Abstract
Cross-sectional associations between attention deficit hyperactivity disorder (ADHD) and posttraumatic stress disorder (PTSD) have been observed, but longitudinal studies assessing this association are lacking. This prospective study evaluated the association between predeployment ADHD and postdeployment PTSD among U.S. Army soldiers. Soldiers who deployed to Afghanistan were surveyed before deployment (T0) and approximately 1 month (T1), 3 months (T2), and 9 months (T3) after their return. Logistic regression was performed to estimate the association between predeployment ADHD and postdeployment (T2 or T3) PTSD among 4,612 soldiers with data at all waves and no record of stimulant medication treatment during the study. To evaluate specificity of the ADHD–PTSD association, we examined associations among predeployment ADHD, postdeployment major depressive episode (MDE), generalized anxiety disorder (GAD), and suicidal ideation. Weighted prevalence of ADHD predeployment was 6.1% (SE = 0.4%). Adjusting for other risk factors, predeployment ADHD was associated with risk of postdeployment PTSD, adjusted odds ratio (AOR) = 2.13, 95% CI [1.51, 3.00], p < .001, including incidence among soldiers with no predeployment history of PTSD, AOR = 2.50, 95% CI [1.69, 3.69], p < .001. ADHD was associated with postdeployment MDE, AOR = 2.80, 95% CI [2.01, 3.91], p < .001, and GAD, AOR = 3.04, 95% CI [2.10, 4.42], p < .001, but not suicidal ideation. Recognition of associations between predeployment ADHD and postdeployment PTSD, MDE, and GAD may inform targeted prevention efforts. Future research should examine whether treatment of ADHD is protective against PTSD and related disorders in trauma-exposed individuals.
Posttraumatic stress disorder (PTSD) has been termed one of the “signature wounds” of the recent military conflicts in Iraq and Afghanistan (Tanielian & Jaycox, 2008). The prevalence rate of PTSD among veterans of these conflicts has been estimated to be between 5–20% (Ramchand et al., 2010). Although combat exposure has been consistently associated with risk for PTSD (Ramchand et al., 2010) and appears to act in a dose–dependent fashion (Hoge et al., 2004), most exposed individuals do not develop the disorder. Individual characteristics play a crucial role in conferring additional risk or resilience in the face of exposure to trauma (Kessler, Rose et al., 2014). Further elucidation of these individual factors will help identify those most vulnerable to the disorder while highlighting possible targets for intervention at the mechanistic level. These considerations are lent added urgency in light of the modest efficacy of current treatments for PTSD (Institute of Medicine, 2008) and the near absence of evidence-based PTSD prevention strategies (Howlett & Stein, 2016).
Preexisting psychiatric history has been consistently implicated as an important risk factor for PTSD (Brewin, Andrews, & Valentine, 2000). One disorder often comorbid with PTSD is attention deficit hyperactivity disorder (ADHD; (A. E. Spencer et al., 2016). Within the last decade, adult ADHD has received increasing attention as a common and often seriously impairing disorder. Along with PTSD and intermittent explosive disorder, ADHD is among the three most common psychiatric conditions in nondeployed U.S. Army soldiers, with a prevalence of 7.0% (Kessler, Heeringa et al., 2014). However, adult ADHD is often unrecognized; only 10.9% of individuals who meet ADHD criteria in the general population have received treatment within the last 12 months, a much lower proportion that which has been reported for other psychiatric conditions (Kessler et al., 2006).
Cross-sectional studies have demonstrated an association between ADHD and PTSD (A. E. Spencer et al., 2016). Given that the onset of ADHD is typically earlier than that of PTSD, these findings have been taken to indicate that ADHD is an antecedent risk factor for PTSD. Furthermore, studies restricted to individuals exposed to trauma have also found an association between ADHD and PTSD, which suggests that ADHD may represent a vulnerability in the face of trauma rather than merely increasing the risk of trauma exposure through greater impulsivity and risk-taking (A. E. Spencer et al., 2016).
Few prospective studies have examined the link between ADHD and PTSD. The authors of one study found no association between ADHD in children and either trauma exposure or PTSD at 4-year follow-up (Wozniak et al., 1999). However, a larger study in children found a significant association between ADHD and increased risk of PTSD at 10-year follow-up (odds ratio [OR] = 2.23; (Biederman et al., 2014). An analysis that examined active duty U.S. military members who were participating in the Millennium Cohort Study found that the use of stimulant medications (a first line treatment for ADHD), but not an ADHD diagnosis itself, was predictive of new-onset PTSD at follow-up (Crum-Cianflone et al., 2015). An important limitation of that study was that ADHD diagnosis was based on medical record review, which might have underestimated the prevalence of the disorder.
More large, high-quality prospective studies are needed to examine the association between ADHD and PTSD in adults. Additionally, a possible link between ADHD and risk of other mental disorders in the context of trauma exposure has been largely unexplored. In the present study, we examined the association between predeployment ADHD and postdeployment PTSD and related disorders using data from the Pre/Post Deployment Study (PPDS), a prospective, longitudinal component of the Army Study to Assess Risk and Resilience in Servicemembers (STARRS; Ursano et al., 2014).
Method
Participants and Procedures
The Pre/Post Deployment Study (PPDS) was a multiwave panel survey of members of three U.S. Army Brigade Combat Teams (BCTs; Kessler, Colpe et al., 2013; Ursano et al., 2014). Baseline (T0) assessment of soldiers occurred during the first quarter of 2012, approximately 1–2 months before the BCTs deployed to Afghanistan. Follow-up evaluations took place within 1 month of the BCTs’ return to the United States (T1), and at time points 3 months (T2) and 9 months (T3) postdeployment. All participants provided written, informed consent for the self-administered questionnaires (SAQs). Baseline SAQ respondents also were asked for consent for the collection of blood samples, linkage of SAQ responses to their Army and Department of Defense (DoD) administrative records, and contact for participation in future assessments. Procedures were approved by the Human Subjects Committees of all collaborating organizations.
At T0, 9,949 soldiers were present for duty in the three BCTs, and 9,488 of these individuals (95.3%) consented to the SAQ. The majority of soldiers who consented (n = 8,558, 86.0%) provided complete SAQ data and consent for linkage of responses to their Army/DoD records. Of those respondents, 7,742 were subsequently deployed to Afghanistan. Because the current study relied on data from all four assessment waves, the eligible baseline sample of 7,742 soldiers was restricted to those who completed all follow-ups (n = 4,645). To rule out the possibility that ADHD treatment (vs. diagnosis) explained differences in risk of postdeployment PTSD and related disorders, we excluded any respondents whose administrative records indicated treatment with any stimulants or other ADHD-specific medications at any time during the 3 months before deployment through the end of deployment (n = 33). To compensate for losses due to T1, T2, and/or T3 attrition, response propensity (based on T0 measures available for baseline SAQ respondents who did and did not consent to administrative data linkage) and poststratification (based on key sociodemographic and Army service variables from consenting SAQ respondents and from administrative data available for the entire Army), weighting factors were developed and applied in all analyses (Heeringa, West, & Berglund, 2010).
Measures
Predeployment ADHD.
The T0 survey assessed ADHD symptoms during the preceding 6 months using items adapted from the self-administered Composite International Diagnostic Interview Screening Scales (CIDI-SC; (Kessler & Ustun, 2004). Prior research has shown the items to detect adult ADHD with good accuracy (Kessler et al., 2007, 2010). Four initial screening questions assessed problems with sustained attention, making careless mistakes, task completion, and task prioritization. Respondents indicated whether they experienced each problem never, rarely, sometimes, often, or very often. If a respondent gave at least two screening items a rating of sometimes or more, eight additional symptoms were queried, using the same response scale (never to very often). These symptoms included problems with task initiation, remembering appointments/obligations, organization, and hyperactivity. A final question asked whether symptoms of inattention and/or hyperactivity had interfered with work and/or personal life none of the time, a little of the time, some of the time, most of the time, or all or almost all of the time during the past 6 months. A diagnostic algorithm based on respondents’ ratings of these ADHD items was validated against structured clinical interviews in the Army STARRS clinical reappraisal study (Kessler, Santiago et al., 2013); this clinically calibrated ADHD diagnosis was the predictor of primary interest for the current study.
The ADHD diagnostic variable did not incorporate an age-of-onset criterion; however, a sensitivity analysis was conducted to evaluate whether results were comparable when predeployment ADHD with probable childhood- or adolescent-onset was considered as a predictor. Probable childhood- or adolescent-onset ADHD was defined as self-reported age-of-onset of 18 years or younger. Sensitivity models estimated associations of childhood- or adolescent-onset ADHD with the postdeployment outcomes of interest.
PTSD.
Past-month PTSD at either postdeployment assessment (i.e., T2 or T3) was the primary outcome of interest. Past-month postdeployment major depressive episode (MDE), generalized anxiety disorder (GAD), and suicidal ideation (SI) at T2 or T3 were examined as secondary outcomes. Predeployment PTSD, MDE, GAD, and SI (current vs. lifetime but not current vs. no diagnosis at T0) were adjusted for in certain models (see the Data Analysis subsection). A sensitivity analysis also added adjustment for predeployment score on the PTSD Checklist (six-item screening version; (Wilkins, Lang, & Norman, 2011) to models of PTSD outcomes.
Other mental disorders and suicidal ideation.
Mental disorder diagnoses were based on items from the CIDI-SC (Kessler & Ustun, 2004) and PTSD Checklist (Weathers, Litz, Herman, Huska, & Keane, 1993) and validated against structured clinical interviews in the Army STARRS clinical reappraisal study (Kessler, Santiago et al., 2013). Suicidal ideation was established using an expanded self-report version of the Columbia Suicide Severity Rating Scale (Posner et al., 2011). Subjects were asked, “Did you ever in your life have thoughts of killing yourself?” If an individual answered yes, they were asked “Did you have these thoughts at any time in the past 30 days?”
We also considered mental disorder symptoms during deployment as secondary outcomes. The T1 survey contained items that assessed core symptoms of PTSD, MDE, and GAD. The five items related to PTSD were highly internally consistent (Cronbach’s α = .84), as were the items related to MDE and GAD (seven items total; Cronbach’s α = .90). Ratings in each domain were summed to create indices of PTSD symptom severity (range: 0–20) and MDE/GAD symptom severity (range: 0–28) during deployment.
Sociodemographic and Army service variables.
Participants completed surveys regarding demographic and military variables at T0. These variables included age, sex, race, ethnicity, education level, BCT, and number of prior deployments. All variables were adjusted for in all models.
Other covariates.
Because both proximal and distal trauma exposures may contribute to risk for PTSD and associated disorders, we adjusted for deployment stress and childhood maltreatment in all models. Level of combat and/or deployment stress (assessed at T1) was quantified using a Deployment Stress Scale (DSS; possible score range: 0–16) as described in a previously published report (Campbell-Sills et al., 2017). Another Army STARRS report explained the derivation of a global childhood maltreatment scale (Stein et al., 2018). Scores on this scale represent the average frequency of exposure to five subtypes of maltreatment subtypes (sexual abuse, physical abuse, emotional abuse, physical neglect, and emotional neglect). The global childhood maltreatment scale is scored on a scale of 1 (never) to 5 (very often) and displayed satisfactory internal consistency in this sample (Cronbach’s α = .78). Finally, because deployment-acquired traumatic brain injury (TBI) has been shown to be associated with postdeployment PTSD and related disorders (Stein et al., 2015) and sequelae of TBI may include attentional symptoms, we adjusted for presence (vs. absence) of deployment-acquired TBI in all models. A history of predeployment lifetime TBI was initially considered for inclusion as a predictor but it was found to be unassociated with ADHD diagnosis at T0, χ2(2, N = 4574) = 3.39, p = .184, and not predictive of postdeployment PTSD or other disorders under consideration.
Data Analysis
We calculated weighted prevalence of ADHD at T0. Weights-adjusted logistic regression was used to estimate the association between predeployment ADHD and postdeployment PTSD (at T2 or T3) in the full sample. A subgroup model also examined the association of predeployment ADHD with incidence of PTSD (at T2 or T3) among soldiers without lifetime PTSD at T0. Analogous models were fit to examine associations among predeployment ADHD and MDE, GAD, and suicidal ideation at T2 or T3. Weights-adjusted linear regression was used to estimate associations between predeployment ADHD and severity of PTSD and MDE and/or GAD symptoms during deployment.
All models were adjusted for age, sex, race, ethnicity, education, BCT, number of prior deployments, combat and/or deployment stress, childhood maltreatment, and deployment-acquired TBI. Analyses conducted using data from the full sample were also adjusted for predeployment history of the disorder in question (e.g., predeployment PTSD was included in the model of postdeployment PTSD and predeployment MDE was included in the model of postdeployment MDE). Models of postdeployment suicidal ideation were adjusted for both predeployment suicidal ideation and predeployment MDE. The PPDS data were clustered by BCT and administration session and weighted; thus, the design-based Taylor series linearization method was used to estimate standard errors. Multivariable significance was examined using design-based Wald chi-square tests. Two-tailed p values less than .05 were considered significant. Logistic regression model fits were assessed using the Hosmer and Lemeshow goodness-of-fit test. Analyses were conducted using R (Version 3.3.2; R Core Team, 2013).
Results
Descriptive Findings
The weighted prevalence of ADHD at T0 (predeployment) was 6.1% (SE = 0.4%). Prevalence of ADHD did not differ by sex, race, ethnicity, or age, ps=0.271–0.818. The weighted prevalence of ADHD at T0 with self-reported age of onset before age 18 years was 3.1% (SE = 0.3%) and did not differ by the aforementioned demographic characteristics, ps = .177–.669. The prevalence of comorbid ADHD and PTSD at T0 was 1.7%.
ADHD as a Predictor of Postdeployment Disorders
PTSD.
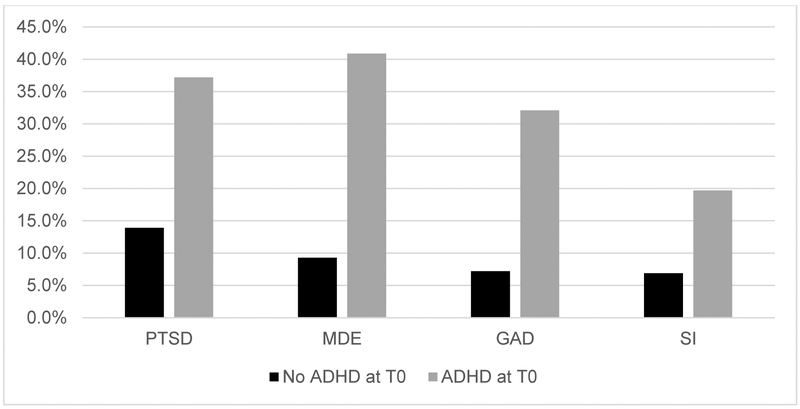
In a logistic regression analysis that was adjusted for demographic characteristics and risk factors (age, sex, ethnicity, race, BCT, number of prior deployments, combat and/or deployment stress, childhood maltreatment, and deployment-acquired TBI) as well as predeployment PTSD, predeployment ADHD was strongly associated with increased odds of past-month postdeployment (T2 or T3) PTSD, adjusted odds ratio (AOR) = 2.13, 95% CI [1.51, 3.00], p < .001 (see Table 1). The Hosmer and Lemeshow goodness-of-fit test revealed a satisfactory model fit, χ2(8, N = 4,534) = 11.39, p = .180. Figure 1 shows prevalence of postdeployment mental disorders by predeployment (T0) ADHD status.
Table 1.
Results of Weights-Adjusted Logistic Regression Evaluating the Association Between Predeployment Attention Deficit Hyperactivity Disorder (ADHD) and Posttraumatic Stress Disorder (PTSD) Diagnosis at 3 or 9 Months Postdeployment
| Variable | AOR | 95% CI | χ2a | df |
|---|---|---|---|---|
| Age (years) | 1.02 | [1.01, 1.04] | 7.66** | 1 |
| Sex | 3.58 | 1 | ||
| Male | 1.00 | |||
| Female | 1.54 | [0.99, 2.41] | ||
| Race | 6.93 | 3 | ||
| White | 1.00 | |||
| Asian | 1.37 | [0.83, 2.27] | ||
| Black | 1.12 | [0.76, 1.65] | ||
| Others | 1.51 | [1.0, 2.17] | ||
| Ethnicity | 1.02 | 1 | ||
| Non-Hispanic | 1.00 | |||
| Hispanic | 1.18 | [0.85, 1.64] | ||
| Education | 11.47** | 2 | ||
| High school degree | 1.00 | |||
| GED | 1.51 | [1.04, 2.19] | ||
| College degree | 0.73 | [0.57, 0.93] | ||
| Prior deployments | 0.27 | 2 | ||
| None | 1.00 | |||
| One | 1.02 | [0.77, 1.36] | ||
| Two or more | 0.96 | [0.73, 1.25] | ||
| Childhood maltreatment (raw score range=1-5) | 1.36 | [1.11, 1.65] | 9.15** | 1 |
| Predeployment PTSD | 96.11*** | 2 | ||
| None | 1.00 | |||
| Lifetime, not current | 2.76 | [2.03, 3.75] | ||
| Current | 5.20 | [3.49, 7.75] | ||
| Deployment stress severity (raw score range=0-16) | 1.23 | [1.20, 1.27] | 215.86*** | 1 |
| Deployment-acquired TBI | 18.42*** | 1 | ||
| None reported | 1.00 | |||
| One or more reported | 1.65 | [1.31, 2.07] | ||
| Predeployment ADHD | 18.78*** | 1 | ||
| No | 1.00 | |||
| Yes | 2.13 | [1.51, 3.00] |
Note. AOR = adjusted odds ratio; df = degrees of freedom; GED = General Equivalency Diploma; TBI = traumatic brain injury.
p < .05.
p < .01.
p < .001.
N = 4,534.
Figure 1.
Prevalence of postdeployment mental disorders by predeployment (T0) attention deficit and hyperactivity disorder (ADHD) status. Postdeployment disorders were considered present if diagnostic criteria were met at 3 or 9 months postdeployment (T2 or T3). Between-groups differences were significant for all outcomes, χ2(1, N = 4612) = 58.5–255.22, ps < .001. However, these comparisons and the estimates shown in the figure are unadjusted whereas the predictive models of the effects of predeployment ADHD on postdeployment posttraumatic stress disorder (PTSD), major depressive episode (MDE), generalized anxiety disorder (GAD), and suicidal ideation (SI) control for a wide range of factors. Predeployment ADHD was not significantly associated with postdeployment SI after imposing these controls but did display independent associations with the other outcomes.
To examine incidence of PTSD at T2 or T3, we tested the same model among soldiers without lifetime PTSD at T0. Predeployment ADHD was again associated with increased odds of postdeployment past-month PTSD, AOR = 2.50, 95% CI [1.69, 3.69], p < .001. The Hosmer and Lemeshow goodness-of-fit test revealed a satisfactory model fit, χ2(8, N = 4,022) = 9.91, p = .272.
We also evaluated whether predeployment ADHD was associated with increased severity of PTSD symptoms during deployment (as assessed at T1). Weights-adjusted linear regression with adjustment for sociodemographic characteristics, BCT, number of prior deployments, childhood maltreatment, deployment stress, deployment-acquired TBI, and predeployment PTSD showed that predeployment ADHD was positively associated with PTSD symptom severity during deployment, b = 1.84, 95% CI [1.27, 2.41], χ2(1, N = 4,511) = 39.72, p < .001 (see Table 2).
Table 2.
Results of Weights-Adjusted Linear Regression Evaluating Effect of Predeployment Attention Deficit Hyperactivity Disorder (ADHD) on Posttraumatic Stress Disorder (PTSD) Symptom Severity During Deployment
| Variable | b | 95% CI | χ2a | df |
|---|---|---|---|---|
| Age (years) | 0.005 | [−0.013, 0.022] | 0.28 | 1 |
| Female sex (Reference: Male) | 0.87 | [0.58, 1.17] | 33.31*** | 1 |
| Race (Reference: White) | 7.88* | 3 | ||
| Asian | 0.60 | [−0.07, 1.28] | ||
| Black | 0.20 | [−0.07, 0.47] | ||
| Others | 0.44 | [0.04, 0.84] | ||
| Education (Reference: High school degree) | 0.27 | 2 | ||
| GED | −0.04 | [−0.40, 0.33] | ||
| College degree | −0.06 | [−0.30, 0.18] | ||
| Prior deployments (Reference: None) | 3.5 | 2 | ||
| One | 0.27 | [−0.01, 0.55] | ||
| Two or more | 0.17 | [−0.10, 0.44] | ||
| Childhood maltreatment (raw score range: 1–5) | 0.31 | [0.12, 0.51] | 9.78** | 1 |
| Predeployment PTSD (Reference: None) | 123.71*** | 2 | ||
| Lifetime, not current | 1.92 | [1.47, 2.37] | ||
| Current | 3.20 | [2.50, 3.90] | ||
| Deployment stress (raw score range: 0-16) | 0.50 | [0.45, 0.55] | 364.2*** | 1 |
| Deployment-acquired TBI | 1.38 | [1.04, 1.72] | 62.33*** | 1 |
| Predeployment ADHD | 1.84 | [1.27, 2.41] | 39.72*** | 1 |
Note. df = degrees of freedom; GED = General Equivalency Diploma; TBI = traumatic brain injury.
p < .05.
p < .01.
p < .001.
N = 4,022
MDE.
After adjusting for predeployment MDE as well as demographics and risk factors, predeployment ADHD was associated with increased odds of past-month MDE at T2 or T3, AOR = 2.80, 95% CI [2.01, 3.91], p < .001, with satisfactory model fit revealed by a Hosmer and Lemeshow goodness-of-fit-test, χ2(8, N = 4534) = 4.34, p = .825. Predeployment ADHD was also associated with increased odds of past-month MDE at time T2 or T3 when soldiers with lifetime MDE predeployment were excluded, AOR = 3.24, 95% CI [2.06, 5.08], p < .001, with satisfactory model fit revealed by a Hosmer and Lemeshow goodness-of-fit test, χ 2(8, N = 4,126) = 13.12, p = .108.
GAD.
Predeployment ADHD was associated with increased odds of past-month GAD at time T2 or T3 after adjusting for predeployment GAD as well as sociodemographic variables and risk factors, AOR = 3.04, 95% CI [2.10, 4.42], p < .001, with satisfactory model fit revealed by a Hosmer and Lemeshow goodness-of-fit test, χ2(8, N = 4,534) = 11.78, p = .161. An otherwise identical model which excluded soldiers with lifetime GAD predeployment also revealed a significant association, AOR = 5.29, 95% CI [3.26, 8.59], p < .001, with satisfactory model fit revealed by a Hosmer and Lemeshow goodness-of-fit test, χ2(8, N = 4,178) = 8.10, p = .424.
Suicidal ideation.
After we adjusted for sociodemographic variables, predeployment suicidal ideation and MDE, and other risk factors, predeployment ADHD was not significantly associated with past-month suicidal ideation at T2 or T3, AOR = 1.46, 95% CI [0.93, 2.30], p = .103, with satisfactory model fit revealed by a Hosmer and Lemeshow goodness-of-fit test, χ2(8, N = 4,534) = 10.89, p = .208. Similarly, no association between predeployment ADHD and incidence of suicidal ideation among soldiers with no predeployment history of suicidal ideation was apparent, AOR = 1.40, 95% CI [0.70, 2.82], p = .342, with satisfactory model fit revealed by a Hosmer and Lemeshow goodness-of-fit test, χ2(8, N = 4,021) = 10.14, p = .256.
Combined MDE and GAD symptoms during deployment.
A weights-adjusted linear regression with adjustment for sociodemographic characteristics, BCT, number of prior deployments, childhood maltreatment, deployment stress, deployment-acquired TBI, and predeployment MDE and GAD showed that predeployment ADHD was associated with a higher level of MDE/GAD symptom severity during deployment, b = 1.64, 95% CI [1.00, 2.28]; χ2(1, N = 4,481) = 25.23, p < .001.
Sensitivity analyses.
We repeated all analyses described earlier using predeployment ADHD with age of onset before 18 years as a predictor. Associations among ADHD and postdeployment PTSD, MDE, and GAD (including new-onset of PTSD, MDE, and GAD among soldiers without predeployment history of the disorder in question) remained statistically significant and of substantial magnitude, AORs = 1.59–4.42. Associations between ADHD and PTSD and MDE/GAD symptom severity during deployment also remained significant and of comparable magnitude.
Finally, to further test robustness of the associations between predeployment ADHD and the primary outcome (PTSD), we ran sensitivity models of PTSD outcomes that also included adjustment for predeployment PTSD symptom severity (T0 score on the six-item PTSD Checklist). In the full sample, predeployment ADHD remained significantly associated with postdeployment PTSD diagnosis, AOR = 1.74, 95% CI [1.22, 2.49], p = .002, and PTSD symptom severity during deployment, b = 1.50, 95% CI [0.92, 2.07], χ2(1, N = 4,511) = 26.00, p < .001. Additionally, ADHD remained significantly associated with PTSD incidence among soldiers without lifetime PTSD as reported at T0, AOR = 1.99, 95% CI [1.30, 3.03], p = .001. The full results of the sensitivity analyses are available upon request.
Discussion
We found that predeployment ADHD in U.S. Army soldiers who were deployed to Afghanistan was strongly associated with PTSD both during and after deployment. This association was present after adjusting for other risk factors, including predeployment PTSD and combat stress severity, whether a dichotomous PTSD diagnosis postdeployment or PTSD symptom severity during deployment was the outcome of interest and whether or not ADHD was restricted to cases with in which individuals had childhood/adolescent- or adult–onset ADHD. Adjusted odds ratios that characterized the association between predeployment ADHD and postdeployment PTSD diagnosis suggested that preexisting ADHD was associated with a roughly doubled risk of postdeployment PTSD.
Although a fairly large body of cross-sectional studies have demonstrated an association between ADHD and PTSD (A. E. Spencer et al., 2016), prospective trials examining this association are extremely sparse. Two prospective studies in children yielded conflicting results (Biederman et al., 2014; Wozniak et al., 1999). However, the negative finding by Wozniak et al. (1999) should be interpreted with caution given the very low rate of trauma exposure that was reported in either group in that study (12% of ADHD probands and 7% of controls) and low rates of development of PTSD (two participants in the ADHD and no controls). A sample of active-duty U.S. military members from the Millennium Cohort Study did not show an association between ADHD diagnosis and subsequent new-onset PTSD but did demonstrate an association between use of stimulant medications and PTSD (Crum-Cianflone et al., 2015). Importantly, the Millennium Cohort Study used medical record data to establish ADHD diagnosis whereas in the present study, all participants completed a survey on ADHD symptoms at baseline. Given that adult ADHD is less likely to be recognized and treated than other psychiatric conditions, this may have been a major contributor to the discrepancy in findings between the two studies. Indeed, the prevalence of ADHD in the Millennium Cohort Study was 1.1% compared to the weighted prevalence of 6.1% reported in the present study whereas the proportion of individuals who were taking a prescription stimulant and had a recorded ADHD diagnosis was only 49.6% (indications for stimulant prescription among the remaining participants were not recorded). It is also unclear whether the individuals receiving stimulants may have had more severe ADHD symptoms than those who were not receiving stimulants, which would to explain the association between stimulants and PTSD in that study. In the present study, the association between ADHD and PTSD cannot be explained by stimulant medications as soldiers who were prescribed stimulants were excluded from analysis. We cannot rule out the possibility, however, that some soldiers took stimulants without a prescription.
The strong association between ADHD and PTSD suggests an underlying shared pathology that may be leveraged in the development of new prevention and treatment strategies. Indeed, several lines of evidence have suggested that ADHD and PTSD may be mechanistically related. There is overlap in the symptomatic manifestations of the two disorders, including irritability, excessive motor activity, concentration difficulties, impulsive behavior, and exaggerated startle response (Daud & Rydelius, 2009). The disorders share prominent prefrontal cortical dysfunction and abnormal catecholamine transmission (Arnsten, 2007). A study of familial transmission concluded that ADHD and PTSD share familial risk factors and that co-occurrence is not due to diagnostic errors (Antshel et al., 2013). Preliminary evidence has identified specific genetic risk factors common to both disorders, including polymorphisms of the dopamine transporter gene and cannabinoid receptor gene (Drury, Brett, Henry, & Scheeringa, 2013; Onaivi, 2009; T. J. Spencer et al., 2013). In addition to genetics, environmental factors may contribute to the association between ADHD and PTSD. Early adversity is among the most consistent risk factors for PTSD (Breslau, 2009) as well as for depression and anxiety disorders (Heim & Nemeroff, 2001) whereas individuals with ADHD also exhibit elevated rates of childhood abuse (Rucklidge, Brown, Crawford, & Kaplan, 2006). In the present study, childhood maltreatment was included as a covariate in analyses in order to adjust for these associations.
Although high comorbidity between ADHD and other psychiatric disorders (including major depressive disorder and GAD) has been previously observed (Kessler et al., 2006), the role of ADHD in the risk of onset of these disorders in the context of trauma exposure has been unclear. The present study found evidence for robust associations between predeployment ADHD and postdeployment MDE and GAD, including incidence of these disorders among soldiers with no lifetime history of MDE and GAD prior to the index deployment. These findings suggest that individuals with ADHD may exhibit generally decreased resilience in the face of trauma as opposed to specific vulnerability to PTSD. Future research can investigate additional potential sequelae of trauma exposure in individuals with ADHD, including possible worsening of ADHD symptoms and overall functional impairment, as well as any factors that differentially predict specific postdeployment symptoms.
In the general population, ADHD has been linked to elevated risk of suicidal ideation, suicide attempts, and completed suicides (Impey & Heun, 2012; James, Lai, & Dahl, 2004); however, in our study, we found that predeployment ADHD was not independently associated with postdeployment suicidal ideation. Given evidence that co-occurring mental disorders (e.g., depression) contribute substantially to an increased risk of suicidal behaviors among individuals with ADHD (Impey & Heun, 2012; James et al., 2004), our models estimating associations between predeployment ADHD and postdeployment suicidal ideation adjusted for both lifetime history of suicidal ideation and predeployment MDE. In the presence of these controls, predeployment ADHD was not associated with postdeployment suicidal ideation.
The increased risk of deployment-related PTSD associated with preexisting ADHD raises the question of whether treatment of preexisting ADHD may be protective in individuals exposed to trauma. Although it has been proposed that stimulant medications (a first-line treatment for ADHD) could heighten the risk of PTSD by increasing brain norepinephrine and thus strengthening traumatic memories (Crum-Cianflone et al., 2015; Herbst, McCaslin, & Kalapatapu, 2017), preclinical and clinical research has suggested that stimulants may have beneficial effects in individuals who have been exposed to trauma. The stimulant methylphenidate appears to enhance extinction of contextual fear in mice (Abraham, Cunningham, & Lattal, 2012) and improve PTSD-like symptoms in a rat model, particularly when combined with the antidepressant desipramine (Aga-Mizrachi et al., 2014). Additionally, a recent randomized controlled trial in humans found that methylphenidate was associated with substantial improvement in PTSD symptoms (McAllister et al., 2016). Additional research is needed to clarify the risks and benefits of stimulant medications in individuals with ADHD who are at elevated risk for exposure to trauma, such as those on combat deployments.
Though the large sample, detailed longitudinal assessments, and exclusion of individuals who had been prescribed stimulants (in order to rule out an effect of medication) were strengths of this study, there were also limitations. Diagnoses were based on self-report rather than structured clinical interviews. As noted above, the diagnostic instruments have been validated against structured clinical interviews in the Army STARRS clinical reappraisal study (Kessler, Santiago et al., 2013). Perhaps more importantly, our ability to assess the age of ADHD onset was limited to subjects’ self-report. The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association [APA], 2013) requires several inattentive or hyperactive–impulsive symptoms to be present prior to age 12 years in order to establish an ADHD diagnosis (American Psychiatric Association, 2013). In clinical practice, childhood ADHD symptoms are typically assessed via parent and teacher report whereas adult symptoms are more often assessed via self-report (Caye et al., 2016), which raises concern that retrospective self-report of childhood symptoms and age of onset of the disorder may have limited accuracy. It is important to note that recent research has called into question the assumption that adult ADHD typically represents a continuation of a childhood disorder and has instead found that a large proportion of adult ADHD cases begin during adulthood and suggested that childhood- and adult-onset ADHD may exhibit distinct clinical and mechanistic characteristics (Agnew-Blais et al., 2016; Caye et al., 2016; Moffitt et al., 2015). In the present study, we decided to perform sensitivity analyses using ADHD with self-reported age of onset before 18 years as the predictor, thereby providing evidence that the established associations between ADHD and PTSD (and some other postdeployment disorders, such as MDE and GAD) were present among those respondents who recalled attentional problems during childhood and/or adolescence. Future studies with access to more complete information regarding childhood symptoms can examine these associations in more detail. A final limitation was the inability to know which individuals were completely stimulant naïve.
In summary, we found robust evidence for strong associations between predeployment ADHD and postdeployment PTSD, MDE, and GAD in a large sample of U.S. Army soldiers who were deployed to Afghanistan. Recognition of the association between ADHD and postdeployment disorders may inform targeted prevention efforts that aim to reduce onset of PTSD and related disorders following military deployment. It remains unclear whether treatment of ADHD is protective against PTSD and other disorders, a question that is an important topic for future study. Furthermore, investigation of possible mechanistic links between ADHD and PTSD may yield advances in prevention and treatment approaches.
Acknowledgments
The Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS) was sponsored by the Department of the Army and funded under cooperative agreement number U01MH087981 (2009–2015) with the U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Mental Health (NIH/NIMH). Subsequently, STARRS-LS was sponsored and funded by the Department of Defense (USUHS grantHU0001-15-2-0004). The contents are solely the responsibility of the authors and do not necessarily represent the views of the Department of Health and Human Services, NIMH, Department of the Army, or Department of Defense.
The Army STARRS Team consists of Co-Principal Investigators: Robert J. Ursano, MD (Uniformed Services University of the Health Sciences) and Murray B. Stein, MD, MPH (University of California San Diego and VA San Diego Healthcare System)
Site Principal Investigators: Steven Heeringa, PhD (University of Michigan), James Wagner, PhD (University of Michigan), and Ronald C. Kessler, PhD (Harvard Medical School)
Army liaison/consultant: Kenneth Cox, MD, MPH (USAPHC (Provisional))
Other team members: Pablo A. Aliaga, MA (Uniformed Services University of the Health Sciences); COL David M. Benedek, MD (Uniformed Services University of the Health Sciences); Laura Campbell-Sills, PhD (University of California San Diego); Chia-Yen Chen DSc (Harvard Medical School); Carol S. Fullerton, PhD (Uniformed Services University of the Health Sciences); Nancy Gebler, MA (University of Michigan); Joel Gelernter (Yale University); Robert K. Gifford, PhD (Uniformed Services University of the Health Sciences); Paul E. Hurwitz, MPH (Uniformed Services University of the Health Sciences); Sonia Jain, PhD (University of California San Diego); Tzu-Cheg Kao, PhD (Uniformed Services University of the Health Sciences); Lisa Lewandowski-Romps, PhD (University of Michigan); Holly Herberman Mash, PhD (Uniformed Services University of the Health Sciences); James E. McCarroll, PhD, MPH (Uniformed Services University of the Health Sciences); James A. Naifeh, PhD (Uniformed Services University of the Health Sciences); Tsz Hin Hinz Ng, MPH (Uniformed Services University of the Health Sciences); Matthew K. Nock, PhD (Harvard University); Nancy A. Sampson, BA (Harvard Medical School); CDR Patcho Santiago, MD, MPH (Uniformed Services University of the Health Sciences); Jordan W. Smoller, MD, ScD (Harvard Medical School); and Alan M. Zaslavsky, PhD (Harvard Medical School).
Footnotes
In the past 3 years, Dr. Stein has been a consultant for Actelion, Aptinyx, Bionomics, Dart Neuroscience, Healthcare Management Technologies, Janssen, Oxeia Biopharmaceuticals, Pfizer, and Resilience Therapeutics. The remaining authors report no competing interests.
References
- Abraham AD, Cunningham CL, & Lattal KM (2012). Methylphenidate enhances extinction of contextual fear. Learn Mem, 19(2), 67–72. 10.1101/lm.024752.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aga-Mizrachi S, Cymerblit-Sabba A, Gurman O, Balan A, Shwam G, Deshe R, . . . Avital A (2014). Methylphenidate and desipramine combined treatment improves PTSD symptomatology in a rat model. Transl Psychiatry, 4(9), e447 10.1038/tp.2014.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnew-Blais JC, Polanczyk GV, Danese A, Wertz J, Moffitt TE, & Arseneault L (2016). Evaluation of the persistence, remission, and emergence of attention-deficit/hyperactivity disorder in young adulthood. JAMA Psychiatry, 73(7), 713–720. 10.1001/jamapsychiatry.2016.0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Association. [Google Scholar]
- Antshel KM, Kaul P, Biederman J, Spencer TJ, Hier BO, Hendricks K, & Faraone SV (2013). Posttraumatic stress disorder in adult attention-deficit/hyperactivity disorder: clinical features and familial transmission. J Clin Psychiatry, 74(3), e197–204. 10.4088/JCP.12m07698 [DOI] [PubMed] [Google Scholar]
- Arnsten AF (2007). Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex, 17(Suppl 1), i6–15. 10.1093/cercor/bhm033 [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty C, Spencer TJ, Woodworth KY, Bhide P, Zhu JM, & Faraone SV (2014). Is ADHD a risk for posttraumatic stress disorder (PTSD)? Results from a large longitudinal study of referred children with and without ADHD. World J Biol Psychiatry, 15(1), 49–55. 10.3109/15622975.2012.756585 [DOI] [PubMed] [Google Scholar]
- Breslau N (2009). The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse, 10(3), 198–210. 10.1177/1524838009334448 [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, & Valentine JD (2000). Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol, 68(5), 748–766. 10.1037//0022-006X.68.5.748 [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Ursano R, Kessler R, Sun X, Heeringa S, Nock M, . . . Stein MB (2017). Prospective longitudinal evaluation of risk for post-deployment alcohol misuse: Results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). In submission.
- Caye A, Rocha TB, Anselmi L, Murray J, Menezes AM, Barros FC, . . . Rohde LA (2016). Attention-deficit/hyperactivity disorder trajectories from childhood to young adulthood: evidence from a birth cohort supporting a late-onset syndrome. JAMA Psychiatry, 73(7), 705–712. 10.1001/jamapsychiatry.2016.0383 [DOI] [PubMed] [Google Scholar]
- Crum-Cianflone NF, Frasco MA, Armenta RF, Phillips CJ, Horton J, Ryan MA, . . . LeardMann C (2015). Prescription stimulants and PTSD among US military service members. J Trauma Stress, 28(6), 585–589. 10.1002/jts.22052 [DOI] [PubMed] [Google Scholar]
- Daud A, & Rydelius PA (2009). Comorbidity/overlapping between ADHD and PTSD in relation to IQ among children of traumatized/non-traumatized parents. J Atten Disord, 13(2), 188–196. 10.1177/1087054708326271 [DOI] [PubMed] [Google Scholar]
- Drury SS, Brett ZH, Henry C, & Scheeringa M (2013). The association of a novel haplotype in the dopamine transporter with preschool age posttraumatic stress disorder. J Child Adolesc Psychopharmacol, 23(4), 236–243. 10.1089/cap.2012.0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa SG, West BT, & Berglund PA (2010). Applied Survey Data Analysis. Boca Raton, FL: Chapman and Hall. [Google Scholar]
- Heim C, & Nemeroff CB (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry, 49(12), 1023–1039. 10.1016/S0006-3223(01)01157-X [DOI] [PubMed] [Google Scholar]
- Herbst E, McCaslin S, & Kalapatapu RK (2017). Use of stimulants and performance enhancers during and after trauma exposure in a combat veteran: a possible risk factor for posttraumatic stress symptoms. Am J Psychiatry, 174(2), 95–99. 10.1176/appi.ajp.2016.16010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, & Koffman RL (2004). Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med, 351(1), 13–22. 10.1056/NEJMoa040603 [DOI] [PubMed] [Google Scholar]
- Howlett JR, & Stein MB (2016). Prevention of trauma and stressor-related disorders: a review. Neuropsychopharmacology, 41(1), 357–369. 10.1038/npp.2015.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey M, & Heun R (2012). Completed suicide, ideation and attempt in attention deficit hyperactivity disorder. Acta Psychiatr Scand, 125(2), 93–102. 10.1111/j.1600-0447.2011.01798.x [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. (2008). Treatment of Posttraumatic Stress Disorder: An Assessment of the Evidence. Washington, DC: The National Academies Press. [Google Scholar]
- James A, Lai FH, & Dahl C (2004). Attention deficit hyperactivity disorder and suicide: a review of possible associations. Acta Psychiatr Scand, 110(6), 408–415. 10.1111/j.1600-0447.2004.00384.x [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, . . . Zaslavsky AM (2006). The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry, 163(4), 716–723. 10.1176/ajp.2006.163.4.716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Adler LA, Gruber MJ, Sarawate CA, Spencer T, & Van Brunt DL (2007). Validity of the World Health Organization Adult ADHD Self-Report Scale (ASRS) Screener in a representative sample of health plan members. Int J Methods Psychiatr Res, 16(2), 52–65. 10.1002/mpr.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Colpe LJ, Fullerton CS, Gebler N, Naifeh JA, Nock MK, . . . Heeringa SG (2013). Design of the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Int J Methods Psychiatr Res, 22(4), 267–275. 10.1002/mpr.1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Green JG, Adler LA, Barkley RA, Chatterji S, Faraone SV, . . . Van Brunt DL (2010). Structure and diagnosis of adult attention-deficit/hyperactivity disorder: analysis of expanded symptom criteria from the Adult ADHD Clinical Diagnostic Scale. Arch Gen Psychiatry, 67(11), 1168–1178. 10.1001/archgenpsychiatry.2010.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Heeringa SG, Stein MB, Colpe LJ, Fullerton CS, Hwang I, . . . Army STARRS Collaborators. (2014). Thirty-day prevalence of DSM-IV mental disorders among nondeployed soldiers in the US Army: results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). JAMA Psychiatry, 71(5), 504–513. 10.1001/jamapsychiatry.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Rose S, Koenen KC, Karam EG, Stang PE, Stein DJ, . . . Carmen Viana M (2014). How well can post-traumatic stress disorder be predicted from pre-trauma risk factors? An exploratory study in the WHO World Mental Health Surveys. World Psychiatry, 13(3), 265–274. 10.1002/wps.20150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Santiago PN, Colpe LJ, Dempsey CL, First MB, Heeringa SG, . . . Ursano RJ (2013). Clinical reappraisal of the Composite International Diagnostic Interview Screening Scales (CIDI-SC) in the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Int J Methods Psychiatr Res, 22(4), 303–321. 10.1002/mpr.1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, & Ustun TB (2004). The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res, 13(2), 93–121. 10.1002/mpr.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Zafonte R, Jain S, Flashman LA, George MS, Grant GA, . . . Stein MB (2016). Randomized placebo-controlled trial of methylphenidate or galantamine for persistent emotional and cognitive symptoms associated with PTSD and/or traumatic brain injury. Neuropsychopharmacology, 41(5), 1191–1198. 10.1038/npp.2015.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Houts R, Asherson P, Belsky DW, Corcoran DL, Hammerle M, . . . Caspi A (2015). Is adult ADHD a childhood-onset neurodevelopmental disorder? evidence from a four-decade longitudinal cohort study. Am J Psychiatry, 172(10), 967–977. 10.1176/appi.ajp.2015.14101266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES (2009). Cannabinoid receptors in brain: pharmacogenetics, neuropharmacology, neurotoxicology, and potential therapeutic applications. Int Rev Neurobiol, 88, 335–369. 10.1016/S0074-7742(09)88012-4 [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, . . . Mann JJ (2011). The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry, 168(12), 1266–1277. 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2013). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Ramchand R, Schell TL, Karney BR, Osilla KC, Burns RM, & Caldarone LB (2010). Disparate prevalence estimates of PTSD among service members who served in Iraq and Afghanistan: possible explanations. J Trauma Stress, 23(1), 59–68. 10.1002/jts.20486 [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ, Brown DL, Crawford S, & Kaplan BJ (2006). Retrospective reports of childhood trauma in adults with ADHD. J Atten Disord, 9(4), 631–641. 10.1177/1087054705283892 [DOI] [PubMed] [Google Scholar]
- Spencer AE, Faraone SV, Bogucki OE, Pope AL, Uchida M, Milad MR, . . . Biederman J (2016). Examining the association between posttraumatic stress disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Clin Psychiatry, 77(1), 72–83. 10.4088/JCP.14r09479 [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Faraone SV, Madras BK, Bonab AA, Dougherty DD, . . . Fischman AJ (2013). Functional genomics of attention-deficit/hyperactivity disorder (ADHD) risk alleles on dopamine transporter binding in ADHD and healthy control subjects. Biol Psychiatry, 74(2), 84–89. 10.1016/j.biopsych.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Campbell-Sills L, Ursano RJ, Rosellini AJ, Colpe LJ, He F, . . . Army STARRS Collaborators. (2018). Childhood maltreatment and lifetime suicidal behaviors among new soldiers in the U.S. Army: results from Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). J Clin Psychiatry, 79(2). 10.4088/JCP.16m10900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Kessler RC, Heeringa SG, Jain S, Campbell-Sills L, Colpe LJ, . . . Army STARRS Collaborators. (2015). Prospective longitudinal evaluation of the effect of deployment-acquired traumatic brain injury on posttraumatic stress and related disorders: results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Am J Psychiatry, 172(11), 1101–1111. 10.1176/appi.ajp.2015.14121572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanielian TL, & Jaycox L (2008). Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery (Vol. 720). Santa Monica, CA: Rand Corporation. [Google Scholar]
- Ursano RJ, Colpe LJ, Heeringa SG, Kessler RC, Schoenbaum M, Stein MB, & Army STARRS Collaborators. (2014). The Army study to assess risk and resilience in servicemembers (Army STARRS). Psychiatry, 77(2), 107–119. 10.1521/psyc.2014.77.2.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, & Keane TM (1993). The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Paper presented at the Annual Convention of the International Society for Traumatic Stress Studies. [Google Scholar]
- Wilkins KC, Lang AJ, & Norman SB (2011). Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety, 28(7), 596–606. 10.1002/da.20837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J, Crawford MH, Biederman J, Faraone SV, Spencer TJ, Taylor A, & Blier HK (1999). Antecedents and complications of trauma in boys with ADHD: findings from a longitudinal study. J Am Acad Child Adolesc Psychiatry, 38(1), 48–55. 10.1097/00004583-199901000-00019 [DOI] [PubMed] [Google Scholar]