Abstract
Background
Chronic kidney disease (CKD) contributes significant morbidity and mortality among Asians; hence interventions should focus on those most at-risk of progression. However, current end stage renal failure (ESRF) risk stratification tools are complex and not validated in multi-ethnic Asians. We hence aimed to develop an ESRF risk prediction model by taking into account ethnic differences within a fairly homogenous socioeconomic setting and using parameters readily accessible to primary care clinicians managing the vast majority of patients with CKD.
Methods
We performed a prospective cohort study of 1970 adults with CKD estimated glomerular filtration rate <60 ml/min/1.73m2 or albuminuria >30 mg/g from the population-based Singapore Epidemiology of Eye Diseases study (n = 10,033). Outcome was incident ESRF, ascertained by linkage to the Singapore Renal Registry until 2015.
Results
Mean follow up was 8.5 ± 1.8 years and ESRF occurred in 32 individuals (1.6%). ESRF incidence rates were 2.8, 0.8 and 2.6 per 1000 patient years in Malays, Indians and Chinese respectively. The best ESRF prediction model included age, gender, eGFR and albuminuria (calibration χ2 = 0.45, P = 0.93; C-statistic 0.933, 95% confidence interval (CI) 0.889–0.978, p = 0.01; AIC 356). Addition of ethnicity improved discrimination marginally (C statistic 0.942, 95% CI 0.903–0.981, p = 0.21). Addition of clinical variables such as diabetes and hyperlipidemia did not improve model performance significantly.
Conclusion
We affirmed the utility of commonly available clinical information (age, gender, eGFR and UACR) in prognosticating ESRF for multi-ethnic Asians with CKD.
Introduction
Chronic kidney disease (CKD) increases risks of end-stage renal failure (ESRF) and cardiovascular disease [1] and ranked among the 10 most frequent causes of death and/or factors driving death and disability in India, Taiwan, China, Indonesia, Malaysia, Singapore and Australia according to the 2016 Global Burden of Disease Study [1, 2]. CKD is estimated to occur in 20–40% of Asian patients attending primary care clinics [3–5], thus placing a significant burden on primary healthcare and specialist nephrology services to evaluate and optimize control of risk factors to reduce progression to ESRF. However, renal function trajectories differ among patients with CKD and healthcare resources should be utilized effectively to focus CKD retardation efforts and more intensive monitoring in high-risk patients. There is thus an urgency to identify those most at-risk of progression to ESRF. The popular Kidney Failure Risk Equation (KFRE) developed in Canada incorporates demographic and biochemical parameters such as estimated glomerular filtration rate (eGFR), albuminuria, serum calcium, phosphate bicarbonate and albumin [6]. A multi-national meta-analysis with few Asian cohorts found that the model achieved good discrimination but over-estimated risk in non-North American groups [7]. Singapore has a multi-ethnic population with three major ethnic groups (Chinese, Malay and Indian) common in Asia thus enabling evaluation of ethnic differences within a fairly homogenous socioeconomic setting. As prevalence and risk factors of CKD appear to differ among the three ethnic groups [8], the broad application of a standard calibration factor for non-North American cohorts derived from the aforementioned meta-analysis may be inappropriate for the local CKD population [7]. Moreover, it is also uncertain if the slight improvement in discrimination and calibration justified the extra complexity of the 8-variable KFRE when compared with other simpler models [9]. Other renal failure prediction models used cystatin C or kidney histology [10–12], parameters not readily obtainable in primary care thus limiting their usefulness to clinicians managing the vast majority of patients with CKD. We aimed to develop and validate a risk prediction model for predicting the risk of ESRF using commonly available clinical variables easy to apply in the primary care setting, using data from the Singapore Epidemiology of Eye Diseases (SEED) study, a prospective, community-based cohort of Chinese, Malays and Indians in Singapore.
Materials and methods
Study population
SEED is a population-based cohort study of Chinese, Malay and Indian adults (n = 10,033) aged 40–80 years at baseline aimed to investigate the prevalence, incidence and risk factors of age-related eye diseases, and also the burden of major systemic diseases such as diabetes, hypertension and CKD. SEED included three independent population-based studies, the Singapore Malay Eye Study (SiMES, 2004–2006), the Singapore Indian Eye Study (SINDI, 2007–2009) and the Singapore Chinese Eye Study (SCES, 2009–2011) [13, 14]. Detailed methodology for these studies was previously reported [15]. Subjects were recruited in the same geographical area using age-stratified random sampling from computer-generated random lists of individuals 40 to 80 years of age residing in Singapore. All 3 studies followed similar protocols and were conducted in the same center (Singapore Eye Research Institute). The current study included 1970 persons who had CKD at baseline and outcome ESRD was obtained by linkage to the national renal registry. Previous investigators evaluating ESRF risk prediction used the Modification of Diet in Renal Diseases (MDRD) formula to estimate GFR [6, 9]. However, this formula tends to under-estimate GFR, possibly misclassifying patients with normal kidney function as mild CKD, while the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula may be more accurate among those with normal or slightly lower GFR and correlated well with adverse outcomes internationally and among Asians [16–18]. Since our cohort was derived from population-based studies and thus more likely to include individuals with better renal function than cohorts selected from nephrology or hospital-based clinics, we considered the CKD-EPI equation to be more suitable to calculate eGFR for subjects in this study. CKD was defined according to modified Kidney Disease: Improving Global Outcomes (KDIGO) 2012 clinical practice guideline as eGFR <60 ml/min/1.73m2 or albuminuria (urinary albumin-to-creatinine ratio, UACR) >30 mg/g [19, 20]. Among 2524 individuals with eGFR <60 ml/min/1.73m2 or UACR >30 mg/g, we excluded those with Stage 5 CKD (eGFR <15 ml/min/1.73 m2, n = 22) or had missing data on serum creatinine (n = 22) or urinary albumin-to-creatinine ratio (n = 510). Thus, 1970 individuals were included in the development cohort.
Data collection
An interviewer-administered questionnaire was used to collect participants’ socio-demographic, lifestyle and medical history. Smoking was classified into current smoker and former or non-smokers. Physical examination included weight, height and clinic blood pressure (BP). Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Systolic BP and diastolic BP were measured using a digital automatic BP monitor (Dinamap model Pro Series DP110X-RW, 100V2; GE Medical Systems Information Technologies Inc., USA) after the participant was seated for at least 5 min. BP was measured twice, 5 minutes apart. A third measurement was made if the systolic BP differed by more than 10 mmHg or the diastolic BP by more than 5 mmHg. The mean between the two closest readings were then taken as the blood pressure for that individual. Hypertension was present if systolic BP was ≥140 mmHg or diastolic ≥90 mmHg or individuals reported previously physician-diagnosed hypertension or use of blood-pressure lowering medication. Diabetes mellitus was defined as random serum glucose ≥11.1 mmol/L or glycosylated haemoglobin (HbA1c) ≥6.5% or self-reported physician-diagnosed diabetes or use of glucose-lowering medication [21]. Hyperlipidemia was defined as total cholesterol ≥6.2 mmol/L or use of lipid lowering medication. Non-fasting venous blood was tested for serum lipids, glucose, HbA1c and creatinine. Serum creatinine was measured by enzymatic method (SiMES) or Jaffe method (SINDI and SCES) calibrated to the National Institute of Standards and Technology (NIST) Liquid Chromatography Isotope Dilution Mass Spectrometry (LC-IDMS) and expressed in micromoles per liter (μmol/L). A single random spot urine sample was used to measure urine albumin to creatinine ratio (UACR, mg/g) using commercial assay (Immulite, DPC, United Kingdom). The lower detection limits for urinary albumin and creatinine were 0.5 mg/L and 0.027 mmol/L respectively. UACR was available only in a third of the Malay participants (those with known diabetes and 1 in 5 with no diabetes). Consequently, 473 of 719 participants from the SiMES cohort with eGFR < 60 ml/min/1.73 m2 were excluded due to missing UACR values. All laboratory investigations were conducted at the National University Hospital Reference Laboratory (SiMES) and Singapore General Hospital (SINDI, SCES) which are accredited by the College of American Pathologists.
Written informed consent was obtained from all participants before enrolment. This study was approved by the Singapore Eye Research Institute Review Board and conduct of the study adhered to the Declaration of Helsinki.
Outcome definition
Primary outcome of interest in this study was incident ESRF, defined as eGFR less than 15 ml/min/1.73 m2, serum creatinine more than 500 μmol/L or 5.7 mg/dL, or if subject received transplantation or chronic dialysis. ESRF was ascertained by linking the study cohort to the Singapore Renal Registry at National Registry of Diseases Office until 2015. The Singapore Renal Registry collates voluntary submissions of new ESRF patients from all public and private centers in Singapore with estimated data capture of 95% of all dialysis patients [22].
Statistical analysis
All statistical analyses were performed using STATA statistical software (Version 14.1, StataCorp, College Station, Texas). Baseline characteristics of the 3 ethnic groups with CKD were compared using analysis of variance (ANOVA) or chi-square test as appropriate for the variable. Uni-variate Cox proportional-hazards regression was performed to examine the association between demographic and clinical characteristics with incident ESRF. Variables associated with ESRF (P < 0.05) in uni-variate analysis and established clinical factors from literature (age, gender, eGFR, UACR, ethnicity, diabetes mellitus, hypertension and hyperlipidemia) were subsequently included in a series of multi-variable models for further analysis. Calibration was assessed using Nam-D’Agostino χ2 statistic to examine how closely each model’s predicted probabilities agreed with actual outcome, where χ2 values >20 with P<0.05 suggest poor agreement between predicted and observed outcomes [23]. Model comparison was done using Harrell’s Concordance statistic (C statistic) and Akaike Information Criterion (AIC). C statistic, the equivalent of the area (AUC) under a receiver operating characteristic (ROC) curve for a binary outcome variable [24], was computed as a measure of discrimination for ESRF in the developed models. AIC was computed to compare the goodness of fit between the various models, accounting for model complexity; difference in AIC >10 is considered significant [25]. Bootstrap sampling with 10,000 replications was then performed for the chosen prediction model to compute the standard errors and 95% CIs to check for robustness of the model coefficients. Lastly, we performed a sensitivity analysis for our best developed model among subjects with CKD stages 3–4.
Results
We identified 1970 participants (382 Malays, 816 Indians and 772 Chinese) to have CKD at baseline (Table 1). Subjects’ mean age was 62.4 ± 10.2 years and half were female. Malay subjects in SiMES were more likely to be hypertensive with higher BMI, BP and cholesterol levels but lower eGFR. Indian participants in SINDI were younger, more likely to have diabetes and had higher glucose and HbA1c levels. Chinese participants in SCES were older but had better renal function. S1 Fig illustrates the distribution of the development cohort categorized in an eGFR and albuminuria grid, where lower eGFR and higher UACR incrementally increase risk of progressive CKD [19]. More than half the cohort (61.3%) had normal or mildly decreased eGFR (eGFR ≥60 ml/min/1.73 m2) with moderately increased albuminuria (30–300 mg/g) and thus at moderately increased risk of progressive CKD. In contrast, few (9.3%) had eGFR <45 ml/min/1.73 m2 and were at high to very high risk of progressive CKD.
Table 1. Baseline characteristics of subjects with chronic kidney disease categorized by ethnicity.
| ALL CKD | SiMES | SINDI | SCES | P-value | |
|---|---|---|---|---|---|
| N = 1970 | n = 382 | n = 816 | n = 772 | ||
| Age, years | 62.4 (10.2) | 62.5 (10.4) | 60.9 (10.0) | 63.8 (10.2) | < 0.001 |
| Female gender, n (%) | 1050 (53.3) | 216 (56.5) | 421 (51.6) | 413 (53.50) | 0.28 |
| Current smoker, n (%) | 237 (12.0) | 64 (16.7) | 92 (11.2) | 81 (10.4) | 0.006 |
| Diabetes mellitus, n (%) | 882 (45.3) | 164 (43.1) | 478 (59.6) | 240 (31.3) | < 0.001 |
| Hyperlipidemia, n (%) | 1092 (56.2) | 201 (52.6) | 465 (58.7) | 426 (55.4) | 0.11 |
| Hypertension, n (%) | 1534 (78.0) | 328 (86.3) | 601 (73.8) | 605 (78.3) | < 0.001 |
| Systolic BP, mmHg | 146 (22) | 159 (23) | 143 (21) | 144 (21) | < 0.001 |
| Diastolic BP, mmHg | 79 (11) | 83 (12) | 78 (11) | 78 (9) | < 0.001 |
| Total cholesterol, mmol/L | 5.32 (1.20) | 5.71 (1.25) | 5.10 (1.18) | 5.36 (1.13) | < 0.001 |
| LDL cholesterol, mmol/L | 3.21 (0.97) | 3.35 (0.98) | 3.19 (0.99) | 3.15 (0.94) | 0.005 |
| HDL cholesterol, mmol/L | 1.22 (0.38) | 1.33 (0.34) | 1.10 (0.34) | 1.30 (0.40) | < 0.001 |
| Body mass index, kg/m2 | 25.5 (4.8) | 26.6 (5.0) | 26.4 (5.2) | 24.0 (3.7) | < 0.001 |
| eGFR, ml/min/1.73 m2 | 75.97 (24.17) | 58.39 (15.67) | 80.13 (23.02) | 80.29 (24.89) | < 0.001 |
| UACR, mg/g | 158.88 (484.50) | 205.55 (652.21) | 148.89 (447.95) | 146.35 (419.33) | 0.11 |
| Serum glucose, mmol/L | 7.8 (4.4) | 7.9 (4.5) | 8.4 (4.6) | 7.2 (4.1) | < 0.001 |
| HbA1c, % | 6.7 (1.59) | 6.8 (1.8) | 6.9 (1.7) | 6.3 (1.2) | < 0.001 |
| Time to ESRD, years | 4.4 (2.3) | 6.0 (2.3) | 3.6 (2.6) | 3.5 (1.6) | 0.01 |
| Follow-up, years | 8.5 (1.8) | 11.1 (1.0) | 8.9 (0.7) | 6.7 (0.9) | < 0.001 |
Abbreviations: BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease; HbA1c, glycosylated hemoglobin A1; HDL, high density lipoprotein; LDL, low density lipoprotein; SCES, Singapore Chinese Eye Study; SiMES, Singapore Malay Eye Study; SINDI, Singapore Indian Eye Study; UACR, urine albumin to creatinine ratio; Values for categorical variables are reported as number (percentage) and continuous variables reported as mean (standard deviation).
Total follow up was 8.5 ± 1.8 years and ESRF occurred in 32 subjects (1.6%) at 4.4 ± 2.3 years. S2 Fig shows incident ESRF categorized by ethnicity. Incidence rates of ESRF were 2.8, 0.8 and 2.6 per 1000 patient years respectively in Malay, Indian and Chinese subgroups. Among those who had ESRF, fewer than half (13 subjects, 40.6%) had early ESRF within 5 years (8, 3 and 2 subjects from SiMES, SINDI and SCES respectively). Table 2 shows the uni-variate analysis for factors associated with ESRF. Indians, compared to Chinese, were less likely to develop ESRF despite a significantly longer follow-up duration (11.1±1.0 versus 6.8±0.9 years, p<0.001). Diabetes, hyperlipidemia, lower eGFR and higher albuminuria, serum glucose and HbA1c were significantly associated with ESRF.
Table 2. Hazard ratios, calibration and goodness of fit for models for end stage renal failure.
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age per 10 years | 0.48 | 0.32, 0.72 | < 0.001 | 0.69 | 0.46, 1.03 | 0.07 | 0.62 | 0.41, 0.93 | 0.021 | 0.68 | 0.45, 1.02 | 0.06 | 0.67 | 0.44, 1.02 | 0.06 |
| Female | 1.06 | 0.53, 2.12 | 0.87 | 0.88 | 0.44, 1.76 | 0.71 | 0.80 | 0.38, 1.67 | 0.55 | 1.06 | 0.52, 2.16 | 0.88 | 1.18 | 0.57, 2.43 | 0.66 |
| Race | |||||||||||||||
| Chinese | Reference | ||||||||||||||
| Malay | 0.53 | 0.23, 1.23 | 0.14 | ||||||||||||
| Indian | 0.28 | 0.10, 0.78 | 0.015 | ||||||||||||
| eGFR per 5 ml/min/1.73 m2 | 0.59 | 0.53, 0.67 | < 0.001 | 0.68 | 0.61, 0.77 | < 0.001 | 0.69 | 0.62, 0.77 | < 0.001 | 0.69 | 0.62, 0.77 | < 0.001 | 0.70 | 0.62, 0.78 | < 0.001 |
| Log Albuminuria | 1.71 | 1.38, 2.12 | < 0.001 | 1.70 | 1.37, 2.11 | < 0.001 | 1.67 | 1.33, 2.09 | < 0.001 | 1.61 | 1.29, 2.00 | < 0.001 | |||
| Diabetes | 2.65 | 0.99, 7.13 | 0.05 | 2.60 | 0.97, 6.97 | 0.06 | |||||||||
| Hypertension | 0.66 | 0.19, 2.34 | 0.52 | ||||||||||||
| Hyperlipidemia | 2.09 | 0.79, 5.49 | 0.14 | ||||||||||||
| C Statistic | 0.89 | 0.82, 0.95 | 0.93 | 0.889, 0.978 | 0.010a | 0.942 | 0.903, 0.981 | 0.21a | 0.946 | 0.914, 0.977 | 0.07a | 0.939 | 0.899, 0.980 | 0.13a | |
| Akaike Information Criterion | 379 | 356 | 353 | 355 | 345 | ||||||||||
| Nam-D’Agostino χ2 | 26.43 | < 0.001 | 0.45 | 0.93 | 0.44 | 0.93 | 1.60 | 0.66 | 0.46 | 0.93 | |||||
Model 1: age, gender, eGFR. Model 2: age, gender, eGFR, albuminuria. Model 3: age, gender, race, eGFR, albuminuria. Model 4: age, gender, eGFR, albuminuria, diabetes, hypertension. Model 5: age, gender, eGFR, albuminuria, diabetes, hyperlipidaemia
aP values are for comparison of C statistics between successive models for model 1–3. Models 4 and 5 were compared with model 2.
eGFR, estimated glomerular filtration rate
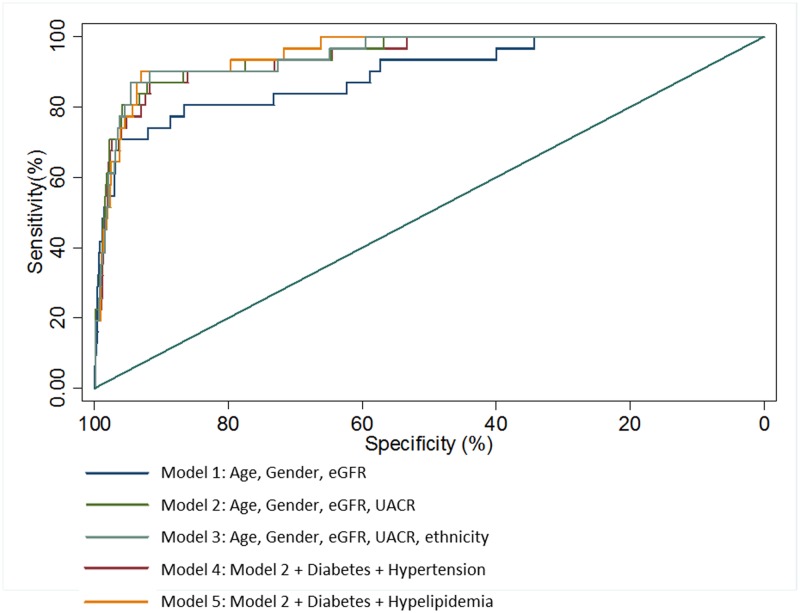
Table 2 shows hazard ratios for the variables and C-statistics and AIC for successive models for ESRF. As it was well-established that baseline renal function measured by eGFR was associated with ESRF risk [19], and confirmed in our cohort (S1 Table), age, gender and eGFR were included in Model 1 and performed fairly well in both discrimination and goodness of fit. However, the Nam–D’Agostino chi-squared test for model 1 indicated inadequate calibration (P<0.001). Addition of albuminuria in Model 2 improved calibration (χ2 = 0.45, P = 0.93), C-statistic (0.933, 95% confidence interval (CI) 0.889–0.978, p = 0.01) and AIC. Further addition of ethnicity in Model 3 improved the C-statistic marginally (0.942, 95% CI 0.903–0.981) but this was not statistically significant. Addition of other commonly available co-morbidity information (diabetes and hypertension in Model 4; diabetes and hyperlipidemia in Model 5) did not significantly alter discrimination and goodness of fit compared to Model 2. Again, addition of ethnicity to Models 4 and 5 did not significantly improve model performance (C-statistic 0.954, 95% CI 0.925–0.982, p = 0.22 and C statistic 0.948, 95% CI 0.911–0.985, p = 0.10 respectively, not presented). Models 3 to 5 all had adequate calibration (all P>0.60). Fig 1 demonstrates the ROC curves for Models 1–5 in predicting ESRF. AUC for Model 1 was ≥0.80, suggesting excellent discriminatory power, while Models 2 to 5 had AUC ≥0.90, suggesting outstanding discriminatory power. Model 2 had significantly better discrimination (p = 0.01) than Model 1 but AUC for Models 3, 4 and 5 were not significantly different compared to Model 2. Sensitivity analysis in subjects with CKD Stage 3 and 4, i.e. those with eGFR 15 to 60 ml/min/1.73 m2, confirmed consistently high discriminatory value and goodness of fit with Model 2 (C-statistic 0.942, AIC 232).
Fig 1. Receiver operating characteristic (ROC) curves for models 1–5 for discriminating persons with and without incident end-stage renal failure (ESRF).
Area under the curve (AUC) and 95% confidence intervals (CI) for Models 1 (AUC 0.933, 95% CI 0.889–0.978, p = 0.01) was significantly better compared to Model 1 (AUC 0.885, 95% CI 0.816–0.953). Discrimination by Models 3 (AUC 0.942, 95% CI 0.903–0.981, p = 0.21), 4 (AUC 0.946, 95% CI 0.914–0.977, p = 0.07) and 5 (AUC 0.939, 95% CI 0.899–0.980, p = 0.13) were not significantly different compared with Model 2. The straight line representing an AUC of 0.5 indicates inability to differentiate between outcomes, whereas the ideal predictive model should have an AUC of 1.0.
We performed internal validation of our model by using bootstrap sampling with 10,000 replications and computed the standard errors and 95% confidence intervals (CIs) of the estimated coefficients to check for robustness of our prediction model (Model 2) coefficients. The bootstrapped results were generally consistent with our original model, except that age which was marginally significantly associated with ESRF (P = 0.07) in our original model was shown to be associated with ESRF based on bootstrap results (P = 0.042). In addition, the bootstrapped standard errors were reasonably similar and do not differ much from the original model, of which the bootstrapped confidence intervals were close to our original CIs (S2 Table). Hence the results suggest that there is little, if any, bias in the coefficients obtained and used for prediction for final chosen model 2.
As the variables in Model 2 were consistent with the 4-variable KFRE in direction but differed in coefficient magnitude (Table 3), we compared calibration of Model 2 with the 4-variable KFRE with and without regional calibration factor (S3 Table) using the Brier score, an aggregate measure of disagreement between observed outcome and its prediction. The score is derived by taking the mean squared difference between predicted risk probability and actual outcome and ranges from 0 to 1, where 0 indicates perfect predictions and a value of 0.33 or greater indicating random predictive ability. The lower the Brier score is for a set of predictions, the better the predictions are calibrated. Brier scores for Model 2, the 4-variable KFRE and the calibrated 4-variable KFRE were 0.0131, 0.0124 and 0.0125 respectively. Comparison of Brier scores using Wilcoxon sign rank test found that the 4-variable KFRE and calibrated 4-variable KFRE had significantly better calibration than Model 2 (p<0.001). We further confirmed that the 4-variable KFRE model demonstrated good discrimination in our study cohort [C Statistic = 0.91; 95% CI (0.86–0.97)].
Table 3. Hazard ratios and beta coefficients for Model 2 and 4-variable Kidney Failure Risk Equation.
| Model 2 | 4-variable KFRE | |||
|---|---|---|---|---|
| HR | Beta | HR | Beta | |
| Male | 1.14 | 0.13 | 1.26 | 0.27 |
| Age per 10yr | 0.69 | -0.37 | 0.80 | -0.22 |
| eGFR per 5 mL/min/1.73 m2 | 0.68 | -0.38 | 0.57 | -0.55 |
| Log Albuminuria | 1.71 | 0.54 | 1.60 | 0.46 |
Abbreviations: eGFR, estimated glomerular filtration rate; KFRE, Kidney Failure Risk Equation
Discussion
We evaluated ESRF risk prediction models for multi-ethnic Asians with CKD using readily available demographic and co-morbidity data and laboratory values for tests commonly performed in primary care [3]. The high C-statistics of 0.933 (95% CI 0.889–0.978) and 0.942 (95% CI 0.903–0.981) for Model 2 (age, gender, eGFR and UACR) and 3 (age, gender, ethnicity, eGFR and UACR) respectively, confirmed excellent discrimination i.e. the ability to differentiate subjects who developed ESRF from those who did not. The discrimination afforded by our models was similar to that achieved by the 4-variable KFRE for predicting ESRF [7], despite lower incidence of ESRF (1.9 per 1000 patient years) in our cohort compared with other studies which reported kidney failure incidence between 10 and 80 per 1000 patients years [7]. Notably, our cohort was derived from population-based studies and had less severe renal dysfunction with higher baseline eGFR (mean 75.9 ±24.1 ml/min/1.73 m2) compared with studies which included subjects with CKD stage 3–5 recruited from hospital-based diabetic or nephrology clinics and hence at higher risk for progressive CKD [6, 26]. We addressed this in the sensitivity analysis which showed that our developed Model 2 performed well among individuals with CKD Stage 3 and 4 with more severe renal dysfunction.
In addition, we confirmed that the ethnicity altered ESRF risks but its’ addition to the highly-discriminating Model 2 (age, gender, eGFR and UACR) did not significantly improve model performance (p = 0.21). This study affirmed the utility of age, gender, eGFR and UACR in prognosticating ESRF, as evaluated by the 4-variable KFRE7. Although a calibration factor for non-North American cohorts was added to the KFRE (S3 Table), this was largely based on European or East Asian populations and thus limited its generalizability to the multi-ethnic Southeast Asian population. In this study, calibration of the 4-variable KFRE was not altered by addition of the regional calibration factor (Brier scores 0.0124 and 0.0125 respectively).
Use of these predictive models to estimate individualized risk of progression to ESRF can improve planning for frequency of follow up and engaging patients in shared decision making, especially since certain treatment options such as referral to nephrology services at tertiary care centers and advance care or dialysis planning involve significant healthcare costs and risks and should be offered to those most at-risk of ESRF. Table 4 demonstrates use of Model 2 and the 4-variable KFRE in estimating absolute risk of ESRF at 5 years. Compared with 4-variable KFRE, model 2 of the current study increases the predicted risk by 1.3% and 0.6% for patients A and B at low risk for ESRF, but decreased the risk by 1.3% and 2.7% in patients C and D at greater risk for ESRF. Although the absolute risk difference is not large, Model 2 tends to predict a lower risk of ESRF among those with more severe renal impairment, possibly because our development cohort had milder CKD compared to the KFRE development cohort from CKD clinics [6].
Table 4. Predicted probability of end stage renal failure at 5 years for 4 Hypothetical Patient Profiles based on Model 2 and the 4-variable Kidney Failure Risk Equation.
| Patient A 50 year old female, eGFR 50 ml/min/1.73 m2 and UACR 100 mg/g |
Patient B 60 year old male, eGFR 50 ml/min/1.73 m2 and UACR 100 mg/g |
Patient C 50 year old female, eGFR 30 ml/min/1.73 m2 and UACR 100 mg/g |
Patient D 60 year old female, eGFR 30 ml/min/1.73 m2 and UACR 300 mg/g |
|
|---|---|---|---|---|
| Model 2 | 2.9 | 2.3 | 12.8 | 15.6 |
| 4-variable KFRE | 1.6 | 1.7 | 14.1 | 18.3 |
Abbreviations: eGFR, estimated glomerular filtration rate; KFRE, Kidney Failure Risk Equation. Predicted probabilities calculated according the risk equations for Model 2 (Risk = 1–0.998^exp (-0.382*[(eGFR/5)-15.19]+0.133*(male-0.467) + 0.536*[ln(UACR)-4.016] -0.374*[(age/10)-6.24]) and the 4-variable KFRE (Risk = 1–0.924^exp(-0.554*[(eGFR/5)-7.22]+0.269*(male-0.560) + 0.456*[ln(UACR)-5.277] -0.217*[(age/10)-7.04]) and presented as percentages.
An alternate prediction model validated locally for risk of CKD progression, defined as worsening of eGFR categories or ≥25% reduction in eGFR from baseline, is limited to diabetics [24]. Among 1582 local diabetic patients followed up for median 5.5 years by a hospital-practice diabetic center, approximately 40% had progressive CKD but inclusion of milder forms of CKD progression, such as from CKD Stage 1 to 2, in the outcome definition makes the alternate prediction model less useful for the purpose of identifying patients most at-need of intensive healthcare resources such as nephrology referrals and dialysis planning.
Strengths of this study include the inclusion of a large, well-characterized multi-ethnic Asian sample, a complete and robust data set and long follow up. However, lack of UACR values in two-thirds of Malay participants in SiMES resulted in excluding a large portion of Malay participants with eGFR <60 ml/min/1.73 m2 from this study. Notably, prevalence of CKD (defined as eGFR <60 or UACR >30 mg/g) was highest among Malays (44.7% versus 27.6% and 25.5% among Indians and Chinese respectively). Selection bias may result if those with missing UACR values had lower or greater risk for ESRF outcome than those who were included in the cohort. Our assessment of diabetes based on a single measure of random blood glucose ≥11.1 mmol/L may have resulted in nondifferential misclassification of diabetes status which is a limitation inherent to large population-based epidemiological studies. The low incidence of ESRF limited the number of variables in the model that could be adjusted for and limited power to detect effect of ethnicity on ESRF risk. We were unable to directly compare our model with the 8-variable KFRE since our dataset did not include biochemistry values for serum calcium, phosphate, albumin and bicarbonate but the discriminatory values of both models were similar. Multiple large cross-sectional studies have shown that serum calcium and phosphate are unlikely to be markedly deranged before CKD Stage 3b (eGFR <45 ml/min/1.73 m2) [27, 28]. The United States Third National Health and Nutrition Examination Survey (NHANES III) studied data from 15,594 subjects and found that serum albumin was <38 g/L in 10% of those with eGFR 60–89 ml/min/1.73 m2 compared to 20% and 51% among those with CKD stage 3 and 4 respectively (P<0.001) [29]. Similarly, serum bicarbonate was <22 mmol/L in 1% of those with eGFR 60–89 ml/min/1.73 m2 compared to 2% and 19% among those with CKD stage 3 and 4 respectively (P<0.001) [29]. Hence we postulate that addition of these variables in prediction models, such as the 8-variable KFRE, is unlikely to markedly alter ESRF risk possibilities in subjects with mild CKD such as our cohort. CKD is under-documented in local primary care clinics and screening for chronic kidney disease among at-risk individuals remains suboptimal in many countries [3, 30, 31]. Hence, additional biochemistry results (serum calcium, phosphate, bicarbonate, albumin) required by the 8-variable KFRE may not be readily available and may increases both patients’ out-of-pocket expenditure and healthcare costs. Instead, a low-cost model that uses commonly available clinical data to accurately predict ESRF in the general population with CKD will be a more useful clinical decision support tool for the primary healthcare practitioner to guide frequency of follow up with renal function tests or need for nephrology referral.
Conclusion
We affirmed the utility of clinical information that are commonly available in the primary care setting (age, gender, eGFR and UACR) in prognosticating ESRF for multi-ethnic Asians with CKD.
Supporting information
(DOCX)
ESRF occurred in 12 Malays.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
As the study involves human participants, the data cannot be made freely available in the manuscript, the supplemental files, or a public repository due to ethical restrictions. Nevertheless, the data are available from the Singapore Eye Research Institutional Ethics Committee for researchers who meet the criteria for access to confidential data. Interested researchers can send data access requests to the Singapore Eye Research Institute using the following email address: seri@seri.com.sg.
Funding Statement
This study was supported by the National Medical Research Council Grants nos. 0796/2003, 1149/2008, STaR/0003/2008, NMRC/OFLCG/001/2017 and Biomedical Research Council no. 08/1/35/19/550. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lim CC, Teo BW, Ong PG, Cheung CY, Lim SC, Chow KY, et al. Chronic kidney disease, cardiovascular disease and mortality: A prospective cohort study in a multi-ethnic Asian population. European journal of preventive cardiology. 2015;22(8):1018–26. Epub 2014/05/27. 10.1177/2047487314536873 . [DOI] [PubMed] [Google Scholar]
- 2.Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England). 2017;390(10100):1151–210. Epub 2017/09/19. 10.1016/s0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lew QLJ, Allen JC, Nguyen F, Tan NC, Jafar TH. Factors Associated with Chronic Kidney Disease and Their Clinical Utility in Primary Care Clinics in a Multi-Ethnic Southeast Asian Population. Nephron. 2017. Epub 2017/12/19. 10.1159/000485110 . [DOI] [PubMed] [Google Scholar]
- 4.Prasannakumar M, Rajput R, Seshadri K, Talwalkar P, Agarwal P, Gokulnath G, et al. An observational, cross-sectional study to assess the prevalence of chronic kidney disease in type 2 diabetes patients in India (START -India). Indian journal of endocrinology and metabolism. 2015;19(4):520–3. Epub 2015/07/17. 10.4103/2230-8210.157857 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chia YC, Ching SM. Hypertension and the development of new onset chronic kidney disease over a 10 year period: a retrospective cohort study in a primary care setting in Malaysia. BMC nephrology. 2012;13:173 Epub 2012/12/25. 10.1186/1471-2369-13-173 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, et al. A predictive model for progression of chronic kidney disease to kidney failure. Jama. 2011;305(15):1553–9. Epub 2011/04/13. 10.1001/jama.2011.451 . [DOI] [PubMed] [Google Scholar]
- 7.Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: A meta-analysis. Jama. 2016;315(2):164–74. 10.1001/jama.2015.18202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabanayagam C, Lim SC, Wong TY, Lee J, Shankar A, Tai ES. Ethnic disparities in prevalence and impact of risk factors of chronic kidney disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2010;25(8):2564–70. Epub 2010/02/27. 10.1093/ndt/gfq084 . [DOI] [PubMed] [Google Scholar]
- 9.Peeters MJ, van Zuilen AD, van den Brand JA, Bots ML, Blankestijn PJ, Wetzels JF. Validation of the kidney failure risk equation in European CKD patients. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2013;28(7):1773–9. Epub 2013/05/07. 10.1093/ndt/gft063 . [DOI] [PubMed] [Google Scholar]
- 10.Mise K, Hoshino J, Ueno T, Hazue R, Sumida K, Hiramatsu R, et al. Clinical and pathological predictors of estimated GFR decline in patients with type 2 diabetes and overt proteinuric diabetic nephropathy. Diabetes/metabolism research and reviews. 2015;31(6):572–81. Epub 2014/12/24. 10.1002/dmrr.2633 . [DOI] [PubMed] [Google Scholar]
- 11.Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. Jama. 2011;305(15):1545–52. Epub 2011/04/13. 10.1001/jama.2011.468 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamanouchi M, Hoshino J, Ubara Y, Takaichi K, Kinowaki K, Fujii T, et al. Value of adding the renal pathological score to the kidney failure risk equation in advanced diabetic nephropathy. PloS one. 2018;13(1):e0190930 Epub 2018/01/18. 10.1371/journal.pone.0190930 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong CW, Lamoureux EL, Cheng C-Y, Cheung GCM, Tai ES, Wong TY, et al. Increased Burden of Vision Impairment and Eye Diseases in Persons with Chronic Kidney Disease—A Population-Based Study. EBioMedicine. 2016;5:193–7. 10.1016/j.ebiom.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavanya R, Jeganathan VS, Zheng Y, Raju P, Cheung N, Tai ES, et al. Methodology of the Singapore Indian Chinese Cohort (SICC) eye study: quantifying ethnic variations in the epidemiology of eye diseases in Asians. Ophthalmic Epidemiol. 2009;16(6):325–36. 10.3109/09286580903144738 . [DOI] [PubMed] [Google Scholar]
- 15.Foong AW, Saw SM, Loo JL, Shen S, Loon SC, Rosman M, et al. Rationale and methodology for a population-based study of eye diseases in Malay people: The Singapore Malay eye study (SiMES). Ophthalmic Epidemiol. 2007;14(1):25–35. 10.1080/09286580600878844 . [DOI] [PubMed] [Google Scholar]
- 16.Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J. Risk implications of the new CKD Epidemiology Collaboration (CKD-EPI) equation compared with the MDRD Study equation for estimated GFR: the Atherosclerosis Risk in Communities (ARIC) Study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2010;55(4):648–59. Epub 2010/03/02. 10.1053/j.ajkd.2009.12.016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2010;56(3):486–95. Epub 2010/06/19. 10.1053/j.ajkd.2010.03.026 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teo BW, Xu H, Wang D, Li J, Sinha AK, Shuter B, et al. GFR estimating equations in a multiethnic Asian population. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2011;58(1):56–63. Epub 2011/05/24. 10.1053/j.ajkd.2011.02.393 . [DOI] [PubMed] [Google Scholar]
- 19.Group K. Kidney Disease: Improving Global Outcomes (KDIGO) 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney international. 2013;3:1–150. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, De Jong PE, Coresh J, Nahas ME, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney international. 2011;80(1):17–28. 10.1038/ki.2010.483 [DOI] [PubMed] [Google Scholar]
- 21.Diagnosis and classification of diabetes mellitus. Diabetes care. 2010;33 Suppl 1:S62–9. Epub 2010/01/29. 10.2337/dc10-S062 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choong H. Seventh report of the Singapore renal registry 2007/2008. Health Promotion Board, National Disease Registry Office, Ministry of Health, Singapore. 2010.
- 23.D’agostino R, Nam B-H. Evaluation of the performance of survival analysis models: discrimination and calibration measures. Handbook of statistics. 2003;23:1–25. [Google Scholar]
- 24.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. Epub 1982/04/01. 10.1148/radiology.143.1.7063747 . [DOI] [PubMed] [Google Scholar]
- 25.Akaike H. A new look at the statistical model identification. IEEE transactions on automatic control. 1974;19(6):716–23. [Google Scholar]
- 26.Low S, Lim SC, Zhang X, Zhou S, Yeoh LY, Liu YL, et al. Development and validation of a predictive model for Chronic Kidney Disease progression in Type 2 Diabetes Mellitus based on a 13-year study in Singapore. Diabetes research and clinical practice. 2017;123:49–54. Epub 2016/12/07. 10.1016/j.diabres.2016.11.008 . [DOI] [PubMed] [Google Scholar]
- 27.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney international. 2007;71(1):31–8. Epub 2006/11/09. 10.1038/sj.ki.5002009 . [DOI] [PubMed] [Google Scholar]
- 28.Vassalotti JA, Uribarri J, Chen SC, Li S, Wang C, Collins AJ, et al. Trends in mineral metabolism: Kidney Early Evaluation Program (KEEP) and the National Health and Nutrition Examination Survey (NHANES) 1999–2004. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2008;51(4 Suppl 2):S56–68. Epub 2008/04/11. 10.1053/j.ajkd.2007.12.018 [DOI] [PubMed] [Google Scholar]
- 29.Eustace JA, Astor B, Muntner PM, Ikizler TA, Coresh J. Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney international. 2004;65(3):1031–40. 10.1111/j.1523-1755.2004.00481.x [DOI] [PubMed] [Google Scholar]
- 30.Rim THT, Byun IH, Kim HS, Lee SY, Yoon JS. Factors associated with diabetic retinopathy and nephropathy screening in Korea: the Third and Fourth Korea National Health and Nutrition Examination Survey (KNHANES III and IV). Journal of Korean medical science. 2013;28(6):814–20. 10.3346/jkms.2013.28.6.814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Assogba G, Couchoud C, Roudier C, Pornet C, Fosse S, Romon I, et al. Prevalence, screening and treatment of chronic kidney disease in people with type 2 diabetes in France: the ENTRED surveys (2001 and 2007). Diabetes & metabolism. 2012;38(6):558–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
ESRF occurred in 12 Malays.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
As the study involves human participants, the data cannot be made freely available in the manuscript, the supplemental files, or a public repository due to ethical restrictions. Nevertheless, the data are available from the Singapore Eye Research Institutional Ethics Committee for researchers who meet the criteria for access to confidential data. Interested researchers can send data access requests to the Singapore Eye Research Institute using the following email address: seri@seri.com.sg.