Abstract
The recognition that atherosclerosis is a complex chronic inflammatory disorder mediated through both adaptive and innate immunity has led to the hypothesis that anti-cytokine therapies targeting specific interleukin signaling pathways could serve as powerful adjuncts to lipid lowering in the prevention and treatment of cardiovascular disease. Cytokines involved in human atherosclerosis can be broadly classified as pro-inflammatory and pro-atherogenic (such as IL-1, IL-6, and TNF) or as anti-inflammatory and anti-atherogenic (such as IL-10 and IL-1rA). The recent Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) has shown that specific targeting of IL-1β can significantly reduce cardiovascular event rates without lipid or blood pressure lowering. In CANTOS, the magnitude of benefit of this cytokine targeted approach to atherosclerosis treatment was associated to the magnitude of reduction of the central signaling cytokine IL-6 and the downstream clinical biomarker high-sensitivity C-reactive protein (hsCRP). By contrast, in the recent Cardiovascular Inflammation Reduction Trial (CIRT), low-dose methotrexate neither reduced IL-1β, IL-6, or hsCRP nor lowered cardiovascular event rates. Taken together, these two contemporary trials provide proof-of-principle that focused cytokine inhibition, not broad spectrum anti-inflammatory therapy, is likely to be crucial for atheroprotection. This review provides an overview of cytokines in atherosclerosis, the potential benefits and risks associated with targeted anti-cytokine therapies, and a look to the future of clinical practices addressing “residual inflammatory risk”.
Keywords: Interleukins, Inflammasome, Cardiovascular Disease, Clinical Trials, Cytokines
Introduction
Despite aggressive control of lipids, blood pressure, and traditional risk factors, major life-threatening atherothrombotic events continue to occur with alarming frequency. The recognition that atherosclerosis is a complex chronic inflammatory disorder mediated through both adaptive and innate immunity (1,2) has led to the clinical concept of “residual inflammatory risk”, a largely untreated but potentially lethal condition more prevalent in contemporary patients than its counterpart “residual cholesterol risk” (3). Within the past 18 months, two major clinical trials that targeted vascular inflammation with markedly different approaches have yielded markedly different outcomes. First, the Canakinumab Antiinflammatory Thrombosis Outcomes Study (CANTOS) demonstrated that specific targeting of interleukin-1β with the monoclonal antibody canakinumab results in significant reductions in major adverse cardiovascular events with a magnitude of effect directly related to the magnitude of interleukin-6 and C-reactive protein (CRP) reduction (4). By contrast, the Cardiovascular Inflammation Reduction Trial (CIRT) showed no benefit in terms of cardiovascular event reduction with an alternative non-specific approach using low-dose methotrexate, an agent that also had no impact on circulating levels of interleukin-1β, interlukin-6, or high-sensitivity CRP (hsCRP) (5). When taken together, these two contemporary trials provide proof-of-principle that focused cytokine inhibition, rather than broad spectrum anti-inflammatory therapy, is likely to be crucial for long-term atheroprotection. CANTOS and CIRT thus provide critical insights into future directions for targeted anti-cytokine therapies as adjuncts to lipid lowering.
Cytokines and Atherothrombosis: Mouse Models
The role played by cytokines and interleukin signaling in atherosclerosis has been the subject of several excellent reviews (6–10). Cytokines are low molecular weight proteins that mediate a wide range of inflammatory responses between neighboring cells that orchestrate neutrophil infiltration. Critical early discoveries of cytokine function were made in the field of fever research where the first “between-white blood cell” signaling molecule or “interleukin-1” was described (11). The cytokine family includes more than 30 subsequently identified interleukins as well as tumor necrosis factors (TNF), interferons, chemokines, colony-stimulating factors, and transforming growth factors (TGF). As a group, the cytokines regulate both innate and adaptive immune responses, and virtually all cells isolated within atherosclerotic plaque both produce and respond to cytokine activity (7). As such, cytokines provide one avenue of cross-talk between the “primitive fast” and “selective slow” forms of innate and adaptive immune response.
Kleeman and colleagues have provided a comprehensive review of studies evaluating cytokines and atherosclerosis in mice (Figure 1, left)(12). Cytokines involved in vascular disease can broadly be classified as pro-inflammatory and pro-atherogenic (such as interleukin-1, interleukin-6, and TNF) or as anti-inflammatory and anti-atherogenic (such as interleukin-10 and IL-1rA), although certain cytokines perform both roles in different settings (Figure 1, right). Multiple properties associated with the initiation, development, and eventual rupture of atherosclerotic plaques including leukocyte recruitment, endothelial dysfunction, inhibition of collagen synthesis, and coagulation activation are mediated by these cytokines.
Figure 1. Cytokines Implicated in the Atherothrombotic Process.
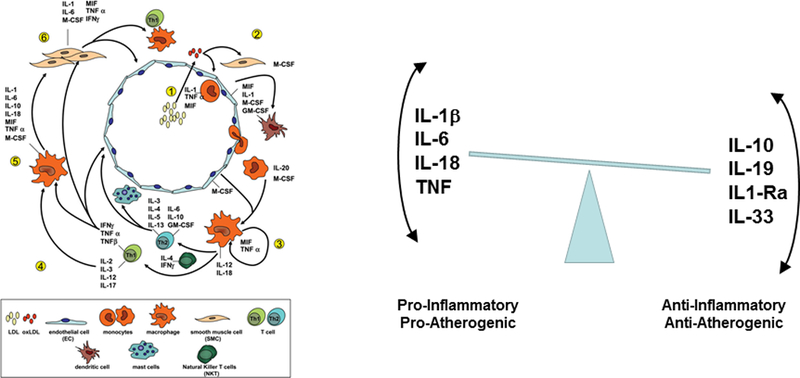
Data derived from mouse studies (left) and human studies (right) suggest an intricate balance between pro- and anti-inflammatory cytokine signaling pathways in the genesis of atherosclerotic lesions and in the probability of plaque rupture leading to clinical events such as myocardial infarction, stoke, and cardiovascular death. Adapted from Kleeman et al, Cardiovascular Research 2008;79:360–376 and Ait-Oufella H et al, Arterioscler Thromb Vasc Biol 2011;31:969–979.
Hyperlipidemic mouse models have long implicated cytokine function and interleukin signaling in the development of atherosclerosis. As critical examples, when compared to control animals, TNF-α−/− ApoE−/− double knockout mice have less atherosclerosis and reduced endothelial adhesion (13), an effect replicated in ApoE−/− mice treated with agents that reduce TNF-α activity (14,15). Similarly, early data indicated that lack of interleukin-1β decreases the severity of atherosclerosis in ApoE-deficient mice (16) and antibodies targeting mouse interleukin-1 have resulted in reduced atherogenesis (17). By contrast, exposure to exogenous interleukin-1β increases intimal medial thickening (18,19). In parallel work, administration of either interluekin-6 or interleukin-18 may promote atherosclerosis and increase lesion size in ApoE deficient mice (20,21) while reduced atherosclerosis has been observed in interleukin-18 deficient Apo-E knockout mice (22,23).
It is important to recognize that murine and human data do not always coincide. As will be described below, the CANTOS trial has demonstrated that interleukin-1β inhibition with canakinumab lowers cardiovascular event rates (4), data consistent with the early IL-1 evidence cited above. Yet, other recent murine work has suggested that interleukin-1α and interleukin-1β may have contrarian atheroprotective effects in advanced lesions in ApoE−/− mice (24,25).
Cytokines and Atherothrombosis: Epidemiologic Evidence in Humans
Human data implicating cytokines in atherogenesis initially came from large-scale prospective cohort studies. Prominent among these was evidence provided 20-years ago from the NIH-funded Physicians Health Study and Women’s Health Study cohorts in which adhesion molecules such as ICAM and VCAM as well as circulating cytokines such as IL-6 and TNF were predictive of future cardiovascular risk in apparently healthy men and women, as was the downstream biomarker high-sensitivity C-reactive protein (hsCRP) (26–29). Similar effects have been reported in unstable angina and in settings of chronic atherosclerosis (30–32).
These human data have repeatedly been replicated, and meta-analyses relating vascular risk to levels of interleukin-6, interleukin-18, MMP-9, sCD40L, and TNF-α present a clear epidemiologic signal (33)( Figure 2). Mendelian randomization studies have also implicated the interleukin-6 signaling system in both atherogenesis and acute plaque rupture (34,35). Such data are useful when considering causal pathways since similar Mendelian randomization studies of the downstream biomarker hsCRP have been neutral. Taken together, these latter genetic epidemiology studies suggest that one active target for cytokine-based atherosclerosis therapy likely sits in the upstream interleukin-1β to interleukin-6 axis; by contrast, hsCRP, while a clinically useful biomarker, is unlikely to be an active target. No Mendelian randomization data is currently available that is specific for interleukin-1β.
Figure 2. Meta-analysis of the Associations of Inflammatory Cytokines and Risks of coronary Disease in Prospective Cohort Studies.
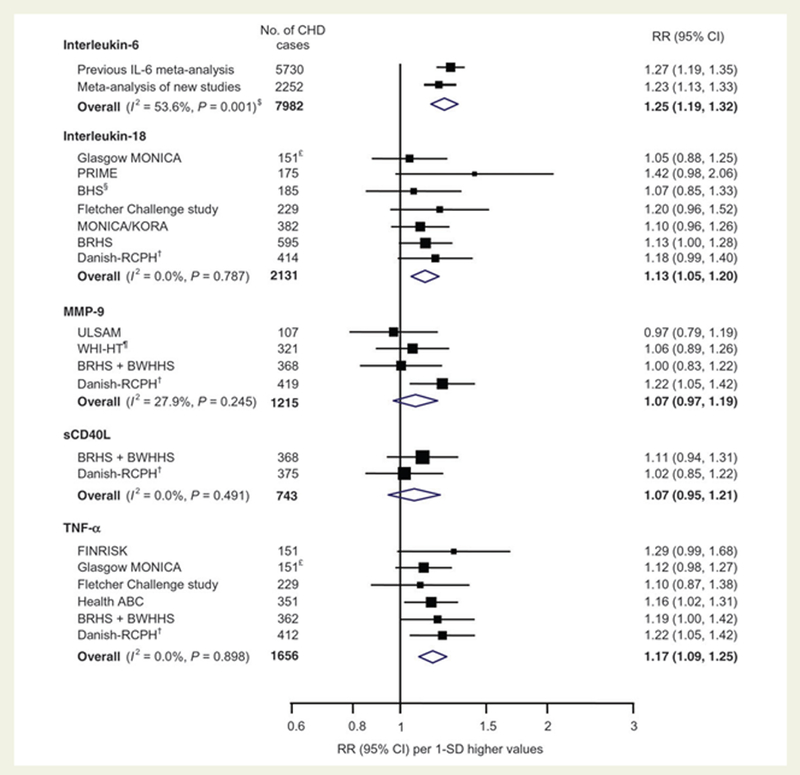
Data are shown for IL-6, IL-18, MMP-9, sCD40L, and TNFa, adjusted for age, gender, smoking status, obesity, blood pressure, and lipid levels. Adapted from Kaptoge et al, Eur Heart J 2014;35:578–589.
Patients with chronic inflammatory disorders including rheumatoid arthritis, psoriasis, inflammatory bowel disease and Crohn’s disease all have increased rates of atherosclerosis, often of early onset. In these settings, observational (non-randomized) data has suggested reduced atherosclerotic event rates in patients taking TNF-inhibitors and low-dose methotrexate (36–38). The recent ENTRACTE trial has found the IL-6 inhibitor tocilizumab to have similar vascular event rates as the TNF-inhibitor etanercept (39).
From a clinical perspective, assessment of low-grade systemic inflammation with hsCRP remains the gold standard (40). As a down-stream biomarker, hsCRP provides a summary read-out of the central inflammatory cascade and captures much of the upstream activity of the interleukin-1 to interleukin-6 signaling pathway.
Despite misconception in the clinical community, large-scale longitudinal biomarker studies demonstrate that the stability and reproducibility of hsCRP over time is virtually identical to that of LDL cholesterol and superior to that of blood pressure (41). hsCRP further adds as much to risk prediction models as does either total or HDL cholesterol (42). As such, the use of hsCRP as a clinical biomarker has increased in practice, a position endorsed in current North American prevention guidelines.
Human data addressing cytokine function and hsCRP were also crucial to understanding the mechanisms by which standard atherosclerosis treatments deliver efficacy. As examples, early human translational work first showed that statins were both anti-inflammatory and lipid lowering agents (43,44). Subsequent analyses of randomized human trials showed that achieving low levels of hsCRP was of similar importance for statin efficacy as was achieving low levels of LDL cholesterol, and that the greatest clinical benefits accrued in patients who achieved low levels of both of these crucial parameters (45–48)(Figure 3). Finally, large-scale outcome trials including JUPITER showed that statins were highly effective in patients with elevated hsCRP and low levels of LDL cholesterol (49,50). Thus, from a clinical perspective, achieving the “dual targets” of low cholesterol and low inflammation maximizes benefit in terms of absolute risk reduction.
Figure 3. Efficacy of Achieving the “Dual Goals” of LDL and hsCRP Reduction.
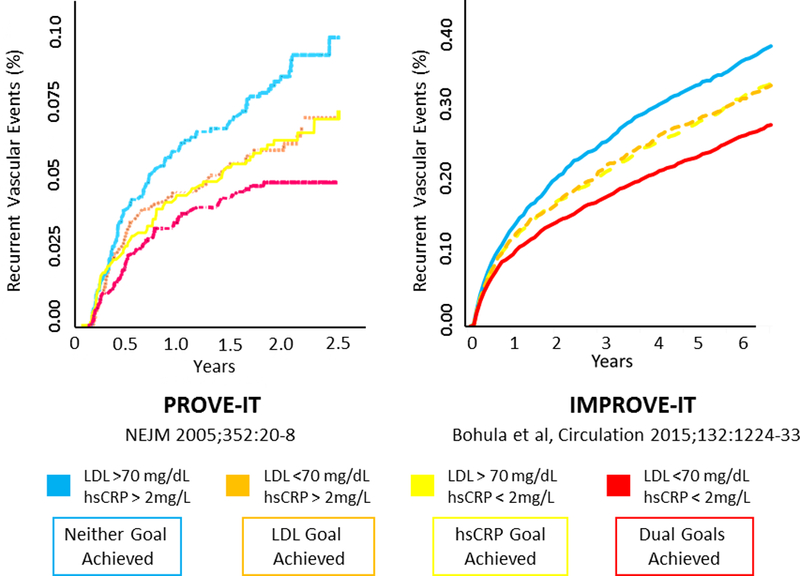
Best cardiovascular outcomes are achieved among atherosclerosis patients treated with statin therapy (left, PROVE-IT) or with statin therapy plus ezetimibe (right, IMPROVE-IT) who not only lowered LDLC below 70mg/dL but who also lowered hsCRP below 2mg/L. Data from Ridker et al, N Engl J Med 2005; 352:20–8 and Bohula et al Circulation 2015; 132:1224–33.
Taken together, these epidemiologic and experimental data suggested that intervention in the central interleukin-1 to interleukin-6 to CRP signaling pathway of innate immunity might lead to clinical benefit for atherosclerosis patients, even in the absence of lipid lowering (51–53). This hypothesis would eventually be tested in the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS)(4).
Initial Human Randomized Trials of Cytokine Inhibition: Targeting TNF in Heart Failure
The first substantive clinical trials of cytokine inhibition in humans were conducted in the setting of heart failure, a logical place to start as patients with acute and chronic heart failure have elevated circulating levels of TNF (54) and TNF promotes left ventricular dysfunction and remodeling (55). Further, initial human intervention data inhibiting TNF activity with the recombinant human TNF receptor etanercept in patients with advanced heart failure suggested dose-dependent improvements in left ventricular structure and function and a trend toward improved functional status (56).
However, subsequent larger trials of etanercept and infliximab (a chimeric monoclonal antibody targeting TNF-α) did not demonstrate benefits on death or hospitalization due to heart failure in patients who already had clinical evidence of left ventricular dysfunction (57,58). Both agents were associated with an increased risk of infection and the infliximab data for clinical outcomes suggested, if anything, a potential hazard.
Despite these data, heart failure remains an attractive target for anti-cytokine therapies. In this regard, small studies with anakinra, an interleukin-1 receptor antagonist, suggest potential benefits on remodeling and inflammatory biomarkers among heart failure patients (59,60). Further, recent findings from CANTOS demonstrate modest dose-dependent benefits with canakinumab for the endpoint of hospitalization for heart failure with the greatest magnitude of benefit tracking with the magnitude of cytokine inhibition.
Initial Human Randomized Trials of Cytokine Inhibition: Targeting Interleukin-1β in Acute Ischemia and Chronic Atherosclerosis
To date, the most successful trial of anti-cytokine therapy has involved inhibition of interleukin-1, in particular interleukin-1β, the dominant circulating form of interleukin-1 and a powerful inducer of innate immune function (61). Interleukin-1β is activated through caspase cleavage in the NLRP3 inflammasome and plays critical roles in early atherosclerotic plaque development and in the processes leading to acute rupture and subsequent vascular hypoxia (52,53). Several pharmacologic agents are available that alter interleukin-1 function including anakinra (an interleukin-1 receptor antagonist that inhibits interleukin-1α and interleukin-1β), rilonocept (an interleukin-1 trap that further inhibits the interleukin-1 receptor), and the selective monoclonal antibodies canakinumab and gevokizumab that directly target interleukin-1β (51,62). The MRC-ILA Heart Study showed initial promise for two weeks anakinra treatment in non-ST elevation acute coronary syndromes where reductions in the area under the CRP curve was observed but with little long-term benefit (63).
Prior to initiating the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) of chronic atherosclerosis, a pilot study was conducted among 566 high-risk patients with diabetes. Overall, canakinumab injections across a dose range of 5, 15, 50, and 150mg/month reduced fibrinogen, interleukin-6, and hsCRP by 15, 50, and 64 percent, respectively, in the absence of any effects on HDL or LDL cholesterol (64). However, no clear dose-response effect on these biomarkers was observed between the 50mg/month and the 150mg/month doses in terms of cytokine inhibition which persisted for as long as 3 to 6 months after injection. For this reason, an “anchor dose” of canakinumab 150mg once every three months was selected for CANTOS with both higher and lower doses introduced to address issues of auto-induction, dose-response, and safety.
CANTOS was designed as a large-scale proof-of-principle trial. 10,061 patients with previous myocardial infarction and hsCRP levels of 2mg or greater were randomly allocated to aggressive standard of care plus placebo or to one of three doses of SC canakinumab given every three months (50mg, 150mg, or 300mg)(4). Virtually all participants were on statin therapy (the median baseline LDL cholesterol was 82mg/dL) and three-quarters had previously undergone coronary revascularization procedures.
After 48 months, when compared to placebo participants, those allocated to canakinumab at the 150 or 300 mg doses had 35 to 40% reductions in interleukin-6 and hsCRP with no change in LDL or HDL cholesterol. Yet, actively treated participants in CANTOS experienced a 15 percent reduction in major adverse cardiovascular events (MACE, hazard ratio=0.85, 95%CI 0.76–0.96, P=0.007 for the pooled 150mg and 300mg canakinumab doses) and a 17 percent reduction in MACE plus the additional endpoint of hospitalization for unstable angina requiring urgent revascularization (MACE+, hazard ratio=0.83, 95%CI 0.74–0.92, P=0.0006 for the pooled 150mg and 300mg canakinumab doses)(Figure 4). By contrast, the lower 50mg dose of canakinumab reduced IL-6 and hsCRP by lesser amounts and resulted in non-significant 7 to 10 percent reductions in MACE and MACE-+, respectively (4).
Figure 4. Anti-cytokine Therapy to Reduce Cardiovascular Risk: CANTOS Main Results.
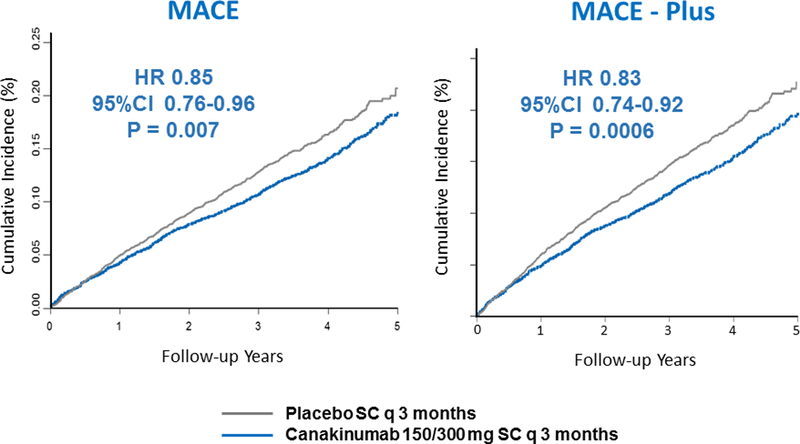
Cumulative incidence of MACE (myocardial infarction, stroke, or cardiovascular death, left) or MACE+ (myocardial infarction, stroke, hospitalization for unstable angina requiring urgent revascularization, or cardiovascular death, right) in the CANTOS trial. Data are shown for the placebo group and for those in the canakinumab 150mg and 300mg groups. These doses of canakinumab resulted in 35 to 40 percent reductions in hsCRP and interleukin-6 with no effect on LDL cholesterol. Data from Ridker et al, N Engl J Med 2017;377:1119–1131
An equally important observation in CANTOS was that the magnitude of cytokine reduction achieved by individual trial participants was a major determinant of clinical efficacy. As commonly observed with many therapeutic agents, there was wide inter-individual variation in response to canakinumab; in pre-specified analyses defined by the magnitude of IL-6 or hsCRP reduction achieved after taking the initial dose, those with greater than average levels of cytokine inhibition in CANTOS had substantially greater clinical benefits (Figure 5). Moreover, these “cytokine responders” had 31 percent reductions in cardiovascular and all-cause mortality (both P-values <0.001)(65,66).
Figure 5. Lower is Better for IL-6 and hsCRP: The CANTOS Trial On-Treatment Findings.
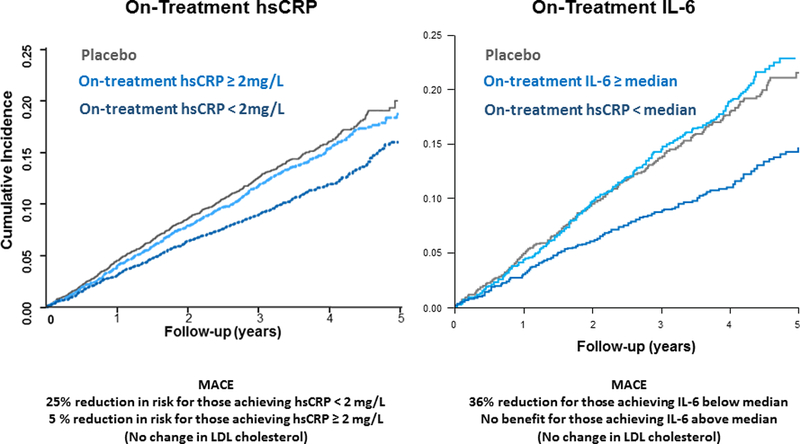
Greater risk reductions were seen in CANTOS with greater hsCRP reductions (left) and greater IL-6 reductions (right). Data are shown for those who achieved hsCRP < 2mg/L or IL-6 levels below the trial median measured three months after a single dose of canakinumab. Adapted from Ridker et al Lancet 2018;391:319–28 and Ridker et al, Eur Heart J 2018:ehy310-ehy310.
The importance of CANTOS for the vascular biology community had been commented on widely (67–74). Fundamentally, CANTOS provides the first hard evidence that inhibiting cytokine function, at least in the central interleukin-1 to interleukin-6 signaling pathway of innate immunity, can lower vascular risk independent of any changes in lipids or blood pressure.
Adverse Effects of Biologic Cytokine Inhibition
As would be anticipated, the major side effect of biologic agents targeting specific cytokines is reduced host defense and consequent increased rates of infection. In CANTOS, where participants underwent interleukin-1β blockade for up to 5 years, this concern was smaller than anticipated by the investigators; while there was a statistically significant absolute increase in fatal infection in roughly 1 participant per thousand treated when all doses of canakinumab were compared to placebo (P=0.02), rates of total infections were similar across study groups (4). The majority of infections were secondary to gram positive organisms with no evidence of opportunistic infection or reactivation of tuberculosis. As such, if detected early using standard clinical surveillance, the infectious risk associated with interleukin-1 inhibition can be mitigated with antibiotic therapy.
The second major adverse concern related to biologic inhibitors has been the potential for increased risks of cancer. In CANTOS, however, no increase in risk of any major cancer was observed. Rather, a significant reduction in total cancer was reported due almost entirely to a reduction in fatal and nonfatal lung cancer (75). Most of this protective effect was for non-small cell lung carcinoma, a tumor class known to be promoted by smoking which is a proinflammatory stimuli. Of particular interest, the benefits on incident lung cancer in CANTOS were dose-dependent (Figure 6) suggesting that cytokine-inhibition in the tumor micro-environment may have substantive effects on tumor progression, invasion, and metastasis. These trial data are consistent with hypotheses raised in the oncology community about the role of inflammation inhibition in certain specific tumors, including non-small cell lung cancer (76–79).
Figure 6. Interleukin-1β Inhibition and Lung Cancer.
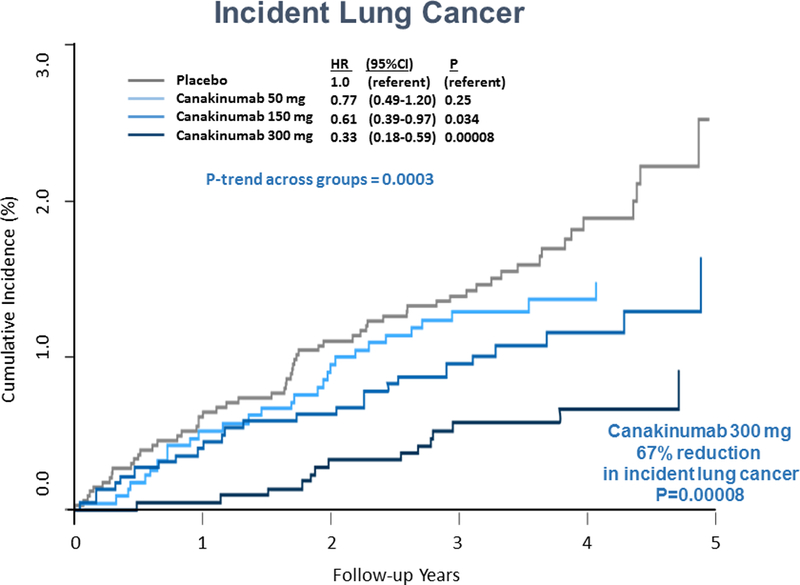
Cumulative incidence of lung cancer (left) and fatal lung cancer (right) among CANTOS participants randomly allocated to placebo, canakinumab 50mg, canakinumab 150mg, or canakinumab 300mg. Adapted from Ridker et al, Lancet 2017;390:1833–42.
A rare, poorly understood, but potentially fatal complication of rituximab, efalizumab, and natalizumab in the setting of autoimmune disease treatment has been the demyelinating disorder progressive multifocal leukoencephalopathy (PML). No cases of PML were reported in CANTOS.
The CIRT Trial: What does the neutral data for methotrexate teach us?
CANTOS tested the inflammation hypothesis of atherothrombosis using a narrow spectrum approach specifically targeting IL-1β with consequent inhibition of the downstream IL-6 to CRP pathway. By contrast, the NHLBI-funded Cardiovascular Inflammation Reduction Trial (CIRT) was designed to address whether an alternative broad-spectrum anti-inflammatory approach using low-dose methotrexate (LDM) might also reduce vascular event rates (5,80). LDM is widely used as primary therapy for systemic inflammatory conditions such as rheumatoid arthritis, psoriatic arthritis, and juvenile idiopathic arthritis. As with biologics, observational data also suggested that inflammatory patients treated with LDM have reduced vascular event rates (81).
To formally test this possibility, CIRT randomly allocated 4,786 North American patients with known atherosclerosis and either diabetes or metabolic syndrome to aggressive standard of care plus placebo or LDM at a target dose of 15 to 20 mg weekly, with all participants being given daily folic acid. As recently reported, during up to 5 years of follow-up, LDM neither reduced interleukin-1β, interleukin-6, nor hsCRP in this patient group nor had any impact on recurrent vascular event rates; overall, the hazard ratio for incident myocardial infarction, stroke, or cardiovascular death was 1.04 (95%CI 0.83–1.30) while the corresponding hazard ratio for these major adverse cardiovascular events plus the additional endpoint of hospitalization for unstable angina requiring revascularization was 0.97 (95%CI 0.79–1.19)(Figure 7)(5).
Figure 7. Low-Dose Methotrexate Neither Reduces IL-1β, IL-6 or CRP, nor Lowers Cardiovascular Event Rates.
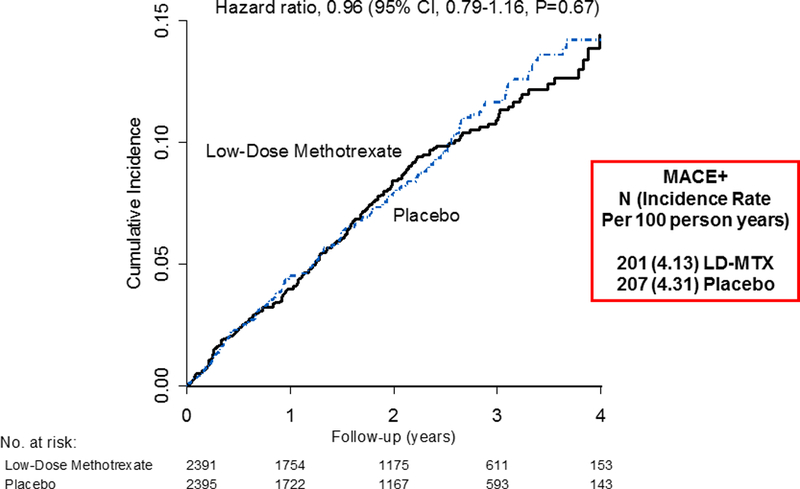
Cumulative incidence of major adverse cardiovascular events plus hospitalization for unstable angina in the Cardiovascular Inflammation Reduction Trial (CIRT). Adapted from Ridker et al, N Engl J Med 2018 (5).
The CIRT and CANTOS trials differ in important ways that are highly informative from a pathophysiologic perspective. CANTOS was specifically designed to address “residual inflammatory risk” (82) and thus only enrolled post-myocardial infarction patients with elevated hsCRP (median hsCRP at baseline 4.2 mg/L). CIRT by contrast had no hsCRP screening criteria and levels were normal at entry (median hsCRP 1.5 mg/L). Thus, one possibility is that anti-cytokine or anti-inflammatory therapies are only effective among atherosclerosis patients with a clear pro-inflammatory signal.
On the other hand, while canakinumab in CANTOS substantially lowered interleukin-6 and hsCRP by 35 to 55 percent through direct interleukin-1 inhibition, LDM had no impact at all on these critical biomarkers of the interleukin-1 signaling pathway. These data emphasize that the underlying mechanisms for the anti-inflammatory and anti-cytokine effects of LDM and canakinumab differ markedly; as such, the data from CIRT for low-dose methotrexate provide an important neutral control for the positive data from CANTOS for canakinumab. This insight should help to focus future work directly on the interleukin-1 to interleukin-6 pathway. The neutral data for LDM in CIRT are also consistent with prior neutral outcome data for the putative anti-inflammatory agents losmapimod and darapladib that also do not chronically reduce the central interleukin-1 to interleukin-6 signaling pathway (83,84).
Cytokine Blockade and Stroke
In parallel with evidence for coronary atherosclerosis, pre-clinical data implicate interleukin-1 inhibition in animal models of stroke including critical findings that interleukin-1β plays a role in blood brain barrier permeability; this non-atherosclerotic effect alters post-stroke edema and hence could reduce stroke severity (85,86). As reviewed elsewhere, a series of small clinical trials testing IL-1Ra in acute stroke have been conducted which generally show safety, reductions in interleukin-6 and CRP, and non-significant trends toward improved outcomes (87). Cytokine reductions in cerebrospinal fluid have also been reported following IL-1Ra therapy in the setting of aneurysmal subarachnoid hemorrhage (88). No stroke study to date has been adequately powered to address efficacy of IL-1Ra on hard clinical endpoints; however, in the recently reported SCIL-STROKE (Subcutaneous Interleukin-1 Receptor Antagonist in Ischemic Stroke) trial in which acute stroke patients were treated with anakinra or placebo, plasma levels of interluekin-6 were reduced and a promising but non-significant reduction observed for the secondary outcome of stroke severity (odds ratio 0.67, 95%CI 0.29–1.52, P=0.34)(89). In provocative recent work, post-ischemic administration of a murine canakinumab analog modestly improved outcomes in experimental stroke; in mice subjected to transient middle cerebral artery occlusion and reperfusion, cerebral infarct volume was reduced, ipsilateral edema declined, and reduced MMP-2 expression and neutrophil infiltration were observed (90).
The above data support consideration for a major outcomes trial of interleukin-1β inhibition in acute stroke (91). In CANTOS, a trial of post-myocardial infarction patients, the incidence rate for stroke was 0.69 per 100 person-years in the combined canakinumab groups as compared to 0.74 per 100 person-years in the placebo group, a non-significant 7 percent reduction (4). It is possible that dose-dependent effects are relevant for stroke prevention as the 300mg canakinumab arm of CANTOS had the lowest incidence rate for total stroke (0.60 events per 100 person years)(HR 0.80, 95%CI 0.57–1.13).
Cytokine Blockade and Atherosclerotic Events in Chronic Kidney Disease
As with atherosclerosis, enhanced activity of the innate and adaptive immune system also plays a role in renal dysfunction. Abundant work implicates the NLRP3 inflammasome in renal failure and experimental work has suggested benefit from interleukin-1 inhibition in several models of renal failure (92,93). However, data from early clinical trials targeting inflammation in kidney disease has been neutral.
CANTOS included 1875 patients with moderate chronic kidney disease (estimated glomerular filtration rate < 60ml/min/1.73m2). As anticipated, this subgroup had substantially higher rates of major cardiovascular events, cardiovascular mortality, and all-cause mortality when compared to CANTOS participants with normal renal function (94). Over the median 3 year follow-up period, canakinumab did not slow progression of eGFR decline nor alter the urinary albumin to creatinine ratio. However, in this high risk chronic kidney disease subgroup, canakinumab significantly reduced major adverse cardiovascular events, particularly among those who had the greatest reduction in interleukin-6 and hsCRP; in the “cytokine responder” population, the observed hazard ratios for MACE, MACE-Plus, and cardiovascular mortality were 0.70, 0.68, and 0.61, respectively (all P-values < 0.01). These data are provocative from both pathophysiologic and clinical perspectives since statin therapy is only modestly effective in renal failure and LDL cholesterol is not a useful risk marker in patients with severe renal failure or during dialysis. By contrast, interleukin-6 and hsCRP levels strongly predict mortality in pre-dialysis patients and those with moderate to severe forms of chronic kidney disease (95,96). Thus, extension of these interleukin signaling data into the setting of severe renal failure is a promising direction (97). The renal subgroup data from CANTOS are hypothesis generating and require direct testing.
Cytokine Blockade and Atherosclerotic Events in Diabetes
Plasma levels of interleukin-6 and hsCRP predict risk of incident type 2 diabetes (98), cytokine activation is a hallmark of insulin resistance and the metabolic syndrome (99), and the NLRP3 inflammasome with consequent production of interleukin-1 and interleukin-18 is intimately involved in glucose regulation (100). It has thus long been hypothesized that inflammation inhibition could play a major role in diabetes management (101). Anakinra, an interleukin-1 receptor antagonist, has been shown to improve beta cell function and modestly lower glycosylated hemoglobin (102), effects similar to that of gevokizumab, a therapeutic antibody targeting interleukin-1β. Salicylate, through inhibition of NF-κβ, also modestly lowers hemoglobin A1c in patients with type 2 diabetes (103) while TNF blockade with etanercept has had mixed effects.
Affirming earlier work, baseline interleukin-6 and hsCRP levels in CANTOS associated with incident type 2 diabetes (104). Further, random allocation to canakinumab reduced major cardiovascular events with similar efficacy among those with prevalent diabetes, pre-diabetes, and normoglycemia. Yet, while interleukin-1β inhibition in CANTOS modestly reduced HbA1c during the first 6 to 9 months of treatment, no long-term benefits on glucose levels were observed nor was there a significant reduction in incident diabetes (105).
Relationships of Clonal Hematopoiesis to Circulating Cytokines and Atherosclerotic Risk
Clonal hematopoiesis of indeterminate potential (CHIP) is a normal variant of aging in which expansion of hematopoietic clones carrying somatic mutations provides a selective advantage and comes to dominate circulating white blood cell lines (106). Several recent cohort studies indicate that carriers of CHIP are at increased cardiovascular risk, independent of traditional risk markers (107,108). Approximately 10 percent of the general population over age 70 has CHIP, typically characterized by common loss-of-function mutations in the genes DNMT3A, TET2, ASXL1, and JAK2. Of these, data for TET2 have been particularly striking as atherosclerosis-prone mice transplanted with TET2 deficient bone marrow have accelerated atherosclerosis and premature heart failure, as well as excess production of interleukin-1β and intereleukin-18 (109,110). Activation of the interleukin-1 associated NRLP3 inflammasome thus appears to be linked closely to clonal hematopoiesis, a finding consistent with parabiotic mouse models that implicate interleukin-1β as a central cytokine linking atherosclerosis to bone marrow and splenic function (111). DNMT3A and JAK2 also play significant roles for inflammation resolution and thrombosis (112). Preliminary genetic analyses suggest that, due to screening for elevated hsCRP, CHIP associated mutations have an altered prevalence in CANTOS and that part of the clinical efficacy of canakinumab likely relates to altering CHIP-associated cardiovascular risk (113).
Beyond IL-1: the NLRP3 inflammasome, IL-18, IL-6, CD40-CD40L and TREM-1 as alternative cytokine targets
As critical components of the innate immune system, inflammasomes comprise macromolecular protein complexes capable of recognizing a variety of immune stimuli including microbial and non-microbial pathogens, exogenous crystalline structures, and cell degradation products such as pathogen-associated molecular patterns (PAMPS) and damage-associated molecular patterns (DAMPS)(114). The sensing component of most inflammasomes contain nucleotide-binding oligomerization domain-containing (NOD)-like receptor (NLR) proteins, and the best described inflammasome relevant to cardiovascular disease is the NLRP3 inflammasome (115).
Activation of caspase 1 is critical for both NLRP3 formation and function, leading to the cleavage of pro-interleukin-1β into locally active interleukin-1β. Caspase 1 within the NLRP3 inflammasome also cleaves pro-interleukin-18 into its active form. Like interleukin-1 and interleukin-6, plasma levels of interleukin-18 correlate with increased cardiovascular risk. Preliminary data from CANTOS further indicates that plasma interleukin-18 levels remain a determinant of residual risk, even after targeted interleukin-1β inhibition. The NLRP3 inflammasome also provides linkage between chronic inflammation and functional decline in aging, thymic immunosenescence, and sarcopenia (116,117). Crystalline structures that can activate the inflammasome include urate crystals (the triggering agent in gout) and cholesterol crystals (118).
Given the success of anti-interleukin-1β therapy demonstrated in CANTOS, it is logical to consider moving one-step upstream to employ NLRP3 inhibitors in cardiovascular disease. Early data with colchicine, a microtubule inhibitor with NLRP3 inhibiting effects, are promising and outcome trials of this agent are underway (119). In apo-E deficient mice, the selective NLRP3 inhibitor MCC950 has shown reduced development of atherosclerotic lesions as well as ICAM and VCAM mRNA expression (120). MCC950 also reduces infarct size and preserves ventricular function in pig models of myocardial infarction and has shown efficacy in the treatment of inflammatory disorders in man (121). Of recent interest, NLRP3 inhibition may have benefits in nonalcoholic steatohepatitis. In the future, orally available NLRP3 inhibitors will likely become available and require testing in various atherosclerotic settings.
If targeting the NLRP3 inflammasome represents a step upstream in the interleukin-1 signaling pathway, direct inhibition of interleukin-6 (an interleukin signaling product of interleukin-1 activation) represents a step downstream. As described above, in CANTOS, much of the benefit of interleukin-1β inhibition was mediated through modulation of interleukin-6 (Figure 6)(66). Thus, direct targeting of interleukin-6 with agents that either block interleukin-6 binding (such as tocilizumab) or that alter interleukin-6 receptor activity (such as sarilumab) merit consideration for atheroprotection. In a small randomized trial of patients with ST elevation myocardial infarction, tocilizumab reduced troponin levels suggesting smaller infarct size (122). This observation is being corroborated in the ongoing Assessing the Effect of Anti-IL-6 Treatment in Myocardial Infarction Study (ASSAIL, NCT03004703). A recent phenome-wide association study has further linked IL-6 receptor variants with increased risks of aortic aneurysm, myocardial infarction, and chronic forms of atherosclerosis (123).
In parallel to the CIRT trial of low-dose methotrexate, a trial of hydroxychloroquine to prevent recurrent events after myocardial infarction has been initiated (124). Hydroxychloroquine is an anti-malarial with anti-inflammatory properties used as second line therapy in rheumatoid arthritis and lupus. It is reported that hydroxychloroquine may reduce TNF, interleukin-1 and interleukin-6 production though the consistency and mechanisms of this effect are uncertain.
The co-stimulatory CD40-CD40L receptor-ligand signaling dyad represents an additional inflammatory pathway relevant for atherothrombosis (125, 126). Inhibition of CD40 signaling reduces atherosclerosis in mice (127) and CD40-CD40L binding leads to recruitment of tumor necrosis factor receptor-associated factors (TRAFs) of which CD40-TRAF6 is involved in atherogenesis (128). Small molecule inhibitors of CD40-TRAF6 have been developed that have the ability to reduce atherosclerosis and markedly slow plaque progression in ApoE−/− mice without inducing major immune suppression or thromboembolic events (129). Whether these effects are independent of interleukin-1 inhibition and can translate to human atherosclerosis requires further investigation (130).
Further translational work is also needed for investigations of TREM-1 (triggering receptor expressed on myeloid cells 1) which has been shown to stimulate the inflammatory response after acute coronary ischemia (131). Supporting this concept, genetic variants in TREM1 may be associated with human coronary disease, an observation requiring large-scale replication (132). Recently, inhibition of TREM-1 has been shown to reduce atherosclerosis progression and vascular inflammation in LDLr-deficient and ApoE deficient mice (133, 134).
Future Directions: Resolvins, Bioelectric Therapy, and Vaccination Strategies
Resolution of acute inflammation is increasingly recognized to play critical roles in innate and adaptive immunity and is no longer believed a passive process. Rather, as reviewed by Serhan and colleagues, the resolution of inflammation and return to homeostasis follows a reproducible and characteristic program involving lipoxins, resolvins, and protectins (135). All of these mediators, generally derived from the omega-3 fatty acids eicosapentanoic acid (EPA) and docosahexanoic acid (DHA), have both anti-inflammatory and pro-resolution properties that can slow neutrophil and eosinophil infiltration, enhance macrophage phagocytosis, and activate host defense. Synthetic versions of these mediators are in development and have shown initial promise in ischemia-reperfusion injury (136).
Bioelectric therapy and stimulation of the autonomic inflammatory reflex represents a particularly novel mechanism to alter cytokine production. Neural control of the acute inflammatory response is partly reflexive through a cholinergic anti-inflammatory pathway, with afferent vagal signaling to the brain triggered by local tissue damage and related inflammatory stimuli, and efferent vagal activity inhibiting cytokine production through ACTH production (137, 138). Direct electrical stimulation of the efferent vagus nerve suppresses inflammation in part by lowering TNF synthesis in liver, spleen, and heart, while vagotomy produces near opposite effects. Whether bioelectric stimulation of the vagus can produce reproducible and sustainable effects on innate immune function with clinical benefit is an ongoing area of research (139).
As expertly reviewed elsewhere, vaccination strategies designed to activate anti-inflammatory components of the adaptive immune response have long shown promise (140–142). In an early pre-clinical trial, blockade of interleukin-12 function through protein vaccination lead to attenuation of atherosclerosis in LDLr−/− mice (143). More recent murine evidence with erlotinib, a selective inhibitor of the epidermal growth factor receptor, also suggests reductions in experimental atherosclerosis (144,145).
Combining PCSK9 Inhibition with Anti-Cytokine Therapy: Can We Eliminate Residual Risk?
Given that atherosclerosis is a disorder driven both by lipid accumulation and inflammation, very aggressive concurrent targeting of cholesterol production and the central cytokine cascade may provide a method to markedly eliminate residual risk.
As described above, it has been known for 20 years that statins are “two-fers” with both anti-inflammatory and lipid-lowering properties reflecting their ability to simultaneously lower hsCRP and LDL cholesterol (43,44,49). This insight eventually led to the JUPITER trial that demonstrated considerable efficacy of high-intensity statin therapy among individuals with low levels of LDL-C but elevated levels of hsCRP (50).
More recently, hard outcome evidence has emerged from the FOURIER, SPIRE1, SPIRE2, and ODYSSEY trials (146,147) that PCSK9 inhibitors – agents with powerful LDL and Lp(a) lowering effects in the virtual absence of anti-inflammatory effects - can reduce “residual cholesterol risk” by 15 to 18 percent with substantially larger benefits of 25 to 30 percent accruing among “LDL responders”, defined as patients achieving the greatest post-treatment reductions in LDL cholesterol. These effects are virtually identical in magnitude to those observed in CANTOS using an agent with powerful anti-inflammatory properties in the absence of lipid lowering.
There are several reasons to believe that therapy combining a PCSK9 inhibitor with an inhibitor of the interluekin-1 to interleukin-6 signaling pathway will provide at least additive effects. First, as shown a decade ago, there is virtually no relationship between on-treatment hsCRP and on-treatment LDLC, at least with statin therapy (46). Second, because of this biologic independence, it has consistently been observed in hard outcome trials that the benefits of hsCRP lowering are present at all levels of LDL cholesterol (46,47). Third, as recently presented in the SPIRE-1, SPIRE-2, and FOURIER trials, there is substantial “residual inflammatory risk” among patients already treated with a PCSK9 inhibitor (148,149). In FOURIER, absolute risk among patients with on-treatment LDLC < 30 mg/dL but elevated hsCRP exceeds that of patients with LDLC levels above 100). The same observation has been made in CANTOS in reverse; absolute risk among those with low levels of hsCRP but elevated levels of LDLC remain substantially high.
All of the above evidence supports the clinical potential for a combination atherosclerosis therapy incorporating very aggressive dual inhibition of lipid production as well as the central interleukin-1 to interleukin-6 pathway of innate immunity. In the simplest case, such combination therapy might include dual injections of both evolocumab and canakinumab. Alternatively, a bi-specific monoclonal antibody could be constructed to simultaneously inhibit PCSK9 and interleukin-1β. As a third example, similar results might be anticipated for concurrent administration of an siRNA targeting LDL cholesterol and an interleukin-6 reducing monoclonal antibody. Such combination therapies, if proven to reduce secondary events in at least an additive model, would thus supercede other lipid lowering or anti-inflammatory strategies in current development. The 2 by 2 factorial trial outlined in Figure 8 provides a conceptual framework for a trial to directly test this hypothesis.
Figure 8. Combining Aggressive LDL Reduction with Concomitant Anti-Cytokine Therapy.
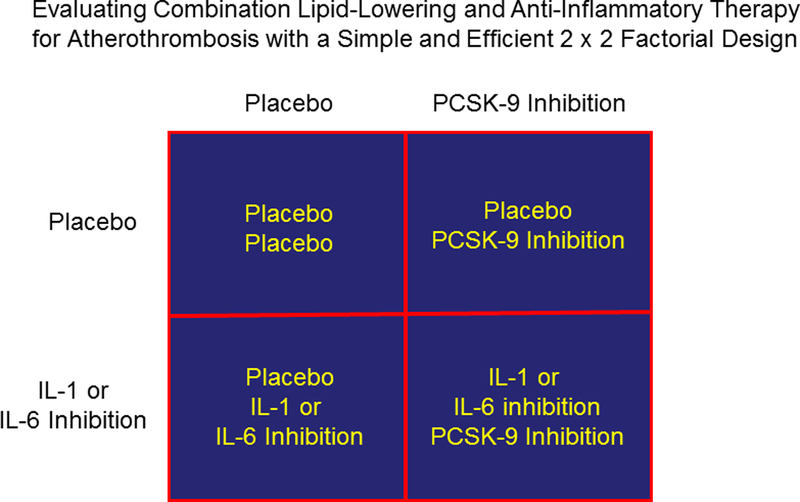
The simple two-by-two factorial design trial shown could be used to test both the independent and additive effects of lipid-lowering and inflammation inhibition in the treatment of atherosclerosis.
Acknowledgments
Source of Funding: National Heart Lung and Blood Institute, Bethesda, MD; Novartis, Basil Switzerland.
Non-standard Abbreviations and Acronyms
- ASSAIL
Assessing the Effect of Anti-IL-6 Treatment in Myocardial Infarction Study
- CANTOS
Canakinumab Antiinflammatory Thrombosis Outcomes Study
- CIRT
Cardiovascular Inflammation Reduction Trial
- FOURIER
Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk
- IMPROVE-IT
Improved Reduction of Outcomes: Vytorin Efficacy International Trial
- JUPITER
Justification for the Use of Statin in Prevention: An Intervention Trial Evaluating Rosuvastatin
- PROVE-IT
Pravastatin or Atorvastatin Evaluation and Infection Trial
- SCIL-STROKE
(Subcutaneous Interleukin-1 Receptor Antagonist in Ischemic Stroke)
- SPIRE
Studies of PCSK9 Inhibition and the Reduction of Vascular Events
Footnotes
Conflicts of Interest. Dr. Ridker designed and served as the Principle Investigator of the JUPITER, CANTOS, SPIRE-1, SPIRE-2, and CIRT trials described here which were funded by AstraZeneca, Novartis, Pfizer, and the NHLBI, respectively. Dr. Ridker has served as a consultant to Novartis, Pfizer, Corvidia, BioCivi, Amgen, Merck, Janssen, and Inflazome and is listed as a co-inventor on patents held by the Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease and diabetes that have been licensed to AstraZeneca and Seimens.
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–95. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317–25 [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM. How common is residual inflammatory risk? Circ Res 2017;120:617–619. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Everett B, Thuren T, Macfadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova A, Lorenzatti A, Forster T, Kobalava Z, Vida-Smith L, Flather M, Shimokowa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ for the CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ for the Cardiovascular Inflammation Reduction Trial (CIRT) Investigators. Low dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2018. (on line November 10)DOI 10.1056/NEJMoa1809798. [DOI] [Google Scholar]
- 6.Tousoulis D, Oikonomou E, Economou EK, Crea F, Kaski JC. Inflammatory cytokines in atherosclerosis: Current therapeutic approaches. Eur Heart J 2016;37:1723–1732. [DOI] [PubMed] [Google Scholar]
- 7.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol 2011;31:969–979. [DOI] [PubMed] [Google Scholar]
- 8.Ramji DP, Davies TS. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev 2015;26:673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tedgui A, Mallat Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiological reviews 2006;86:515–581. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello CA. Anti-inflammatory agents: Present and future. Cell 2010;140:935–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinarello CA. Demonstration of a human pyrogen-inducing factor during mixed leukocyte reactions. J Exp Med 1981;153:1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: A comprehensive review of studies in mice. Cardiovasc Res 2008;79:360–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohta H, Wada H, Niwa T, Kirii H, Iwamoto N, Fujii H, Saito K, Sekikawa K, Seishima M. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in apoe-deficient mice. Atherosclerosis 2005;180:11–17. [DOI] [PubMed] [Google Scholar]
- 14.Canault M, Peiretti F, Mueller C, Kopp F, Morange P, Rihs S, Portugal H, Juhan-Vague I, Nalbone G. Exclusive expression of transmembrane tnf-alpha in mice reduces the inflammatory response in early lipid lesions of aortic sinus. Atherosclerosis 2004;172:211–218. [DOI] [PubMed] [Google Scholar]
- 15.Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein e knockout mice. Arterioscler Thromb Vasc Biol 2004;24:2137–2142. [DOI] [PubMed] [Google Scholar]
- 16.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in apoe-deficient mice. Arterioscler Thromb Vasc Biol 2003;23:656–660. [DOI] [PubMed] [Google Scholar]
- 17.Merhi-Soussi F, Kwak BR, Magne D, Chadjichristos C, Berti M, Pelli G, James RW, Mach F, Gabay C. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein e-knockout mice. Cardiovasc Res 2005;66:583–593. [DOI] [PubMed] [Google Scholar]
- 18.Shimokawa H, Ito A, Fukumoto Y, Kadokami T, Nakaike R, Sakata M, Takayanagi T, Egashira K, Takeshita A. Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. J Clin Invest 1996; 97:769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton AC, Arnold ND, Gunn J, Varcoe R, Francis SE, Dower SK, Crossman DC. Interleukin-1 receptor antagonist alters the response to vessel wall injury in a porcine coronary artery model. Cardiovasc Res 2005;68:493–501. [DOI] [PubMed] [Google Scholar]
- 20.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol 1999;19:2364–2367. [DOI] [PubMed] [Google Scholar]
- 21.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein e(−/−) mice through release of interferon-gamma. Circ Res 2002;90:E34–38. [DOI] [PubMed] [Google Scholar]
- 22.Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, Bayard F, Hansson GK. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein e-knockout mice. Cardiovasc Res 2003;59:234–240. [DOI] [PubMed] [Google Scholar]
- 23.Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, Humbert Y, Chvatchko Y, Tedgui A. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res 2001;89:E41–45. [DOI] [PubMed] [Google Scholar]
- 24.Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL, Owens GK. Genetic inactivation of il-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest 2012;122:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez D, Baylis RA, Durgin BG, Newman AAC, Alencar GF, Mahan S, St Hilaire C, Muller W, Waisman A, Francis SE, Pinteaux E, Randolph GJ, Gram H, Owens GK. Interleukin-1beta has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat Med 2018;24(9):1418–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336:973–9. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000; 342:836–843. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000; 101:1767–1772. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet 1998;351:88–92. [DOI] [PubMed] [Google Scholar]
- 30.Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, Maseri A. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med 1994; 331:417–24. [DOI] [PubMed] [Google Scholar]
- 31.Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB. Production of c-reactive protein and risk of coronary events in stable and unstable angina. European concerted action on thrombosis and disabilities angina pectoris study group. Lancet 1997;349:462–466. [DOI] [PubMed] [Google Scholar]
- 32.Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: Effects of an early invasive or noninvasive strategy. JAMA 2001; 286:2107–13. [DOI] [PubMed] [Google Scholar]
- 33.Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A, Di Angelantonio E, Gudnason V, Rumley A, Lowe GD, Jorgensen T, Danesh J. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J 2014; 35:578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarwar N, Butterworth AS, Freitag DF, et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 2012; 379:1205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swerdlow DI, Holmes MV, Kuchenbaecker KB, et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet 2012; 379:1214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: Anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res 2011;63:522–529. [DOI] [PubMed] [Google Scholar]
- 37.Westlake SL, Colebatch AN, Baird J, Kiely P, Quinn M, Choy E, Ostor AJ, Edwards CJ. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 2010;49:295–307. [DOI] [PubMed] [Google Scholar]
- 38.Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernan MA, Ridker PM, Mozaffarian D. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol 2011;108:1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giles JT, Sattar N, Gabriel S, et al. Cardiovascular safety of tocilzumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Lancet 2018. (in review). [DOI] [PubMed] [Google Scholar]
- 40.Ridker PM. A test in context: High-sensitivity C-reactive protein. J Am Coll Cardiol 2016;67:712–723. [DOI] [PubMed] [Google Scholar]
- 41.Glynn RJ, MacFadyen JG, Ridker PM. Tracking of high-sensitivity C-reactive protein after an initially elevated concentration: the JUPITER Study. Clin Chem 2009; 55:305–12. [DOI] [PubMed] [Google Scholar]
- 42.Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, Butterworth AS, Amouyel P, Assmann G, Bakker SJ, Barr EL, Barrett-Connor E, Benjamin EJ, Bjorkelund C, Brenner H, Brunner E, Clarke R, Cooper JA, Cremer P, Cushman M, Dagenais GR, D’Agostino RB Sr., Dankner R, Davey-Smith G, Deeg D, Dekker JM, Engstrom G, Folsom AR, Fowkes FG, Gallacher J, Gaziano JM, Giampaoli S, Gillum RF, Hofman A, Howard BV, Ingelsson E, Iso H, Jorgensen T, Kiechl S, Kitamura A, Kiyohara Y, Koenig W, Kromhout D, Kuller LH, Lawlor DA, Meade TW, Nissinen A, Nordestgaard BG, Onat A, Panagiotakos DB, Psaty BM, Rodriguez B, Rosengren A, Salomaa V, Kauhanen J, Salonen JT, Shaffer JA, Shea S, Ford I, Stehouwer CD, Strandberg TE, Tipping RW, Tosetto A, Wassertheil-Smoller S, Wennberg P, Westendorp RG, Whincup PH, Wilhelmsen L, Woodward M, Lowe GD, Wareham NJ, Khaw KT, Sattar N, Packard CJ, Gudnason V, Ridker PM, Pepys MB, Thompson SG, Danesh J. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 2012; 367:1310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, Flaker GC, Braunwald E for the Cholesterol and Recurrent Events (CARE) Investigators. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation 1998; 98:839–844. [DOI] [PubMed] [Google Scholar]
- 44.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of c-reactive protein. The cholesterol and recurrent events (care) investigators. Circulation 1999;100:230–235. [DOI] [PubMed] [Google Scholar]
- 45.Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, Orazem J, Magorien RD, O’Shaughnessy C, Ganz P. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005; 352:29–38. [DOI] [PubMed] [Google Scholar]
- 46.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352:20–8. [DOI] [PubMed] [Google Scholar]
- 47.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, Group CT. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: A secondary analysis from the cantos randomised controlled trial. Lancet 2018;391:319–328. [DOI] [PubMed] [Google Scholar]
- 48.Bohula EA, Giugliano RP, Cannon CP, Zhou J, Murphy SA, White JA, Tershakovec AM, Blazing MA, Braunwald E. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of Ezetimibe to Simvastatin and associated with better outcomes in IMPROVE-IT. Circulation 2015; 132:1224–33. [DOI] [PubMed] [Google Scholar]
- 49.Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, Gotto AM Jr, for the Airforce/Texas Coronary Atherosclerosis Prevention Study Investigators. Measurement of C-reactive protein for targeting statin therapy in the primary prevention of acute coronary events. N Engl J Med 2001; 344:26:1959–1965. [DOI] [PubMed] [Google Scholar]
- 50.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–207. [DOI] [PubMed] [Google Scholar]
- 51.Van Tassell BW, Toldo S, Mezzaroma E, Abbate A. Targeting interleukin-1 in heart disease. Circulation 2013;128:1910–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: Moving upstream to identify novel targets for atheroprotection. Circ Res 2016; 118:145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Libby P Interleukin-1 beta as a target for atherosclerosis therapy. Biological basis of CANTOS and beyond. J Am Coll Cardiol 2017;70:2278–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990;323:236–241. [DOI] [PubMed] [Google Scholar]
- 55.Bozkurt B, Kribbs SB, Clubb FJ Jr., Michael LH, Didenko VV, Hornsby PJ, Seta Y, Oral H, Spinale FG, Mann DL. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation 1998;97:1382–1391. [DOI] [PubMed] [Google Scholar]
- 56.Bozkurt B, Torre-Amione G, Warren MS, Whitmore J, Soran OZ, Feldman AM, Mann DL. Results of targeted anti-tumor necrosis factor therapy with etanercept (enbrel) in patients with advanced heart failure. Circulation 2001;103:1044–1047. [DOI] [PubMed] [Google Scholar]
- 57.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT, Anti TNFTACHFI. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation 2003;107:3133–3140. [DOI] [PubMed] [Google Scholar]
- 58.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: Results of the randomized etanercept worldwide evaluation (renewal). Circulation 2004;109:1594–1602. [DOI] [PubMed] [Google Scholar]
- 59.Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, Oddi C, Roberts CS, Melchior RD, Mueller GH, Abouzaki NA, Rengel LR, Varma A, Gambill ML, Falcao RA, Voelkel NF, Dinarello CA, Vetrovec GW. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-anakinra remodeling trial (2) (VCU-ART2) pilot study]. Am J Cardiol 2013;111:1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, Straino S, Biondi-Zoccai GG, Houser JE, Qureshi IZ, Ownby ED, Gustini E, Biasucci LM, Severino A, Capogrossi MC, Vetrovec GW, Crea F, Baldi A, Kukreja RC, Dobrina A. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation 2008;117:2670–2683. [DOI] [PubMed] [Google Scholar]
- 61.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011;117:3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fearon WF, Fearon DT. Inflammation and cardiovascular disease: Role of the interleukin-1 receptor antagonist. Circulation 2008;117:2577–2579. [DOI] [PubMed] [Google Scholar]
- 63.Morton AC, Rothman AM, Greenwood JP, Gunn J, Chase A, Clarke B, Hall AS, Fox K, Foley C, Banya W, Wang D, Flather MD, Crossman DC. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur Heart J 2015; 36:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation 2012; 126:2739–48 [DOI] [PubMed] [Google Scholar]
- 65.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ on behalf of the CANTOS Trial Group. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomized controlled trial. Lancet 2018;391:319–28. [DOI] [PubMed] [Google Scholar]
- 66.Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM, Glynn RJ on behalf of the CANTOS Trial Group. Relationships of interleukin-6 reduction to atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J 2018:ehy310–ehy310. [DOI] [PubMed] [Google Scholar]
- 67.Baylis RA, Gomez D, Mallat Z, Pasterkamp G, Owens GK. The CANTOS trial. One important step for clinical cardiology but a giant leap for vascular biology. Arterioscler Thromb Vasc Biol 2017;37(11):e174–e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hansson GK. Inflammation and atherosclerosis. The end of a controversy. Circulation 2017;136:1875–1877. [DOI] [PubMed] [Google Scholar]
- 69.Ibanez B, Fuster V. CANTOS. A gigantic proof-of-concept trial. Circ Res 2017;121:1320–1322. [DOI] [PubMed] [Google Scholar]
- 70.Weber C, von Hundelshausen P. CANTOS trial validates the inflammatory pathogenesis of atherosclerosis. Setting the stage for a new chapter in therapeutic targeting. Circ Res 2017;121:1119–1121. [DOI] [PubMed] [Google Scholar]
- 71.Crea F, Liuzzo G. Addressing acute coronary syndromes: New challenges and opportunities after the CANTOS trial (Canakinumab Anti-inflammatory Thrombosis Outcomes Study). Circulation 2018;137:1100–1102. [DOI] [PubMed] [Google Scholar]
- 72.Koenig W Inflammation revisited: Atherosclerosis in the post-CANTOS era. Eur Cardiol Rev 2017;12:89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verma S, Leiter LA, Bhatt DL. CANTOS ushers in a new calculus of inflammasome targeting for vascular protection – and maybe more. Cell Metab 2017;26:703–705. [DOI] [PubMed] [Google Scholar]
- 74.Abbate A Why the CANTOS is a game changer in cardiovascular medicine. J Cardiovasc Pharm 2017;70:353–355. [DOI] [PubMed] [Google Scholar]
- 75.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ on behalf of the CANTOS Trial Group. Effect of interleukin-1 inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomized, double-blind, placebo-controlled trial. Lancet 2017;390:1833–42. [DOI] [PubMed] [Google Scholar]
- 76.Dinarello CA. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev 2010; 29:317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev 2006; 25:387–408. [DOI] [PubMed] [Google Scholar]
- 79.Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol 2012; 22:33–40. [DOI] [PubMed] [Google Scholar]
- 80.Everett BM, Pradhan AD, Solomon DH, Paynter N, MacFadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, Ridker PM. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J 2013;166:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernan MA, Ridker PM, Mozaffarian D. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol 2011;108:1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ridker PM. Residual inflammatory risk: addressing the obverse side of the atherosclerosis prevention coin. Eur Heart J 2016;37:1720–22. [DOI] [PubMed] [Google Scholar]
- 83.O’Donoghue ML, Braunwald E, White HD, Lukas MA, Tarka E, Steg PG, Hochman JS, Bode C, Maggioni AP, Im K, Shannon JB, Davies RY, Murphy SA, Crugnale SE, Wiviott SD, Bonaca MP, Watson DF, Weaver WD, Serruys PW, Cannon CP, for the SOLID-TIMI 52 Investigators. Effect of darapladib on major coronary events after an acute coronary syndrome: The SOLID-TIMI 52 randomized clinical trial. JAMA 2014;312:1006–1015. [DOI] [PubMed] [Google Scholar]
- 84.O’Donoghue ML, Glaser R, Cavender MA, Aylward PE, Bonaca MP, Budaj A, Davies RY, Dellborg M, Fox KA, Gutierrez JA, Hamm C, Kiss RG, Kovar F, Kuder JF, Im KA, Lepore JJ, Lopez-Sendon JL, Ophuis TO, Parkhomenko A, Shannon JB, Spinar J, Tanguay JF, Ruda M, Steg PG, Theroux P, Wiviott SD, Laws I, Sabatine MS, Morrow DA, for the LATITUDE-TIMI60 Investigators. Effect of losmapimod on cardiovascular outcomes in patients hospitalized with acute myocardial infarction: A randomized clinical trial. JAMA 2016;315:1591–1599. [DOI] [PubMed] [Google Scholar]
- 85.Relton JK, Martin D, Thompson RC, Russell DA. Peripheral administration of interleukin-1 receptor antagonist inhibits brain damage after focal cerebral ischemia in the rat. Exp Neurol 1996;138:206–213. [DOI] [PubMed] [Google Scholar]
- 86.Blamire AM, Anthony DC, Rajagopalan B, Sibson NR, Perry VH, Styles P. Interleukin-1 induced changes in blood-brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: a magnetic resonance study. J Neurosci 2000;20:8153–8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sobowale OA, Parry-Jones AR, Smith CJ, Tyrell PJ, Rothwell NJ, Allan SM. Interleukin-1 in stroke. From bench to bedside. Stroke 2016;47:2160–2167. [DOI] [PubMed] [Google Scholar]
- 88.Galea J, Ogungbenro K, Hulme S, Patel H, Scarth S, Hoadley M, Illingworth K, McMahon CJ, Tzerakis N, King AT, Vail A, Hopkins SJ, Rothwell N, Tyrell P. Reduction of inflammation after administration of interleukin-1 receptor antagonist following aneurysmal subarachnoid hemorrhage: results of the Subcutaneous Interleukin-1Ra in SAH (SCIL-SAH) study. J Neurosurg 2018;128:515–523. [DOI] [PubMed] [Google Scholar]
- 89.Smith CJ, Hulme S, Vail A, Heal C, Parry-Jones AR, Scarth S, Hopkins K, Hoadley M, Allan SM, Rothwell NJ, Hopkins SJ, Tyrrell PJ. SCIL-stroke (subcutaneous interleukin-1 receptor antagonist in ischemic stroke): A randomized controlled phase 2 trial. Stroke 2018;49:1210–1216. [DOI] [PubMed] [Google Scholar]
- 90.Liberale L, Diaz-Cañestro C, Bonetti NR, Paneni F, Akhmedov A, Beer JH, Montecucco F, Lüscher TF, Camici GG. Post-ischaemic administration of the murine canakinumab-surrogate antibody improves outcome in experimental stroke. Eur Heart J 2018:ehy286–ehy286. [DOI] [PubMed] [Google Scholar]
- 91.Ridker PM. Interleukin-1 inhibition and ischaemic stroke: Has the time for a major outcomes trial arrived? Eur Heart Journal 2018:ehy360–ehy360. [DOI] [PubMed] [Google Scholar]
- 92.Chang A, Ko K, Clark MR. The emerging role of the inflammasome in kidney diseases. Curr Opin Nephrol Hypertens 2014;23:204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hutton HL, Ooi JD, Holdsworth SR, Kitching AR. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology 2016;21:736–744. [DOI] [PubMed] [Google Scholar]
- 94.Ridker PM, MacFadyen JG, Glynn RJ, Koenig W, Libby P, Everett BM, Lefkowitz M, Thuren T, Cornel JH. Inhibition of interleukin-1β by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol 2018;71:2405–2414. [DOI] [PubMed] [Google Scholar]
- 95.Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 2000;35:469–476. [DOI] [PubMed] [Google Scholar]
- 96.Barreto DV, Barreto FC, Liabeuf S, Temmar M, Lemke HD, Tribouilloy C, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work G. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int 2010;77:550–556. [DOI] [PubMed] [Google Scholar]
- 97.Cherney DZI, Lytvyn Y, McCullough PA. Cardiovascular risk reduction in patients with chronic kidney disease. Potential for targeting inflammation with canakinumab. J Am Coll Cardiol 2018;21:2415–8. [DOI] [PubMed] [Google Scholar]
- 98.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–34. [DOI] [PubMed] [Google Scholar]
- 99.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 2017;127:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC, O’Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced il-1beta in type 2 diabetes. Nature immunology 2010;11:897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goldfine AB, Shoelson SE. Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk. J Clin Invest 2017;127:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Larsen CM, Faulenbach M, Vaag A et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007;356:1517–26. [DOI] [PubMed] [Google Scholar]
- 103.Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA, Shoelson SE, Targeting Inflammation Using Salsalate in Type 2 Diabetes Study T. Salicylate (salsalate) in patients with type 2 diabetes: A randomized trial. Ann Intern Med 2013;159:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Everett BM, Donath MY, Pradhan A, Thuren T, Pais P, Nicolau JC, Glynn RJ, Libby P, Ridker PM. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol 2018;71:2392–401. [DOI] [PubMed] [Google Scholar]
- 105.Verma S, Verghese M, Farkouh ME. Targeting inflammation in the prevention and treatment of type 2 diabetes. Insights from CANTOS. J Am Coll Cardiol 2018;21:2402–4. [DOI] [PubMed] [Google Scholar]
- 106.Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, Ebert BL. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015;126:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014;371:2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med 2017; 377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andres V, Hirschi KK, Martin KA, Walsh K. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017; 355:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, Zuriaga MA, Yoshiyama M, Goukassian D, Cooper MA, Fuster JJ, Walsh K. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the il-1beta/nlrp3 inflammasome. J Am Coll Cardiol 2018;71:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sager HB, Heidt T, Hulsmans M, Dutta P, Courties G, Sebas M, Wojtkiewicz GR, Tricot B, Iwamoto Y, Sun Y, Weissleder R, Libby P, Swirski FK, Narhrendorf M. Targeting interleukin-1 reduces leucocyte production after acute myocardial infarction. Circulation 2015;132:1880–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li X, Zhao D, Liu Y, Wang C, Zhang X, Su X, Liu J, Ge W, Levine RL, Li N, Cao X. Tet2 is required to resolve inflammation by recruiting hdac2 to specifically repress il-6. Nature 2015;525:389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Svennson EC, Madar A, Campbell CD, He Y, Sultan M, Healey ML, D’Aco K, Fernandez A, Wache-Mainier C, Ridker PM, Beste MT, Basson CT. TET-2-driven clonal hematopoiesis predicts response to canakinumab in the CANTOS trials: An exploratory analysis. Abstract presented at American Heart Association Scientific Session, November 12, 2018. [Google Scholar]
- 114.Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proil-beta. Molecular cell 2002;10:417–426. [DOI] [PubMed] [Google Scholar]
- 115.Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol 2018;15:203–214. [DOI] [PubMed] [Google Scholar]
- 116.McBride MJ, Foley KP, D’Souza DM, Li YE, Lau TC, Hawke TJ, Schertzer JD. The NLRP3 inflammasome contributes to sarcopenia and lower muscle glycolytic potential in old mice. American journal of physiology. Endocrin Metab 2017;313:E222–E232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Youm YH, Grant RW, McCabe LR, Albarado DC, Nguyen KY, Ravussin A, Pistell P, Newman S, Carter R, Laque A, Munzberg H, Rosen CJ, Ingram DK, Salbaum JM, Dixit VD. Canonical nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab 2013;18:519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010; 464:1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013;61:404–410. [DOI] [PubMed] [Google Scholar]
- 120.van der Heijden T, Kritikou E, Venema W, van Duijn J, van Santbrink PJ, Slutter B, Foks AC, Bot I, Kuiper J. NLRP3 inflammasome inhibition by mcc950 reduces atherosclerotic lesion development in apolipoprotein e-deficient mice-brief report. Arterioscler Thromb Vasc Biol 2017;37:1457–1461. [DOI] [PubMed] [Google Scholar]
- 121.van Hout GP, Bosch L, Ellenbroek GH, de Haan JJ, van Solinge WW, Cooper MA, Arslan F, de Jager SC, Robertson AA, Pasterkamp G, Hoefer IE. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur Heart J 2017;38:828–836. [DOI] [PubMed] [Google Scholar]
- 122.Kleveland O, Kunszt G, Bratlie M, Ueland T, Broch K, Holte E, Michelsen AE, Bendz B, Amundsen BH, Espevik T, Aakhus S, Damas JK, Aukrust P, Wiseth R, Gullestad L. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2 trial. Eur Heart J 2016; 37:2406–13. [DOI] [PubMed] [Google Scholar]
- 123.Cai T, Zhang Y, Ho YL, Link N, Sun J, Huang J, Cai TA, Damrauer S, Ahuja Y, Honerlaw J, Huang J, Costa L, Schubert P, Hong C, Gagnon D, Sun YV, Gaziano JM, Wilson P, Cho K, Tsao P, O’Donnell CJ, Liao KP, Program VAMV. Association of interleukin 6 receptor variant with cardiovascular disease effects of interleukin 6 receptor blocking therapy: A phenome-wide association study. JAMA Cardiol 2018. . [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hartman O, Kovanen PT, Lehtonen J, Eklund KK, Sinisalo J. Hydroxychloroquine for the prevention of recurrent cardiovascular events in myocardial infarction patients: rationale and design of the OXI trial. Eur Heart J Cardiovasc Pharmacology 2017;3:92–97. [DOI] [PubMed] [Google Scholar]
- 125.Schonbeck U, Libby P. Cd40 signaling and plaque instability. Circ Res 2001;89:1092–1103. [DOI] [PubMed] [Google Scholar]
- 126.Lutgens E, Lievens D, Beckers L, Donners M, Daemen M. Cd40 and its ligand in atherosclerosis. Trends Cardiovas Med 2007;17:118–123. [DOI] [PubMed] [Google Scholar]
- 127.Mach F, Schonbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of cd40 signalling. Nature 1998;394:200–203. [DOI] [PubMed] [Google Scholar]
- 128.Lutgens E, Lievens D, Beckers L, Wijnands E, Soehnlein O, Zernecke A, Seijkens T, Engel D, Cleutjens J, Keller AM, Naik SH, Boon L, Oufella HA, Mallat Z, Ahonen CL, Noelle RJ, de Winther MP, Daemen MJ, Biessen EA, Weber C. Deficient cd40-traf6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med 2010;207:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Seijkens TTP, van Tiel CM, Kusters PJH, Atzler D, Soehnlein O, Zarzycka B, Aarts S, Lameijer M, Gijbels MJ, Beckers L, den Toom M, Slutter B, Kuiper J, Duchene J, Aslani M, Megens RTA, van ‘t Veer C, Kooij G, Schrijver R, Hoeksema MA, Boon L, Fay F, Tang J, Baxter S, Jongejan A, Moerland PD, Vriend G, Bleijlevens B, Fisher EA, Duivenvoorden R, Gerdes N, de Winther MPJ, Nicolaes GA, Mulder WJM, Weber C, Lutgens E. Targeting cd40-induced traf6 signaling in macrophages reduces atherosclerosis. J Am Coll Cardiol 2018;71:527–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arditi M, Shah PK. Stop the traffic and reduce the plaque. J Am Coll Cardiol 2018;71:543–546. [DOI] [PubMed] [Google Scholar]
- 131.Boufenzer A, Lemarie J, Simon T, Derive M, Bouazza Y, Tran N, Maskali F, Groubatch F, Bonnin P, Bastien C, Bruneval P, Marie PY, Cohen R, Danchin N, Silvestre JS, Ait-Oufella H, Gibot S. Trem-1 mediates inflammatory injury and cardiac remodeling following myocardial infarction. Circ Res 2015;116:1772–1782. [DOI] [PubMed] [Google Scholar]
- 132.Golovkin AS, Ponasenko AV, Khutornaya MV, Kutikhin AG, Salakhov RR, Yuzhalin AE, Zhidkova II, Barbarash OL, Barbarash LS. Association of tlr and trem-1 gene polymorphisms with risk of coronary artery disease in a russian population. Gene 2014;550:101–109. [DOI] [PubMed] [Google Scholar]
- 133.Joffre J, Potteaux S, Zeboudj L, Loyer X, Boufenzer A, Laurans L, Esposito B, Vandestienne M, de Jager SC, Henique C, Zlatanova I, Taleb S, Bruneval P, Tedgui A, Mallat Z, Gibot S, Ait-Oufella H. Genetic and pharmacological inhibition of trem-1 limits the development of experimental atherosclerosis. J Am Coll Cardiol 2016;68:2776–2793. [DOI] [PubMed] [Google Scholar]
- 134.Bentzon JF. Targeting inflammation in atherosclerosis. J Am Coll Cardiol 2016;68:2794–2796. [DOI] [PubMed] [Google Scholar]
- 135.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 2008;8:349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014;510:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tracey KJ. The inflammatory reflex. Nature 2002;420:853–859 [DOI] [PubMed] [Google Scholar]
- 138.Tracey KJ. Physiology and immunology of the cholinergic anti-inflammatory pathway. J Clin Invest 2007;117:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405:458–462. [DOI] [PubMed] [Google Scholar]
- 140.Nilsson J, Wigren M, Shah PK. Vaccines against atherosclerosis. Expert review of vaccines 2013;12:311–321. [DOI] [PubMed] [Google Scholar]
- 141.Shah PK, Chyu KY, Dimayuga PC, Nilsson J. Vaccine for atherosclerosis. J Am Coll Cardiol 2014;64:2779–2791. [DOI] [PubMed] [Google Scholar]
- 142.Kimura T, Tse K, Sette A, Ley K. Vaccination to modulate atherosclerosis. Autoimmunity 2015;48:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hauer AD, Uyttenhove C, de Vos P, Stroobant V, Renauld JC, van Berkel TJ, van Snick J, Kuiper J. Blockade of interleukin-12 function by protein vaccination attenuates atherosclerosis. Circulation 2005;112:1054–1062. [DOI] [PubMed] [Google Scholar]
- 144.Zeboudj L, Maitre M, Guyonnet L, Laurans L, Joffre J, Lemarie J, Bourcier S, Nour-Eldine W, Guerin C, Friard J, Wakkach A, Fabre E, Tedgui A, Mallat Z, Tharaux PL, Ait-Oufella H. Selective egf-receptor inhibition in cd4(+) t cells induces anergy and limits atherosclerosis. J Am Coll Cardiol 2018;71:160–172. [DOI] [PubMed] [Google Scholar]
- 145.Libby P, Hansson GK. Taming immune and inflammatory responses to treat atherosclerosis. J Am Coll Cardiol 2018;71:173–176. [DOI] [PubMed] [Google Scholar]
- 146.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 147.Ridker PM, Revkin J, Amarenco P, Brunell R, Curto M, Civeira F, Flather M, Glynn RJ, Gregoire J, Jukema JW, Karpov Y, Kastelein JJP, Koenig W, Lorenzatti A, Manga P, Masiukiewicz U, Miller M, Mosterd A, Murin J, Nicolau JC, Nissen S, Ponikowski P, Santos RD, Schwartz PF, Soran H, White H, Wright RS, Vrablik M, Yunis C, Shear CL, Tardif JC. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med 2017; 376:1527–1539. [DOI] [PubMed] [Google Scholar]
- 148.Bohula EA, Giugliano RP, Leiter LA, Verma S, Park J-G, Sever PS, Pineda AL, Honarpour N, Wang H, Murphy SA, Keech A, Pedersen TR, Sabatine MS. Inflammatory and cholesterol risk in the FOURIER trial (Further cardiovascular outcomes research with PCSK9 inhibition in patients with elevated risk). Circulation 2018;138(2):131–140. [DOI] [PubMed] [Google Scholar]
- 149.Pradhan A, Aday AW, Rose LM, Ridker PM. Residual inflammatory risk on treatment with PCSK9 inhibition and statin therapy. Circulation 2018;138(2):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]


