Abstract
The winter 2018/19 influenza season in Hong Kong has been predominated by influenza A(H1N1)pdm09 as at January 2019. We enrolled 2,016 children in three public hospitals in Hong Kong between 2 September 2018 and 11 January 2019. Using the test-negative approach, we estimated high early season effectiveness of inactivated influenza vaccine against influenza A or B of 90% (95% confidence interval (CI): 80–95%) and 92% (95% CI: 82–96%) against influenza A(H1N1)pdm09.
Keywords: influenza, H1N1, vaccine effectiveness, public health, Hong Kong, inactivated influenza vaccination, children, hospital, winter
In Hong Kong, the 2018/19 influenza season started in October 2018, with influenza infections reaching epidemic levels in the final week of 2018, and influenza A(H1N1)pdm09 viruses circulating predominantly [1,2]. Previous influenza A(H1N1)pdm09 epidemics occurred in 2012/13, 2013/14 and 2015/16 [3,4]. Here we continued an ongoing study [3-5] using the test-negative design [6,7] to estimate influenza vaccine effectiveness (VE) in hospitalised children in Hong Kong in the early part of the 2018/19 winter influenza season.
Study participants
In Hong Kong, ca 90% of inpatients are treated in public hospitals. We conducted a test-negative study among children aged 6 months–17 years in three public hospitals; Queen Mary Hospital, Princess Margaret Hospital and Yan Chai Hospital, which together have a catchment area covering ca 17% of all children living in Hong Kong. We enrolled children who were admitted with a febrile acute respiratory illness (ARI) to the general paediatric wards in these three hospitals. Febrile ARI was defined as fever measured ≥ 38 °C plus any respiratory symptom such as cough, sore throat or runny nose [3-5]. Healthcare staff collected nasopharyngeal aspirates from all enrolled children and tested for influenza viruses by reverse transcriptase PCR.
Influenza vaccination history of each patient was obtained by interviewing parents or legal guardians using a standardised questionnaire. Detailed vaccination history was further clarified by reviewing vaccination cards, contacting private clinics and/or checking electronic medical records. Children vaccinated within 6 months before hospital admission with an appropriate schedule according to the Advisory Committee on Immunization Practices [8], with the last appropriate dose at least 14 days before the hospital admission were categorised as vaccinated. Those who received influenza vaccination within 14 days of the hospitalisation were excluded from the analysis. Conditional logistic regression models were used to estimate VE, accounting for confounding by adjusting for age and age-squared and matching by calendar week. VE was estimated via 1 minus the adjusted odds ratio of vaccination [9]. All statistical analyses were performed in R version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria).
The study protocol was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority of Hong Kong West Cluster and the Kowloon West Cluster Research Ethics Committee. Verbal consent was obtained from parents or legal guardians of participants.
Influenza vaccine effectiveness estimate
In order to prevent influenza infections and severe outcomes inactivated influenza vaccines (northern hemisphere formulation) are available, almost all of which are quadrivalent, and more than 1 million doses have been administered in Hong Kong during the 2018/19 season [10] in the local population of 7.4 million. Priority groups for vaccination include older adults, adults with chronic medical conditions, pregnant women, healthcare workers and children up to the age of 12 years.
From 2 September 2018 to 11 January 2019, a total of 2,016 children admitted to the three study hospitals were eligible to be included in the study (Figure). Of these, 344 (17.1%) tested positive for influenza A or B. Among the test-positives, 85% (292/344) were positive for influenza A(H1N1)pdm09 and 13% (45/344) were infected by influenza A(H3N2) viruses (Figure, Table). Among the influenza-positive children only 2.9% (10/344) were vaccinated, compared with 10.2% (170/1,672) among influenza-negative controls (p value < 0.001). Among the 180 vaccinated children, most (n = 160: 88.9%) received the quadrivalent inactivated influenza vaccine, compared with 10 (5.6%) who reported receipt of a trivalent inactivated influenza vaccine and 10 (5.6%) unknown. Influenza VE was 90% (95% confidence interval (CI): 80–95) against influenza A or B, and 92% (95% CI: 82–96) against influenza A(H1N1)pdm09.
Figure.
(A) Influenza activity based on laboratory surveillancea (B) timeline of recruitment of hospitalised children with acute respiratory illness testing positive or negative for influenza virus by type/subtype, Hong Kong, 2 September 2018–11 January 2019 (n = 2,016)
a Reported by the Public Health Laboratory Services.
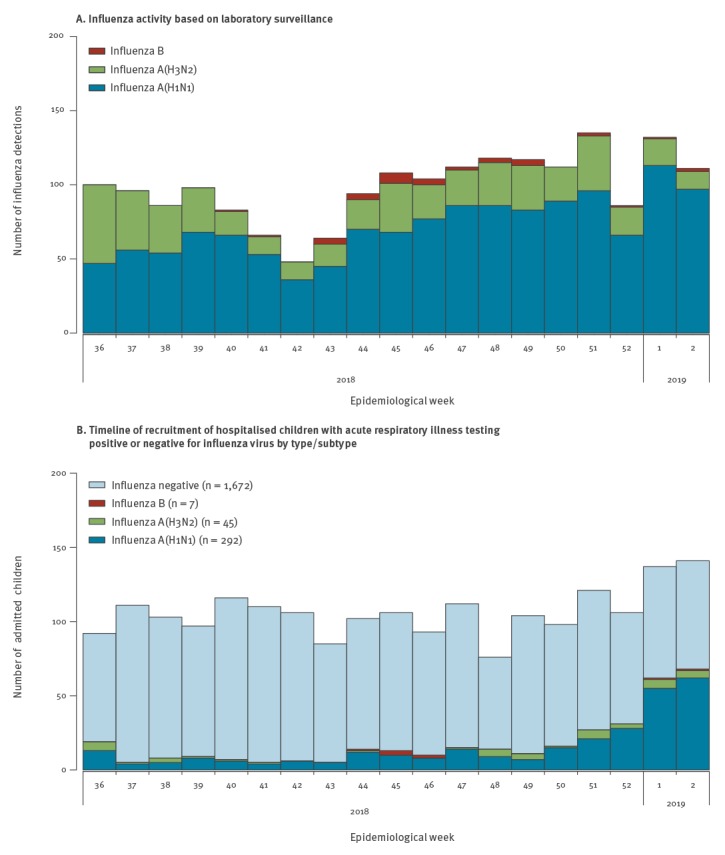
Table. Comparison of cases testing positive for any influenza virus and test-negative controls, Hong Kong, September–December 2018 (n = 2,016).
| Characteristics | Influenza-positive N = 344 |
Influenza-negative N = 1,672 |
P value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age group | |||||
| 6 months–2 years | 130 | 37.8 | 846 | 50.6 | < 0.001a |
| 3–5 years | 141 | 41.0 | 468 | 28.0 | |
| 6–17 years | 73 | 21.2 | 358 | 21.4 | |
| Sex | |||||
| Male | 159 | 46.2 | 949 | 56.8 | |
| Female | 185 | 53.8 | 723 | 43.2 | < 0.001a |
| Receipt of influenza vaccinationb | |||||
| Overall | 10 | 2.9 | 170 | 10.2 | < 0.001c |
| By age group | |||||
| 6 months–2 years | 1 | 0.8 | 47 | 5.6 | |
| 3–5 years | 5 | 3.5 | 68 | 14.5 | |
| 6–17 years | 4 | 5.5 | 55 | 15.4 | |
| By sex | |||||
| Male | 0 | 0.0 | 94 | 9.9 | |
| Female | 10 | 5.4 | 76 | 10.5 | |
a P values estimated by chi-squared tests.
b Receipt of influenza vaccination defined as receipt of a quadrivalent or trivalent inactivated influenza vaccine with an age-appropriate schedule within 6 months prior to admission to one of the three study hospitals.
C P value estimated by Fishers exact test.
To confirm that there was no evidence of a change in VE concurrent with the rising phase of the winter epidemic, we conducted a sensitivity analysis. We restricted the analysis to the 783 children admitted between 25 November 2018 and 11 January 2019. In this subgroup we estimated a VE of 87% (95% CI: 75–94) against influenza A or B overall and 92% (95% CI: 81–96) against influenza A(H1N1)pdm09.
Discussion
In this study, we found high VE for influenza A(H1N1)pdm09. Our results suggest that the influenza vaccine was effective in reducing hospitalisations due to influenza A(H1N1)pdm09 in children in the early phase of the 2018/19 winter influenza season; highlighting the importance of promoting influenza vaccination in Hong Kong. Vaccination coverage was only 10% in the test-negative controls. A pilot school-based influenza vaccination programme was launched in October 2018 and expansion of this scheme could increase vaccination coverage in school-age children. Further strategies may be needed to improve vaccination coverage in children below 6 years of age, particularly in the age range of 6 months–2 years.
This is one of the first in-season estimates of influenza VE for the 2018/19 influenza season. Our findings were consistent with previous estimates against influenza A(H1N1)pdm09 in children in Hong Kong from 2012/13 to 2016/17 [4]. In that study, we assessed VE estimates by different intervals between vaccination and hospitalisation and estimated a VE of 96% (95% CI: 74%–100%) against influenza A(H1N1)pdm09 for children vaccinated no more than 2 months before hospitalisation. In the present study, we found that most children were vaccinated 14 days–2 months before hospitalisation and that the VE estimates during the early part of the season were also high. This suggests the influenza vaccine confers strong protection soon after its administration. However, in the previous study the influenza VE declined as the season progressed, emphasising the importance of appropriate timing of vaccination [11,12]. Our findings were also consistent with the recently reported VE estimate of 91% (67%-98%) against A(H1N1)pdm09 in children 1-8 years of age in Canada for 2018/19 [13].
This study had several limitations. First, we did not have data on the genetic or antigenic characteristics for the circulating influenza A(H1N1)pdm09 strains in Hong Kong to compare with the A/Michigan/45/2015(H1N1) strain included in the 2018/19 northern hemisphere formulation of the influenza vaccine. This strain was previous included in the 2017 and 2018 southern hemisphere vaccines, and the 2017/18 northern hemisphere vaccine. Nevertheless, our high VE estimates suggest that substantial antigenic drift away from the vaccine strain was unlikely. Moreover, this study may have suffered sparse data bias [14] with few vaccinated and influenza-positive cases. Finally, our estimate of high VE is largely based on protection against influenza A(H1N1)pdm09 and we were unable to estimate VE against influenza A(H3N2) or B which could affect end-of-season VE estimates if influenza A(H3N2) predominates later in the season.
Conclusions
In early part of the 2018/19 winter influenza season in Hong Kong, we found evidence that influenza vaccination provided very good protection against laboratory-confirmed influenza A(H1N1)pdm09 virus hospitalisation in children aged 6 months–17 years.
Acknowledgements
We thank Elaine Yee Lin Lo for research support.
This work was supported in part by the Research Grants Council of Hong Kong Special Administrative Region, China (Project No.T11-705/14N).
Conflict of interest: BJC reports receipt of honoraria from Sanofi and Roche for advisory boards. The authors report no other potential conflicts of interest.
Authors’ contributions: Authors SSC and BJC designed the study, authors SF and HC performed the statistical analyses of the data, authors SSC, MYWK and BJC drafted the manuscript, SSC, MYWK, SF, ELYC, HC, JSCW, JSMP and BJC participated in the interpretation of the analyses, read and approved the final version.
References
- 1.Centre for Health Protection (CHP). Flu Express. Volume 15, Number 52. Hong Kong: CHP; 2019. Available from: https://www.chp.gov.hk/files/pdf/fluexpress_week52_3_1_2019_eng.pdf.
- 2.Centre for Health Protection (CHP). Flu Express. Volume 16, Number 01. Hong Kong: CHP; 2019. Available from: https://www.chp.gov.hk/files/pdf/flux_wk1_10_1_2019_eng.pdf
- 3. Cowling BJ, Chan K-H, Feng S, Chan EL, Lo JY, Peiris JS, et al. The effectiveness of influenza vaccination in preventing hospitalizations in children in Hong Kong, 2009-2013. Vaccine. 2014;32(41):5278-84. 10.1016/j.vaccine.2014.07.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feng S, Chiu SS, Chan ELY, Kwan MYW, Wong JSC, Leung C-W, et al. Effectiveness of influenza vaccination on influenza-associated hospitalisations over time among children in Hong Kong: a test-negative case-control study. Lancet Respir Med. 2018;6(12):925-34. 10.1016/S2213-2600(18)30419-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiu SS, Kwan MYW, Feng S, Wong JSC, Leung C-W, Chan ELY, et al. Influenza vaccine effectiveness against influenza A(H3N2) hospitalizations in children in Hong Kong in a prolonged season, 2016/2017. J Infect Dis. 2018;217(9):1365-71. 10.1093/infdis/jiy027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31(17):2165-8. 10.1016/j.vaccine.2013.02.053 [DOI] [PubMed] [Google Scholar]
- 7. Sullivan SG, Feng S, Cowling BJ. Potential of the test-negative design for measuring influenza vaccine effectiveness: a systematic review. Expert Rev Vaccines. 2014;13(12):1571-91. 10.1586/14760584.2014.966695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC). CDC's Advisory Committee on Immunization Practices (ACIP) Recommends Universal Annual Influenza Vaccination. Atlanta: CDC; 2010. Available from: https://www.cdc.gov/media/pressrel/2010/r100224.htm
- 9. Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical Basis of the Test-Negative Study Design for Assessment of Influenza Vaccine Effectiveness. Am J Epidemiol. 2016;184(5):345-53. 10.1093/aje/kww064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centre for Health Protection (HCP). Statistics on 2018/19 Vaccination Programmes / Schemes. Hong Kong: CHP; Last accessed on Jan 18 2019. Available from: https://www.chp.gov.hk/en/features/26734.html.
- 11. Rubin R. Is It Possible to Get a Flu Shot Too Early? JAMA. 2018;320(22):2299-301. 10.1001/jama.2018.18373 [DOI] [PubMed] [Google Scholar]
- 12.Lipsitch M. Challenges of vaccine effectiveness and waning studies. Clin Infect Dis. 2018. [DOI] [PMC free article] [PubMed]
- 13. Skowronski DM, Leir S, Sabaiduc S, Murti M, Dickinson JA, Olsha R, et al. Interim estimates of 2018/19 vaccine effectiveness against influenza A(H1N1)pdm09, Canada, January 2019. Euro Surveill. 2019;24(4):1900055 10.2807/1560-7917.ES.2019.24.4.1900055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greenland S, Mansournia MA, Altman DG. Sparse data bias: a problem hiding in plain sight. BMJ. 2016;352:i1981. 10.1136/bmj.i1981 [DOI] [PubMed] [Google Scholar]


