Abstract
Vector-borne diseases are an increasing issue to public health, endangering billions of people worldwide. Controlling vector mosquitoes is widely accepted as the most effective way to prevent vector-borne disease outbreaks. Mosquito surveillance is critical for the development of control strategies under the integrated vector management framework. We hypothesize that the effectiveness and reliability of using BG-Sentinel traps for the surveillance strongly depend on the bait used to attract mosquitoes. The objective of this study was to compare the effectiveness of BG-Sentinel traps baited with CO2 and BG-Lure. A total of 72 traps were deployed for 48 hours once a week for four weeks. For the initial 24-hour period, the traps were baited with CO2, and then for an additional 24 hours using the BG-Lure. Collected mosquitoes were analyzed using the Generalized Estimating Equation for repeated measures analysis. Biodiversity was assessed by the Shannon and Simpson indices and by individual rarefaction curves and SHE profiles. A total of 5,154 mosquitoes were collected, from which 3,514 by traps baited with CO2 and 1,640 mosquitoes by traps baited with BG-Lure. Aedes aegypti and Culex quinquefasciatus were the most abundant and dominant species. Results from the Generalized Estimating Equation models indicated that more than twice as many mosquitoes were attracted CO2 than to the BG-Lure. The comparison of attractiveness of CO2 and BG-Lure to Ae. aegypti and Cx. quinquefasciatus was non-significant, suggesting that both species were equally attracted by the baits. The individual rarefaction curves for Ae. aegypti and Cx. quinquefasciatus imply that traps baited with BG-Lure underestimated mosquito species richness compared to those baited with CO2. BG-Lure were less effective in attracting mosquitoes with low abundances and failed to collect Cx. coronator and Cx. nigripalpus, which were consistently collected by traps baited with CO2. According to our results, CO2 significantly (P<0.05) attracted more mosquitoes (2.67 adjusted odds ratios) than the BG-Lure when adjusted for time and species, being more effective in assessing the relative abundance of vector mosquitoes and yielding more trustworthy results. Traps baited with CO2 collected not only more specimens, but also more species in a more consistent pattern.
Introduction
Vector-borne diseases (VBD) are an increasing problem to public health [1–3]. Billions of people worldwide are at risk of being infected by arboviruses, and millions of cases are reported every year. Recent estimates indicated that the dengue virus (DENV) endangers more than 3 billion people globally, infecting more than 390 million people every year [4]. The Zika virus (ZIKV) outbreak in 2016 took the world by storm, the estimated number of cases in the American continent revolves around 700,000 cases, distributed among the 45 countries that have reported local transmission of ZIKV [5–7]. Notwithstanding the morbidity caused by the infection, ZIKV was also responsible for pregnancy and congenital neurologic malformations in fetuses [8–10].
Controlling mosquitoes is accepted as the most effective way to prevent VBD outbreaks [11]. However, it is not an easy task. Aedes (Stegomyia) aegypti (Linnaeus), Aedes (Stegomyia) albopictus (Skuse), Culex (Culex) quinquefasciatus Say and Culex (Culex) nigripalpus Theobald are among the most adapted species to live alongside humans in urban environments. They are exceptionally adapted to thrive in urban and suburban areas, blood feeding in human hosts and laying eggs in artificial breeding sites, widely benefiting from anthropogenic alterations in the environment [12–16]. Aedes aegypti and Ae. albopictus are the primary vector for many arboviruses, including DENV, chikungunya virus (CHIKV), yellow fever virus (YFV) and ZIKV [5,17–21]. Aedes albopictus was also found infected with YFV in a transmission hotspot in Brazil. However, Haemagogus leucocelaenus is considered the main vector of YFV in Brazil and further investigation is still needed to define the role of Ae. albopictus in the transmission of YFV to humans [22,23]. Culex quinquefasciatus and Cx. nigripalpus are the primary vectors for, among others, West Nile virus (WNV) and Eastern Equine encephalitis (EEE) [24–27].
Vector-borne disease outbreaks are becoming more frequent in previously non-endemic areas, notably the ZIKV outbreak in the Americas [5], and the outbreak of CHIKV in Italy [28–30]. Due to the lack of vaccines and drugs for most arboviruses and the unfeasibility of stopping travelers carrying arbovirus from coming and going, controlling mosquito populations is the only feasible alternative.
The integrated vector management (IVM) is the gold standard for controlling mosquitoes [11]. It encompasses the use of scientifically-driven strategies to control mosquito populations, taken into account the ecosystem, management of breeding sites, education of the general public and the use of insecticides to control adult mosquito populations when needed. Many new strategies for controlling mosquitoes have been proposed, however, according to the Vector Control Advisory Group (VCAG) of the World Health Organization (WHO) none of them has yet been proven effective and safe to be included under the IVM framework [31,32]. Therefore, rendering mosquito surveillance and traditional control strategies an essential part of IVM.
Having a reliable surveillance network of traps to assess the relative abundance of vector mosquitoes in a given area is critical to inform control actions and prevent outbreaks. BG-Sentinel traps (BioGents, Regensburg, Germany) baited with BG-Lure (BioGents, GmbH, Regensburg, Germany) as attractant are considered the gold standard for collecting Aedes vectors, especially from the subgenus Stegomyia [33,34]. Previous studies have also reported the effectiveness of BG-Sentinel traps baited with BG-Lure in collecting Culex species [35,36], especially when baited with CO2 in addition to the BG-Lure [37–39].
Miami-Dade County, Florida is a major gateway to the United States with an increased number of people coming and going from and to endemic areas. Therefore, the development of a reliable and dependable surveillance system is critical to guide and support a successful surveillance program aimed to prevent future outbreaks, both in Miami and elsewhere. Considering the paramount need to assess the presence and abundance of vector mosquitoes considering the tropical climate and unique conditions of Miami-Dade County, Florida we hypothesize that the effectiveness and reliability of using BG-Sentinel traps for the surveillance of vector mosquitoes strongly depend on the bait used to attract mosquitoes. Taking that into account, the objective of this study was to compare the effectiveness of BG-Sentinel traps baited with CO2 and BG-Lure in Miami-Dade County, Florida.
Methods
Study design
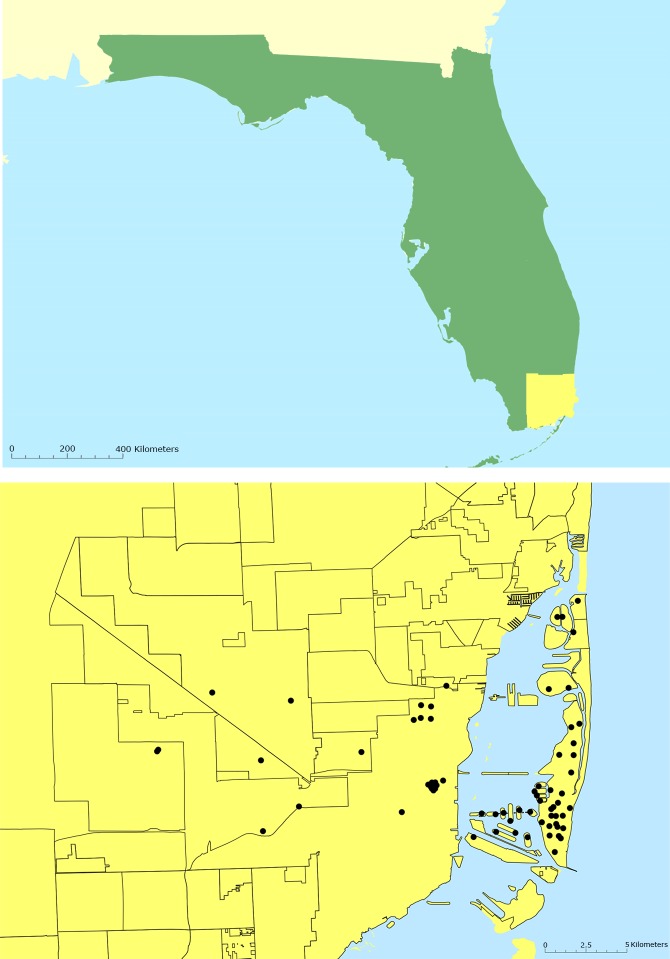
Collection of mosquitoes was conducted using 72 BG-Sentinel traps (Biogents AG, Regensburg, Germany) across 21 neighborhoods of Miami-Dade County, Florida. The location of the traps was chosen based on areas previously affected by the ZIKV outbreak in Miami, namely Miami Beach, Wynwood and Little River [40]. These areas are still considered to be at higher risk for the introduction of arboviruses due to the increased number of travelers and outdoor activities [41], with much of the surveillance and control efforts directed to control mosquito populations in those areas (Fig 1).
Fig 1. Maps displaying the BG-Sentinel trap locations in Miami-Dade County, Florida.
Map above: in green, state of Florida and in yellow Miami-Dade County; Map below: Miami-Dade County, Florida (latitude, 25.761681; longitude, -80.191788).
The sampling effort was standardized for all collections. Each BG-Sentinel trap was deployed 4 times from September to October 2018, for 48 hours (24 hours using each bait) once a week for 4 weeks. For the initial 24 hours period, the traps were baited with CO2 using containers filled with 1 Kg of dry ice pellets, and then subsequently serviced and immediately baited with BG-Lure (BioGents, Regensburg, Germany) for additional 24 hours. Collected mosquitoes were transported to the Miami-Dade County Mosquito Control Laboratory and subsequently morphologically identified using taxonomic keys [42]. Considering that BG-Sentinel traps mimic a host and, therefore, only actively attract female mosquitoes seeking for blood feeding, the male mosquitoes collected were considered accidental catches and were not included in the analyzes.
Since this study poses less than minimal risk to participants and did not involve endangered or protected species the Institutional Review Board at the University of Miami determined that the study was exempt from institutional review board assessment (IRB Protocol Number: 20161212).
Data analysis
We performed a Generalized Estimating Equation (GEE) for repeated measures analysis and using Poisson for the dependent variable distribution for mosquito counts. We had traps as the unit, species, and attractor type, and weeks in the longitudinal model. We tested the interaction species by attractor type and it was non-significant, so it was removed from the model. We kept the scale parameter at 1 and did not estimate it. The Quasi-likelihood under Independence Model Criterion (QICC) for the goodness of fit of the model was 5676. Having species in the model allowed us to analyze all the data in one model. The Poisson link was transformed considering Exp(Beta), resulting in the rate ratio. We also used the ANOVA Type III sum of squares method to analyze the number of mosquitoes collected by BG-Sentinel traps baited with different attractants.
The main goal of the Miami-Dade Mosquito control surveillance system is to monitor the relative abundance of primary vectors (i.e., Ae. aegypti and Cx. quinquefasciatus) and guide control interventions when needed. However, many other neglected vectors are present in Miami and an effective surveillance system has obligatorily to be able to detect species such as Cx. coronator, Cx. nigripalpus, and Cx. erraticus. Failing in attracting a wide range of species will result in the unfeasibility of using a given mosquito attractant for surveillance purposes in Miami. Therefore, biodiversity patterns and differences in the mosquito assembly for the collections comprising female mosquitoes collected by BG-Sentinel traps using CO2 and BG-Lure were analyzed by the Shannon (H) and Simpson (1-D) biodiversity indices [43]. Subsequently, the individual rarefaction curves were generated to estimate sampling sufficiency and the expected occurrence of species for smaller samples. Plots of cumulative species abundance (ln S), Shannon index (H) and log evenness (ln E) (SHE) profiles were also calculated for all collected mosquitoes; changes in the direction of the lines indicate ecological heterogeneity of mosquito assembly [44]. Analyses were carried out with 10,000 randomizations without replacement and a 95% confidence interval using Past software (v.3.16) [45,46]. Fig 1 was produced using ArcGIS 10.2 (Esri, Redlands, CA). Climate data was obtained at the National weather services (available at: https://www.weather.gov/mfl/) (S1 Table).
Results
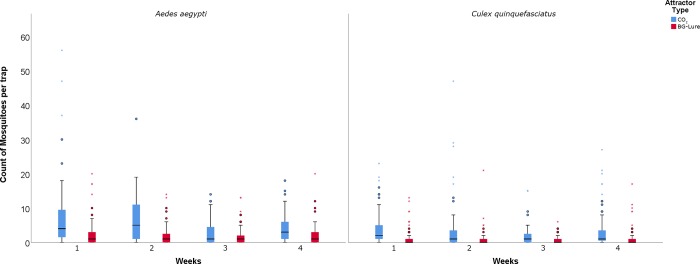
A total of 5,154 female mosquitoes were collected, from which 3,514 were collected by BG-Sentinel traps baited with CO2 and 1,640 by BG-Sentinel traps baited with BG-Lure. BG-Sentinel traps baited with CO2 collected 12 species of mosquitoes distributed among 4 genera. On the other hand, BG-Sentinel traps baited with BG-Lure collected 5 species of mosquitoes from 3 genera. Aedes aegypti was the most collected species totaling 2,674 specimens collected, from which 1,730 were collected by traps baited with CO2 and 944 by traps baited with BG-Lure. Culex quinquefasciatus was the second most abundant mosquito species, adding a total of 2,213 specimens collected, from which 1,529 were collected by traps baited with CO2 and 684 by traps baited with BG-Lure (Table 1, Fig 2).
Table 1. Mosquitoes collected by the 72 BG-Sentinel traps baited with CO2 and BG-Lure in Miami-Dade County, Florida.
| CO2 | BG-Lure | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | Total CO2 | Week 1 | Week 2 | Week 3 | Week 4 | Total BG-Lure | |
| Aedes aegypti | 543 | 478 | 292 | 417 | 1730 | 276 | 197 | 237 | 234 | 944 |
| Aedes albopictus | 6 | 3 | 1 | 10 | 2 | 2 | 5 | 9 | ||
| Aedes taeniorhynchus | 1 | 1 | ||||||||
| Aedes tortilis | 41 | 2 | 43 | |||||||
| Aedes triseriatus | 3 | 3 | 1 | 1 | 8 | |||||
| Anopheles quadrimaculatus | 1 | 1 | ||||||||
| Culex coronator | 16 | 9 | 7 | 5 | 37 | |||||
| Culex erraticus | 1 | 1 | ||||||||
| Culex nigripalpus | 5 | 59 | 7 | 71 | ||||||
| Culex quinquefasciatus | 422 | 417 | 240 | 450 | 1529 | 234 | 150 | 171 | 129 | 684 |
| Wyeomyia mitchelli | 25 | 34 | 2 | 61 | 1 | 1 | ||||
| Wyeomyia vanduzeei | 5 | 10 | 7 | 22 | 2 | 2 | ||||
| Total | 1068 | 1016 | 557 | 873 | 3514 | 512 | 352 | 408 | 368 | 1640 |
Fig 2. Box-plot graphs of collected Aedes aegypti and Culex quinquefasciatus over the 4 weeks of collection in Miami-Dade, Florida using BG-Sentinel traps baited with CO2 and BG-Lure.
Results from the Generalized Estimating Equation models indicated that more than twice as many Ae. aegypti and Cx. quinquefasciatus were attracted to traps baited with CO2 than to traps baited with BG-Lure (P = 0.001; exp(0.984) = 2.67). Furthermore, despite of the Cx. quinquefasciatus relative abundance being consistently higher than for Ae. aegypti according to the Miami-Dade Mosquito Control surveillance database (unpublished results) the comparison of the attractiveness of CO2 and BG-Lure to Ae. aegypti and Cx. quinquefasciatus was found to be non-significant. Ae. aegypti was significantly more attracted to CO2 and BG-Lure, yielding almost 70% more specimens collected in the traps (P = 0.000; exp(0.512) = 1.67). The difference between CO2 and BG-Lure was also significantly different adjusting for species. After the removal of the effects of the difference of species in the data variability, were there still differences between attractants (Table 2, S2 and S3 Tables, Fig 3).
Table 2. Results of Generalized Estimating Equation models for Aedes aegypti and Culex quinquefasciatus collected by BG-Sentinel traps baited with CO2 and BG-Lure.
| Coefficients | Adjusted Rate Ratio | 95%LCL | 95%UCL |
|---|---|---|---|
| Week 1 vs. 4* | 1.41 | 1.12 | 1.76 |
| Week 2 vs. 4 | 1.23 | 1 | 1.51 |
| Week 3 vs. 4* | 0.68 | 0.55 | 0.85 |
| CO2 vs BG-Lure* | 2.67 | 2.04 | 3.51 |
| Aedes aegypti vs. Culex quinquefasciatus* | 1.67 | 1.27 | 2.18 |
* = significant values at an alpha level of 0.05.
Adjusted Rate Ration are significant different than 1. Dependent variable: outcome; Model: (Intercept), weeks, bait type, species; a. set to zero because this parameter is redundant; scale = 1; LCL = lower confidence interval. UCL = upper confidence interval.
Fig 3. Graphs of means of collected mosquitoes over the 4 weeks of collections in Miami-Dade, Florida using BG-Sentinel traps baited with CO2 and BG-Lure.
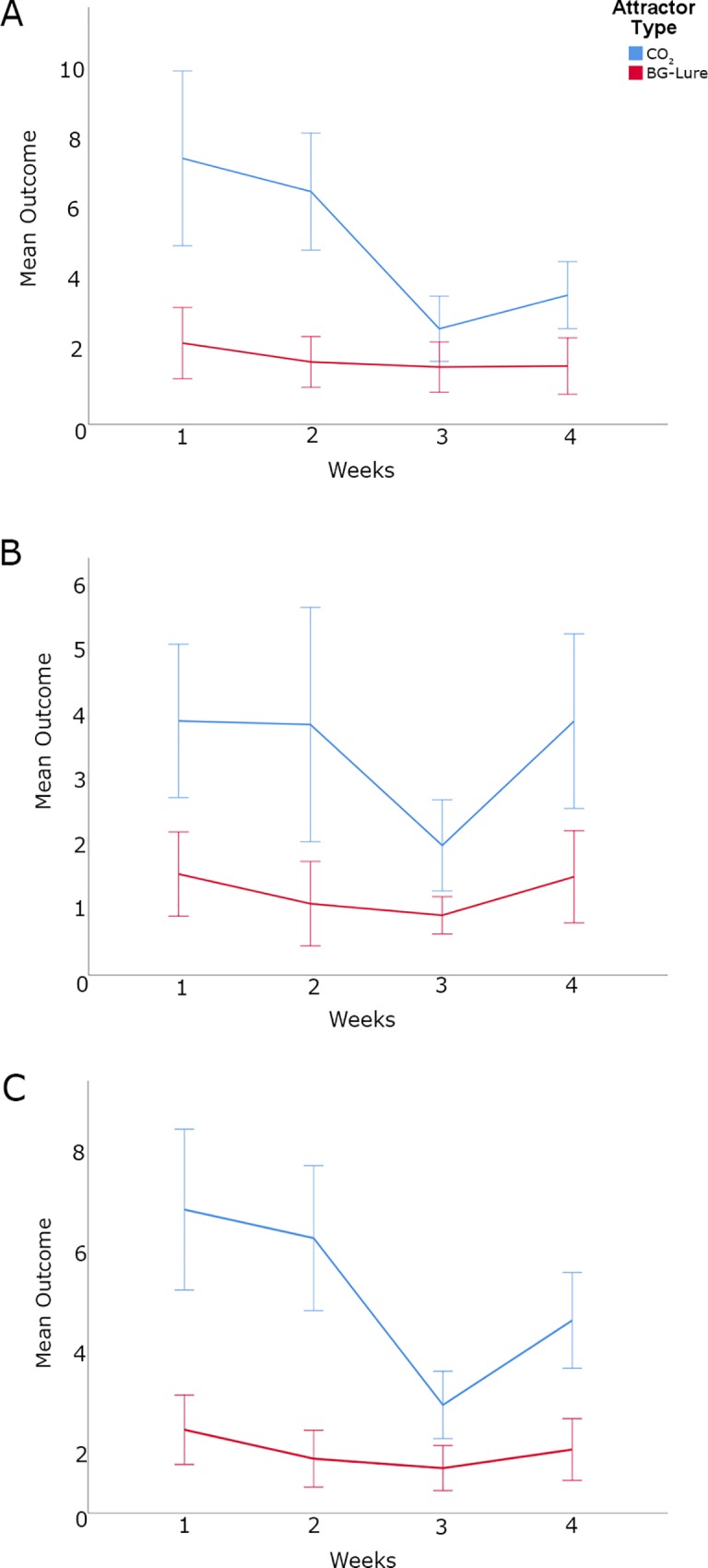
(A) Aedes aegypti; (B) Culex quinquefasciatus; (C) sum of Aedes aegypti and Culex quinquefasciatus.
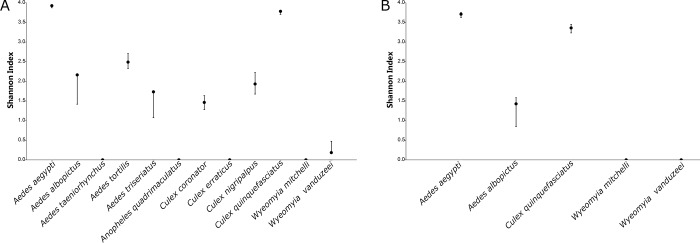
Despite the difference in the species richness and abundance comprising the mosquitoes collected by BG-Sentinel traps baited with CO2 and BG-Lure, the biodiversity indices indicated a similar scenario, in which Ae. aegypti and Cx. quinquefasciatus were the most dominant species. The average of the Shannon (H) index of BG-Sentinel traps baited with CO2 was 1.472 (95% CI: 1.295–1.557) and of BG-Sentinel traps baited with BG-Lure was 1.699 (95% CI: 1.508–1.757). Aedes aegypti collected by BG-Sentinel traps baited with CO2 yielded the highest value in the Shannon (H) index, 3.926 (95% CI: 3.872–3.942), followed by Cx. quinquefasciatus 3.783 (95% CI: 3.705–3.815). Similar results were obtained by BG-Sentinel traps baited with BG-Lure, in which Ae. aegypti yielded the highest values 3.711 (95% CI: 3.624–3.751), followed by Cx. quinquefasciatus 3.360 (95% CI: 3.235–3.456) (Fig 4).
Fig 4.
Shannon (H) index for mosquitoes collected in Miami-Dade County, Florida using BG-Sentinel traps baited with (A) CO2 and (B) BG-Lure.
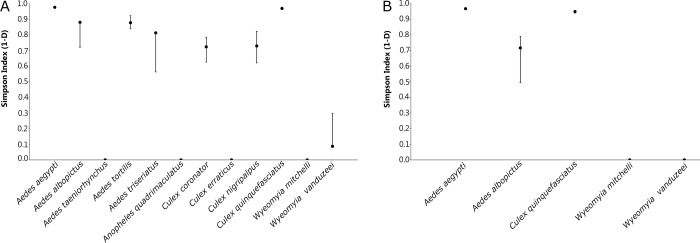
Aedes aegypti yielded the highest value in the Simpson (1-D) index, 0.976 (95% CI: 0.974–0.976) in BG-Sentinel traps baited with CO2, followed by Cx. quinquefasciatus 0.968 (95% CI: 0.964–0.970). BG-Sentinel traps baited with BG-Lure had similar results, Ae. aegypti was the most dominant species according to the Simpson (1-D) index, 0.967 (95% CI: 0.961–0.969), followed by Cx. quinquefasciatus 0.948 (95% CI: 0.939–0.956) (Fig 5).
Fig 5.
Simpson (1-D) index for mosquitoes collected in Miami-Dade County, Florida using BG-Sentinel traps baited with (A) CO2 and (B) BG-Lure.
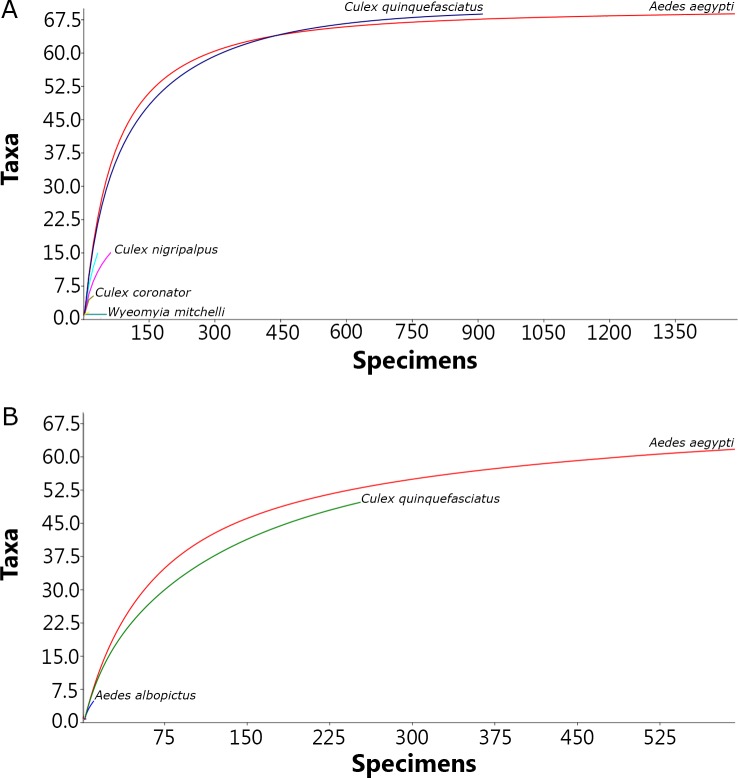
The individual rarefaction curves resulted in two distinct scenarios. In the same timeframe and with identical sampling effort, BG-Sentinel traps baited with CO2 yielded highly asymptotic curves for Ae. aegypti and Cx. quinquefasciatus indicating a high degree of confidence for assessing the relative abundance of these species. The individual rarefaction curves for BG-Sentinel traps baited with BG-Lure, on the other hand, did not reach the asymptote for Cx. quinquefasciatus and reached a moderate asymptote curve for Ae. aegypti. These results are indicating that sampling sufficiency was not reached by traps using BG-Lure as bait (Fig 6).
Fig 6.
Individual rarefaction curves of mosquitoes collected in Miami-Dade, Florida using BG-Sentinel traps baited with (A) CO2 and (B) BG-Lure.
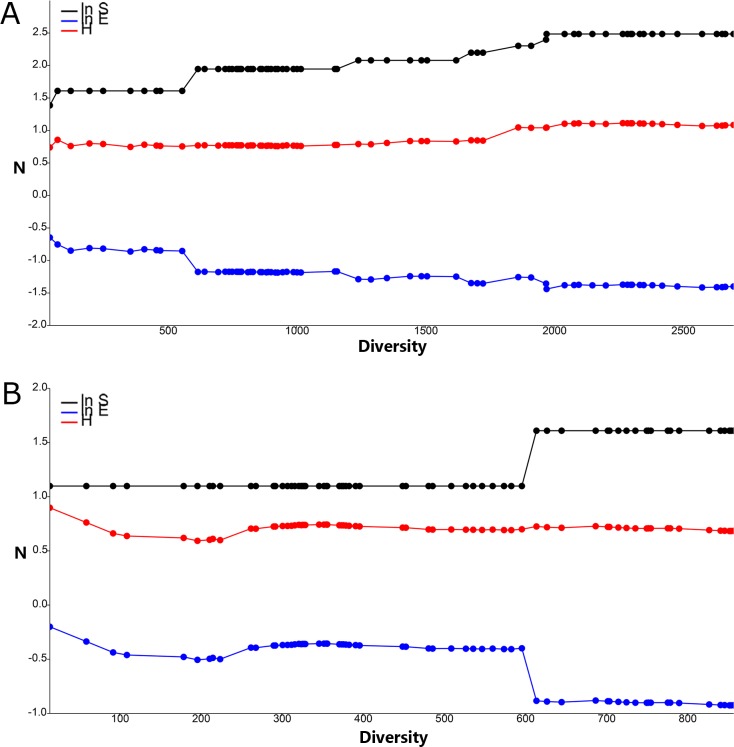
The changes in the direction of the lines in the cumulative SHE analysis exposed two different scenarios comprising species composition, diversity and evenness. BG-Sentinel traps baited with CO2 displayed substantial deviations from a straight line in the SHE analysis indicating a higher degree of heterogeneity in the composition of species. It was possible to observe multiple increases in value in the cumulative species abundance (Ln S) of traps baited with CO2, indicating an increased number of species collected when compared to traps baited with BG-Lure. A similar result was found for the log evenness (ln E), in which traps baited with BG-Lure displayed an unbalanced assembly of mosquitoes with mostly Ae. aegypti and Cx. quinquefasciatus comprising all collected specimens. BG-Sentinel traps baited with CO2 had the opposite results considering the Shannon index (H) with its gradual increase, contrasting with the decrease for traps baited with BG-Lure (Fig 7).
Fig 7.
Plots of cumulative species abundance (ln S), Shannon index (H) and log evenness (ln E) profiles (SHE) of mosquitoes collected in Miami-Dade, Florida using BG-Sentinel traps baited with (A) CO2 and (B) BG-Lure.
Discussion
Mosquito control programs rely on surveillance programs to guide their control operations. The relative abundance of adult mosquitoes in a given area is widely used to trigger control efforts such as management of breeding sites and spraying of insecticides. Therefore, achieving sampling sufficiency to consistently, correctly and reliably assess the relative abundance of adult mosquitoes is critical to prevent VBD outbreaks and protect residents and tourists. Our results indicated that BG-Sentinel traps baited with BG-Lure underestimated the richness and abundance of species when compared to the results obtained by BG-Sentinel traps baited with CO2. Furthermore, traps baited with CO2 collected twice as many specimens and species providing a more realistic panorama of the mosquito assembly and relative abundance of adult mosquitoes.
Even though the results are indicating that BG-Sentinel traps baited with BG-Lure consistently collected Ae. aegypti, even though in fewer numbers when compared to traps baited with CO2, the same was not true for the remaining species. Traps baited with BG-Lure were substantially less effective in collecting mosquitoes with low abundances such as Wyeomyia mitchelli and Wyeomyia vanduzeei that were only collected once (week 2), contrasting with traps baited with CO2, in which they were collected in three occasions (weeks 1, 2 and 3). Similar results were found for Cx. coronator and Cx. nigripalpus, in which neither species was collected by traps baited with BG-Lure but were consistently collected by traps baited with CO2.
Failing to collect important vector species can lead to the incorrect underestimation of their relative abundance and erroneously lead to inaccurately guided mosquito control operations or, in this case, the lack of it. Culex species, such as Cx. coronator and Cx. nigripalpus are primary vectors of WNV and failing to detect them may expose the human population to vector-borne pathogens [24,27,47]. Migratory birds, the natural reservoir of WNV, often stop in suburban and urban areas when they may come in contact with Culex mosquitoes, with the potential of triggering an outbreak [48–50].
Furthermore, an effective mosquito surveillance system based on relative abundance of adult mosquitoes has to account not only for Ae. aegypti, but if possible to all vector species in the region. Many pathogens are circulating under the radar that can be vectored by species from the genera Culex and Wyeomyia, among many others [51,52]. In a more pessimistic scenario, one can also speculate that there are many more arboviruses than we are aware of, such as ZIKV was once in the past [53].
We were not able to collect data across all weather and season variations that would provide more data on the effectiveness of BG-Sentinel traps baited with CO2 and BG-Lure in assessing mosquito abundance and species richness.
Conclusion
According to our results, BG-Sentinel traps baited with CO2 were more effective in assessing the relative abundance of adult vector mosquitoes in comparison with BG-Sentinel traps baited with BG-Lure. Traps baited with CO2 provided more trustworthy results, collecting not only more specimens, but also more species in a more consistent pattern. For this reason, we believe that BG-Sentinel traps baited only with BG-Lure should not be used for the surveillance of vector mosquitoes other than Ae. aegypti. In the specific case of Miami, in which many vector mosquitoes are present, and the surveillance program is not limited to only survey Ae. aegypti, the surveillance system has to be able to detect and provide reliable estimates of the presence and abundance of several species of vector mosquitoes rendering the use of BG-Sentinel traps baited with BG-Lure significantly less effective and, therefore, not recommended.
Supporting information
(TIF)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank the staff of Miami-Dade County Mosquito Control Division for their help with field collections and mosquito identification.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was supported by CDC (https://www.cdc.gov/) grant 1U01CK000510–01: Southeastern Regional Center of Excellence in Vector- Borne Diseases: The Gateway Program. CDC had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. JCB is the recipient of the grant.
References
- 1.Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, Mead PS, et al. Vital Signs: Trends in Reported Vectorborne Disease Cases—United States and Territories, 2004–2016. MMWR Morb Mortal Wkly Rep. 2018;67: 496–501. 10.15585/mmwr.mm6717e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496: 504–507. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee BY, Alfaro-Murillo JA, Parpia AS, Asti L, Wedlock PT, Hotez PJ, et al. The potential economic burden of Zika in the continental United States. PLoS Negl Trop Dis. 2017;11: e0005531 10.1371/journal.pntd.0005531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496: 504–7. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faria NR, Quick J, Claro IM, Thézé J, de Jesus JG, Giovanetti M, et al. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature. 2017;546: 406–410. 10.1038/nature22401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grubaugh ND, Ladner JT, Kraemer MUG, Dudas G, Tan AL, Gangavarapu K, et al. Genomic epidemiology reveals multiple introductions of Zika virus into the United States. Nature. 2017;546: 401–405. 10.1038/nature22400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Sun K, Chinazzi M, Pastore y Piontti A, Dean NE, Rojas DP, et al. Spread of Zika virus in the Americas. Proc Natl Acad Sci. 2017;114: E4334–E4343. 10.1073/pnas.1620161114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paixão ES, Teixeira MG, Costa M da CN, Barreto ML, Rodrigues LC. Symptomatic Dengue during pregnancy and congenital neurologic malformations. Emerg Infect Dis. 2018;24: 1748–1750. 10.3201/eid2409.170361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WTGH, do Carmo GMI, Henriques CMP, Coelho GE, et al. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed zika virus transmission during the first trimester of pregnancy—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65: 242–247. 10.15585/mmwr.mm6509e2 [DOI] [PubMed] [Google Scholar]
- 10.Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JLM, Guimarães KP, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534: 267–271. 10.1038/nature18296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Handbook for integrated vector management. Outlooks Pest Manag. 2013;24: 142–143. [Google Scholar]
- 12.Wilke ABB, Vasquez C, Mauriello PJ, Beier JC. Ornamental bromeliads of Miami-Dade County, Florida are important breeding sites for Aedes aegypti (Diptera: Culicidae). Parasit Vectors. 2018;11: 283 10.1186/s13071-018-2866-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chitolina RF, Anjos FA, Lima TS, Castro EA, Costa-Ribeiro MC V. Raw sewage as breeding site to Aedes (Stegomyia) aegypti (Diptera, culicidae). Acta Trop. 2016;164: 290–296. 10.1016/j.actatropica.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 14.Ramasamy R, Surendran SN, Jude PJ, Dharshini S, Vinobaba M. Larval development of Aedes aegypti and Aedes albopictus in peri-urban brackish water and its implications for transmission of arboviral diseases. PLoS Negl Trop Dis. 2011;5: e1369 10.1371/journal.pntd.0001369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilke ABB, Wilk-da-Silva R, Marrelli MT. Microgeographic population structuring of Aedes aegypti (Diptera: Culicidae). PLoS One. 2017;12: e0185150 10.1371/journal.pone.0185150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Multini LC, Wilke ABB, Suesdek L, Marrelli MT. Population genetic structure of Aedes fluviatilis (Diptera: Culicidae). PLoS One. 2016;11: e0162328 10.1371/journal.pone.0162328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown JE, Evans BR, Zheng W, Obas V, Barrera-Martinez L, Egizi A, et al. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution. 2014;68: 514–525. 10.1111/evo.12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gubler DJ. Dengue, urbanization and globalization: The unholy trinity of the 21st Century. Trop Med Health. 2011;39: S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couto-Lima D, Madec Y, Bersot MI, Campos SS, Motta M de A, Santos FB dos, et al. Potential risk of re-emergence of urban transmission of yellow fever virus in Brazil facilitated by competent Aedes populations. Sci Rep. 2017;7: 4848 10.1038/s41598-017-05186-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa-da-Silva AL, Ioshino RS, Petersen V, Lima AF, Cunha M dos P, Wiley MR, et al. First report of naturally infected Aedes aegypti with chikungunya virus genotype ECSA in the Americas. PLoS Negl Trop Dis. 2017;11: e0005630.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraemer MUG, Sinka ME, Duda KA, Mylne A, Shearer FM, Brady OJ, et al. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci Data. 2015;2: 150035 10.1038/sdata.2015.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Epidemiological Update Yellow Fever. 2017; 1–4.
- 23.Cardoso J da C, de Almeida MAB, dos Santos E, da Fonseca DF, Sallum MAM, Noll CA, et al. Yellow fever virus in Haemagogus leucocelaenus and Aedes serratus mosquitoes, Southern Brazil, 2008. Emerg Infect Dis. 2010;16: 1918–1924. 10.3201/eid1612.100608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vitek CJ, Richards SL, Mores CN, Day JF, Lord CC. Arbovirus transmission by Culex nigripalpus in Florida, 2005. J Med Entomol. 2008;45: 483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zinser M, Ramberg F, Willott E. Culex quinquefasciatus (Diptera: Culicidae) as a potential West Nile virus vector in Tucson, Arizona: Blood meal analysis indicates feeding on both humans and birds. J Insect Sci. 2004;4: 1–3. 10.1673/031.004.3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samy AM, Elaagip AH, Kenawy MA, Ayres CFJ, Peterson AT, Soliman DE. Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus, vector of West Nile virus and lymphatic filariasis. PLoS One. 2016;11: e0163863 10.1371/journal.pone.0163863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turell MJ, Guinn MLO, Dohm DJ, Jones JW. Vector competence of North American mosquitoes for West Nile virus. J Med Entomol. 2001;38: 130–134. 10.1603/0022-2585-38.2.130 [DOI] [PubMed] [Google Scholar]
- 28.Poletti P, Messeri G, Ajelli M, Vallorani R, Rizzo C, Merler S. Transmission potential of chikungunya virus and control measures: The case of italy. PLoS One. 2011;6: e18860 10.1371/journal.pone.0018860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roche B, Léger L, L’Ambert G, Lacour G, Foussadier R, Besnard G, et al. The spread of Aedes albopictus in Metropolitan France: Contribution of environmental drivers and human activities and predictions for a near future. PLoS One. 2015;10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zannoli S, Morotti M, Denicolò A, Tassinari M, Chiesa C, Pierro A, et al. Chikungunya virus and Zika virus in Europe Chikungunya and Zika viruses. Elsevier; 2018. pp. 193–214. 10.1016/B978-0-12-811865-8.00006-4 [DOI] [Google Scholar]
- 31.Wilke ABB, Beier JC, Benelli G. Transgenic mosquitoes–Fact or fiction? Trends Parasitol. 2018;34: 456–465. 10.1016/j.pt.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Vector Control Advisory Group (VCAG) on New Paradigms; 2017. Available at: http://www.who.int/neglected_diseases/vector_ecology/VCAG/en/ 2016. Available: http://www.who.int/neglected_diseases/vector_ecology/VCAG/en/
- 33.Li Y, Su X, Zhou G, Zhang H, Puthiyakunnon S, Shuai S, et al. Comparative evaluation of the efficiency of the BG-Sentinel trap, CDC light trap and Mosquito-oviposition trap for the surveillance of vector mosquitoes. Parasit Vectors. 2016;9: 446 10.1186/s13071-016-1724-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krockel U, Rose A, Eiras AE, Geier M. New tools for surveillance of adult yellow fever mosquitoes: comparison of trap catches with human landing rates in an urban environment. J Am Mosq Control Assoc. 2006;22: 229–38. 10.2987/8756-971X(2006)22[229:NTFSOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 35.Maciel-de-Freitas R, Eiras ÁE, Lourenço-de-Oliveira R. Field evaluation of effectiveness of the BG-Sentinel, a new trap for capturing adult Aedes aegypti (Diptera: Culicidae). Mem Inst Oswaldo Cruz. 2006;101: 321–325. [DOI] [PubMed] [Google Scholar]
- 36.Figuerola J, Muñoz J, Soriguer R, Roussel M, Roiz D, Ruiz S. Efficacy of mosquito traps for collecting potential West Nile mosquito vectors in a natural mediterranean wetland. Am J Trop Med Hyg. 2012;86: 642–648. 10.4269/ajtmh.2012.11-0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Ázara TMF, Degener CM, Roque RA, Ohly JJ, Geier M, Eiras ÁE. The impact of CO2 on collection of Aedes aegypti (Linnaeus) and Culex quinquefasciatus say by BG-sentinel traps in Manaus, Brazil. Mem Inst Oswaldo Cruz. 2013;108: 229–232. 10.1590/0074-0276108022013016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roiz D, Duperier S, Roussel M, Boussès P, Fontenille D, Simard F, et al. Trapping the tiger: Efficacy of the novel BG-Sentinel 2 with several attractants and carbon dioxide for collecting Aedes albopictus (Diptera: Culicidae) in Southern France. J Med Entomol. 2016;53: 460–465. 10.1093/jme/tjv184 [DOI] [PubMed] [Google Scholar]
- 39.Pombi M, Jacobs F, Verhulst NO, Caputo B, Della Torre A, Takken W. Field evaluation of a novel synthetic odour blend and of the synergistic role of carbon dioxide for sampling host-seeking Aedes albopictus adults in Rome, Italy. Parasites and Vectors. 2014;7: 1–5. 10.1186/1756-3305-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Likos A, Griffin I, Bingham AM, Stanek D, Fischer M, White S, et al. Local mosquito-borne transmission of Zika virus—Miami-Dade and Broward Counties, Florida, June-August 2016. MMWR Morb Mortal Wkly Rep. 2016;65: 1032–8. 10.15585/mmwr.mm6538e1 [DOI] [PubMed] [Google Scholar]
- 41.Stoddard PK. Managing Aedes aegypti populations in the first Zika transmission zones in the continental United States. Acta Trop. 2018;187: 108–118. 10.1016/j.actatropica.2018.07.031 [DOI] [PubMed] [Google Scholar]
- 42.Darsie RF Jr., Morris CD. Keys to the adult females and fourth-instar larvae of the mosquitoes of Florida (Diptera, Culicidae). 1st ed Vol. 1 Tech Bull Florida Mosq Cont Assoc; 2000 [Google Scholar]
- 43.Cardoso J da C, de Paula MB, Fernandes A, dos Santos E, de Almeida MAB, da Fonseca DF, et al. Ecological aspects of mosquitoes (Diptera: Culicidae) in an Atlantic forest area on the north coast of Rio Grande do Sul State, Brazil. J Vector Ecol. 2011;36: 175–186. 10.1111/j.1948-7134.2011.00155.x [DOI] [PubMed] [Google Scholar]
- 44.Buzas MA, Hayek LAC. SHE analysis for biofacies identification. J Foraminifer Res. 1998;28: 233–239. [Google Scholar]
- 45.Morris EK, Caruso T, Buscot F, Fischer M, Hancock C, Maier TS, et al. Choosing and using diversity indices: Insights for ecological applications from the German Biodiversity Exploratories. Ecol Evol. 2014;4: 3514–3524. 10.1002/ece3.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammer Ø, Harper DATT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4: 9. [Google Scholar]
- 47.Alto BW, Connelly CR, O’Meara GF, Hickman D, Karr N. Reproductive biology and susceptibility of Florida Culex coronator to Infection with West Nile Virus. Vector-Borne Zoonotic Dis. 2014;14: 606–614. 10.1089/vbz.2013.1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hile JA, Mangiafico JA, Clippinger TL, Raphael BL, Smith JF, Danner DK, et al. An outbreak of West Nile virus in a New York City captive wildlife population. Am J Trop Med Hyg. 2002;67: 67–75. [DOI] [PubMed] [Google Scholar]
- 49.Mcnamara TS, Mclean RG, Saito EMIK, Wolff PL, Gillin CM, Fischer JR, et al. Surveillance of wildlife diseases: Lessons from the West Nile Virus Outbreak. Microbiol Spectr. 2014;1: 1–11. [DOI] [PubMed] [Google Scholar]
- 50.Watts J, July I. West Nile virus detected in mosquitoes in Central Park. World Health. 2000;78: 8–9. [Google Scholar]
- 51.Lorenz C, Azevedo TS, Virginio F, Aguiar BS, Chiaravalloti-Neto F, Suesdek L. Impact of environmental factors on neglected emerging arboviral diseases. PLoS Negl Trop Dis. 2017;11: e0005959 10.1371/journal.pntd.0005959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lednicky J, De Rochars VMB, Elbadry M, Loeb J, Telisma T, Chavannes S, et al. Mayaro virus in child with acute febrile illness, Haiti, 2015. Emerg Infect Dis. 2016;22: 2000–2002. 10.3201/eid2211.161015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kindhauser MK, Allen T, Frank V, Santhana RS, Dye C. Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ. 2016;94: 675–686C. 10.2471/BLT.16.171082 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.