a randomised study. Gut. 2011;60(8):1068–75. [DOI] [PubMed] [Google Scholar]
- 93.Sandborn WJ, Feagan BG, Wolf DC, D'Haens G, Vermeire S, Hanauer SB, et al. Ozanimod Induction and Maintenance Treatment for Ulcerative Colitis. N Engl J Med. 2016;374(18):1754–62. [DOI] [PubMed] [Google Scholar]
- 94.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157(3):1261–70. [PubMed] [Google Scholar]
- 95.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14(5):329–42. [DOI] [PubMed] [Google Scholar]
- 96.Danese S, Rudzinski J, Brandt W, Dupas JL, Peyrin-Biroulet L, Bouhnik Y, et al. Tralokinumab for moderate-to-severe UC: a randomised, double-blind, placebo- controlled, phase IIa study. Gut. 2015;64(2):243–9. [DOI] [PubMed] [Google Scholar]
- 97.Reinisch W, Panes J, Khurana S, Toth G, Hua F, Comer GM, et al. Anrukinzumab, an anti-interleukin 13 monoclonal antibody, in active UC: efficacy and safety from a phase IIa randomised multicentre study. Gut. 2015;64(6): 894–900. [DOI] [PubMed] [Google Scholar]
- 98.Nanau RM, Neuman MG. Metabolome and inflammasome in inflammatory bowel disease. Transl Res. 2012;160(1):1–28. [DOI] [PubMed] [Google Scholar]
- 99.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 Protein Engages IL-12p40 to Form a Cytokine, IL-23, with Biological Activities Similar as Well as Distinct from IL-12. Immunity. 2000;13(5):715–25. [DOI] [PubMed] [Google Scholar]
- 100.Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21(7):719–29. [DOI] [PubMed] [Google Scholar]
- 101.Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, et al. Ustekinumab as Induction and Maintenance Therapy for Crohn's Disease. N Engl J Med. 2016;375(20):1946–60. [DOI] [PubMed] [Google Scholar]
- 102.Panaccione R, Sandborn WJ, Gordon GL, Lee SD, Safdi A, Sedghi S, et al. Briakinumab for treatment of Crohn's disease: results of a randomized trial. Inflamm Bowel Dis. 2015;21(6):1329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013;8:477–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467(7318):967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. [DOI] [PubMed] [Google Scholar]
- 108.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X- linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27(1): 18–20. [DOI] [PubMed] [Google Scholar]
- 109.Yu QT, Saruta M, Avanesyan A, Fleshner PR, Banham AH, Papadakis KA. Expression and functional characterization of FOXP3+ CD4+ regulatory T cells in ulcerative colitis. Inflamm Bowel Dis. 2007;13(2):191–9. [DOI] [PubMed] [Google Scholar]
- 110.Himmel ME, Yao Y, Orban PC, Steiner TS, Levings MK. Regulatory T-cell therapy for inflammatory bowel disease: more questions than answers. Immunology. 2012; 136(2):115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Galien R Janus kinases in inflammatory bowel disease: Four kinases for multiple purposes. Pharmacol Rep. 2016;68(4):789–96. [DOI] [PubMed] [Google Scholar]
- 112.O'Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2017;376(18):1723–36. [DOI] [PubMed] [Google Scholar]
- 114.Paramsothy S, Paramsothy R, Rubin DT, Kamm MA, Kaakoush NO, Mitchell HM, et al. Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J Crohns Colitis. 2017;11(10):1180–99. [DOI] [PubMed] [Google Scholar]
- 115.Dave M, Mehta K, Luther J, Baruah A, Dietz AB, Faubion WA Jr.. Mesenchymal Stem Cell Therapy for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Inflamm Bowel Dis. 2015;21(11):2696–707. [DOI] [PMC free article] [PubMed] [Google Scholar]