Abstract
Background
Malpractice and excess use of antimicrobials have been associated with multiple costs, including the development of resistant bacteria, which has become a threat to the human health. The aim of this study, therefore, was to assess the antibiotic use practice and to identify predictors of hospital outcome to uncover targets for stewardship.
Methods
An Institution-based prospective observational study was performed from 9 April to 7 July 2014 in the internal medicine wards of Tikur Anbessa Specialized Hospital. Patients with suspected systemic bacterial infections during this period were strictly followed and data were abstracted using data abstraction format. Descriptive statistics and binary logistic regression were used for statistical analysis.
Results
About half of the attended patients had suspected systemic bacterial infections, in which pneumonia is the most common. Cephalosporins were the most widely prescribed class of drugs in all the wards. Initial antibiotics were empiric in almost all of the cases. About 28% of the ward and 59% of the ICU patients died during the in-hospital stay. The mean length of stay (LoS) was 18.5+12.2 in the wards and 8.9+4.9 days in the ICU. Whilst digestive disease (AOR = 6.94, 95% CI: 2.24, 21.49), different signs and symptoms of disease (AOR = 2.43, 95% CI: 1.30, 4.56), sepsis (AOR = 2.59, 95% CI: 1.12, 5.99) and vancomycin use (AOR = 2.60, 95% CI: 1.30, 5.21) were independent positive predictors, antibiotic days (> 10) (AOR = 0.37, 95% CI: 0.20, 0.70) was a negative predictor for mortality. On the other hand, hospital-acquired infection (AOR = 3.01, 95% CI: 1.05, 8.62), beyond the median antibiotic days (> 10) (AOR = 4.05, 95% CI: 1.96, 8.37) and agent days beyond 21 days (AOR = 2.18, 95% CI: 1.01–4.68) were independently associated with prolonged LoS.
Conclusion
Generally, this observation entails an appropriate infection management and antimicrobial use policy. Any future policy should better start by addressing cases like pneumonia, and sepsis and drugs like cephalosporins.
Introduction
About half of the antimicrobial agents prescribed to hospital in-patients are considered inappropriate [1]. This malpractice has been associated with multiple costs like the development of resistant bacteria [2,3]. As a result, it is more difficult than ever to challenge infections caused by antibiotic-resistant microbes [3].
The identification of infected patients at risk of poor hospital outcomes (e.g. in-hospital mortality) is important to provide an effective healthcare service [4,5]. Predicting hospital outcomes at admission and during the hospital stay may facilitate the healthcare delivery, as it can allow staff to manage healthcare resources optimally [2].
Different approaches have been promoted to save these precious drugs from the threat of resistant bacterial selection [6]. Antimicrobial stewardship is currently considered as the promising approach and has been promoted for all hospitals [5,7,8].
Although resistance is a global concern, it is primarily a local problem where single and multiple drug resistance to the commonly used antibiotics was high among bacterial isolates in different areas of Ethiopia [9,10], warranting rational use of drugs in the local environment. One study conducted in Tikur Anbessa Specialized Hospital (TASH) reported a high prevalence of multi-drug resistant bacterial strains that cause blood stream infection [11]. Thus, it needs a widespread effort at the individual institutional level to impact antimicrobial usage and, by extension (hopefully), antimicrobial resistance.
To the best of our knowledge, there was a dearth of studies done on the prudent use of antibiotics in TASH as well as in the country. However, other studies conducted in TASH reported suboptimal microbiologic reports utilization practice of healthcare professionals [12] and important gaps in their perception towards antimicrobial resistance [13]. The aim of the present study was therefore to perform a systematic and comprehensive assessment of antibiotic use practice and to identify predictors of hospital outcomes in hospitalized patients with systemic bacterial infections, in order to identify institutional targets for better antibiotic and health care resource stewardship. On the other hand, the aforementioned preliminary study on the perception of healthcare professionals in the hospital (conducted for a similar propose but after our study period) reported the need for specific educational priorities and implementation strategies [13]. Therefore, the study would have invaluable worth to supplement the hospital therapeutic decision, to the local health, for governmental decisions in the area, and for further studies.
Materials and methods
Study setting and period
TASH is a full-service 800-bed governmental University-affiliated tertiary care hospital in the country, Ethiopia. It provides ambulatory and in-hospital care. The in-hospital care is diversified, majorly involving the 120-bed internal medicine wards including a 6-bed medical intensive care unit (ICU). The study was conducted in all these internal medicine wards including the medical ICU since most adult cases were supposed to be seen in this unit. It was conducted from 9 April to 7 July 2014 for 3 consecutive months. Almost all the recording systems of the hospital during the study period were carried out manually. During the study period, the hospital had 4 infectious disease specialists and 2 microbiologists. An antimicrobial stewardship committee was fully established in November 2017 and currently active.
Study Design: The design was an institution based prospective observational study.
Study population & sampling
All patients attending the adult internal medicine wards, including the medical ICU, of TASH during the study period and who had suspected systemic bacterial (non-mycobacterial) infections formed the study population.
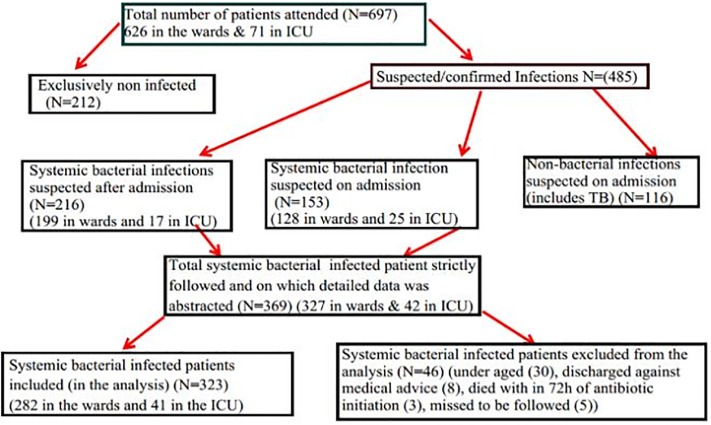
Inclusion criteria: Patients attending care in the adult internal medicine wards with suspected systemic bacterial infection and dispensed with systemic antibacterial agents during the study period were included. Patients taking antibacterial for < 72 hours, aged <18 years, lost to be followed up, or discharged against medical advice; patients taking anti-mycobacterial, non-systemic antibacterial, prophylactic antibacterial were excluded (Fig 1).
Fig 1. Patients included in the study among hospitalized patients with systemic bacterial infection in the internal medicine ward of Tikur Anbessa Specialized Hospital.
Study variables
Dependent Variables: In-hospital mortality and prolonged length of stay (LoS) (taking the median value). Independent Variables: Socio-demographic factors like sex and age; Disease-related factors like primary/admission diagnosis, infection diagnosis, immunocompromised status, and glomerular filtration rates; Drug-related factors like different antibiotics, changes from initial therapy and antibiotic metrics; and Antibiotic use quality indicators for appropriateness of therapy.
Data collection process
Data abstraction format was adopted from different literatures. The data collectors were four clinical pharmacy staff members of the hospital. To maximize the quality of the data, training, regular supervision and monitoring of the data collectors were performed. In addition, a pre-test was conducted on 5% of the initially assumed antibiotic user patients, outside of the study period. Patients who had (on admission) and developed (in the hospital stay) systemic bacterial (except mycobacterial) infections were strictly followed. The data collection for new admissions and abstraction of follow-up updates were performed on odd days bases by reviewing the patient sheet and consulting the attending healthcare provider(s). Demographic characteristics, admission diagnosis, suspected infection diagnosis, laboratory procedures performed (culture & gram staining) and the date of each laboratory report were documented properly. In addition, antibiotics administered, follow-up adjustments to the antibiotic regimen, and the dates’ of each antibiotic initiation and adjustment were also recorded properly. Criteria’s used for diagnosis, the microbiologic techniques, the decision to prescribe and modify antibiotics were left for the physician’s discretion. The data collectors deal with gaps evident during the data abstraction process with the health staffs, especially the attending physician. On biweekly basis, the principal investigator evaluates the work of the data collectors and gets unclear issues to the infectious diseases specialist.
Data analysis
The collected data was checked, cleaned and double entered into epi info 7. Those records that did not satisfy the inclusion criteria and the intent of the data interpretation were excluded from the analysis, except for the rate of infection interpretation (Fig 1).
SPSS for windows version 21.0 was used for data analysis. Descriptive statistics and binary logistic regression were used for statistical analysis. Variables that exhibited a p-value of ≤0.05 in the univariable models were included in the multivariable logistic regression analysis in order to control confounding variables. Crude and Adjusted Odds Ratio (COR/ AOR) at 95% confidence level were calculated and finally the association was declared significant at p<0.05.
Ethical consideration
Ethical approval was obtained from the Ethics committee of the School of Pharmacy, Addis Ababa University. In addition, the hospital management was requested for permission. Since the data collection was primarily dependent on patient charts, no written consent was requested from patients. However, information was given to patients, their physicians, and other health workers, as required. To ensure confidentiality, name and other identifiers of patients and prescribers were not recorded. The collected data was kept in a locked cabinet and only the researchers had access to it.
Operational definitions
Appropriateness of therapy: It is based on the five quality indicators (File 1) developed after reviewing the patients’ records and the collected data, as proposed by van den Bosch et al. for adult non-ICU [14] and for sepsis [15]. These quality indicators were developed to fit our perspective based on the scientific requirements.
Length of stay (LoS): LoS was defined as the number of days (referenced by midnights) between admission and discharge, regardless of the number of hours, because the precise time was often not available. Prolonged LoS is a LoS above the median (> 16 days for the wards and > 10 days for the ICU).
Signs and symptoms of the disease: It is based on international classification 10 (ICD 10), which refers to the signs and symptoms of the underlying diseases (e.g. hemiparesis, secondary to hypertension) that were not classified elsewhere under the primary admission diagnosis but which had been the primary reasons for admission.
Adjustment: Changes made to the antibiotic/regimen after 48–72 hours of the initial therapy that refers to either of the following: Discontinued: meaning discontinuation of all antibiotics found to be unnecessary (e.g. no suspected infection); Modified: meaning either de-escalation (narrowing by either discontinuation of either agent or using the narrower spectrum option) or broadening (addition or using a much broader spectrum instead) of therapy.
Antibiotic metrics: Refers to the following antibiotic use measures: Antibiotic courses: a period during which the same systemic antibiotic (regardless of dose or route) was administered to the same patient on consecutive days; Treatment periods: a period of consecutive days on which any systemic antibiotic or combination of antibiotics was administered to a patient; Agent days: the number of days that a patient received a particular systemic antibiotic during the ward admission period; Antibiotic days: the number of days on which a patient received any systemic antibiotics during the ward admission period.
Results
Socio-demographic and disease characteristics of the patient
Patients had a mean age of 41.8± 17.8 (range: 18–85). Females accounted for about 52% of the study participants. Of all, 75.2% of the patients had suspected infection during ward admission. Patients with circulatory disease (34.4%) accounted for the second higher category of primary admission diagnosis (Table 1).
Table 1. Socio-demographic and disease characteristics of hospitalized patients with bacterial infection in the internal medicine ward of TASH in 2014, Addis Ababa, Ethiopia.
| Variables | Wards, N = 282 (Freq, %) | ICU, N = 41 (Freq, %) | Total, N = 323 (Freq, %) |
|---|---|---|---|
| Average age (mean ± standard deviation (range)) | 41.7± 17.7 (18–85) | 42.9 ± 18.9 (18–84) | 41.8± 17.8 (18–85) |
| Sex of patient | |||
| Female | 149 (52.8) | 20(48.8) | 169(52.3) |
| Male | 133(47.2) | 21(51.2) | 154(47.7) |
| Admission diagnosis (ICD 10)† | |||
| Infectious diseases | 209 (74.1) | 34(82.9) | 243(75.2) |
| Human Immunodeficiency Virus | 45(16.0) | 3(7.3) | 48(14.9) |
| Circulatory disease | 88(31.2) | 23(56.1) | 111(34.4) |
| Neoplasm | 78(27.7) | 2(4.9) | 80(24.8) |
| Signs and symptoms of a disease | 77(27.3) | 8(19.5) | 85(26.3) |
| Endocrine and metabolic disorders | 33(11.7) | 4(9.8) | 37(11.5) |
| Digestive disorders | 18(6.4) | 3(7.3) | 21(6.5) |
| Genitourinary disorders | 23(8.2) | 1(2.4) | 24(7.4) |
| Blood-related disorders | 22(7.8) | 1(2.4) | 23(7.1) |
| Respiratory disorders | 20(7.1) | 6(14.6) | 26(8.1) |
| Other diagnosis†† | 19(6.7) | 3(7.3) | 22(6.8) |
| Abnormal organ functions | |||
| Abnormal Renal Function Test | 35(12.4) | 11(26.8) | 46 (14.2) |
| Glomerular filtration rate below 50 mL/min/1.73 m2 | 21(7.4) | 6(14.6) | 27 (8.4) |
| Microbiologic reports | |||
| Gram stain reported | 36 (12.8) | 11(26.8) | 47(14.6) |
| Culture reported | 34(12.1) | 4(10.81) | 38 (14.9) |
| susceptibility was done* | 9(81.8) | 1(100) | 10(83.3) |
| Origin of infection ♦ | |||
| Community-acquired | 80 (28.4) | 9(21.9) | 89(27.6) |
| Hospital-acquired | 35(12.4) | 10(24.4) | 45(13.9) |
| Unknown | 167(59.2) | 22(53.6) | 189(58.5) |
| MDR risk ♦ ♦ | |||
| Absent | 7(2.5) | 2(4.9) | 9(2.8) |
| Present | 28(9.9) | 12(29.3) | 40(12.4) |
| Not enough evidence | 247(87.6) | 27(65.8) | 274(84.8) |
| Immunocompromised ♦ ♦ ♦ | |||
| Yes | 121(42.9) | 7(17.1) | 128(39.6) |
| No | 161(57.1) | 34(82.9) | 195(60.4) |
†a given patient may have >1 diagnosis, based on the International classification of disease (ICD);
††Other Diagnosis in wards: Drug adverse outcomes (8), Seizure/Epilepsy (4), gynecology (3), Arthritis (2), Communicable hydrocephalus (1) & Cholestatic calculi (1); ICU: Injury (2) Drug-related adverse outcomes (1));
*Denominator-all positive culture reports-12 for the total, 11 in the wards and 1 in the ICU;
♦ Origin of infection was classified based on the source of the infection labeled by the physician;
♦ ♦ Multi-drug resistance risk (MDR) criteria: prior antibiotic receipt in the past 3 months, previous hospital admission during the last 3 months, late-onset hospital-acquired infections (HAIs) (as defined by the physicians and/or the date of antibiotic administration relative to the admission date) (i.e. >5 days after admission), and presence of preexisting immunosuppressive disease;
♦ ♦ ♦ patients with febrile neutropenia, cirrhosis, disseminated TB & HIV infection were classified as immunosuppressed.
Rate of infection
Using the total internal medicine ward admissions (697: 42 for ICU and 327 for wards) as the denominator, the systemic bacterial infection rate was 45.1% (282/626) for the wards and 57.7% (41/71) for the ICU. If the excluded patients with systemic bacterial infection were counted, the rate would have increased to 52.2% (327/626) for the wards and 59.1% (42/71) for the ICU (Fig 1). This rate, however, did not reflect the emergency department since the data collection was exclusively undertaken after the patients were admitted to the internal medicine wards.
Infection diagnosis
Of all the patients, 48.0% had pneumonia. Community-acquired pneumonia in wards (25.2%) and aspiration pneumonia in ICU (36.6%) were the commonest types of pneumonia suspected (Table 2).
Table 2. Types of infections suspected in hospitalized patients in the internal medicine ward of TASH in 2014, Addis Ababa, Ethiopia.
| Bacterial Diagnosis* | Wards, N = 282(Freq, %) | ICU,N = 41(Freq, %) | Total, N = 323(Freq, %) |
|---|---|---|---|
| Pneumonia | 124(44.0) | 31(75.6) | 155(48.0) |
| Community-acquired | 71(25.2) | 7(17.1) | 78(24.2) |
| Aspiration | 27(9.6) | 15(36.6) | 42(13.0) |
| Hospital-acquired | 21(7.5) | 8(19.5) | 29(9.0) |
| Ventilation associated | 0 | 1(2.4) | 1(0.3) |
| Other Pneumonia | 5(1.8) | 0 | 5(1.6) |
| Urinary Tract Infection | 39(13.8) | 3(7.3) | 42(13.0) |
| Sepsis | 34(12.1) | 5(12.2) | 39(12.1) |
| Fever of neutropenia | 34(12.1) | 1(2.4) | 35(10.8) |
| Meningitis | 18(6.4) | 2(4.9) | 20(6.2) |
| Abscess | 15(5.3) | 0 | 15(4,6) |
| Spon. Bacterial Peritonitis | 11(3.9) | 2(4.9) | 13(4.0) |
| Gastroenteritis | 11(3.9) | 0 | 11(3.4) |
| Diabetic foot ulcer | 10(3.5) | 0 | 10(3.1) |
| Infective endocarditis | 9(3.2) | 0 | 9(2.8) |
| Skin infections | 7(2.5) | 1(2.4) | 8(2.5) |
| Unknown infections | 7(2.5) | 0 | 7(2,2) |
| Parapneumonic effusion/empyema | 6(2.1) | 0 | 6(1.9) |
| Tetanus | 0 | 2(4.9) | 2(0.6) |
| Surgical site infections | 0 | 1(2.4) | 1(0.3) |
| Other Bacterial infections** | 23(8.2) | 1(2.4) | 24(7.4) |
*As per the labeling of the prescribing physician a given patient may have ≥ 1 bacterial diagnosis;
**Acute bronchitis (1), Acute Post Streptococcal Glomerulonephritis (1), Acute febrile illnesses (2), Chronic diarrhea (2), Cough (1), Emphysema (1), H. pylori (1), Intra-abdominal infections (4), lymphadenitis (Pyogenic) (2), Odontogenic infections (4), Osteomyelitis (2), Otitis Media (1), Pneumothorax (1), and Sore throat (1); for ICU: acute bronchitis; ICU: Intensive care unit
Antibiotics and antibiotic related factors
Class of and specific antibiotics used
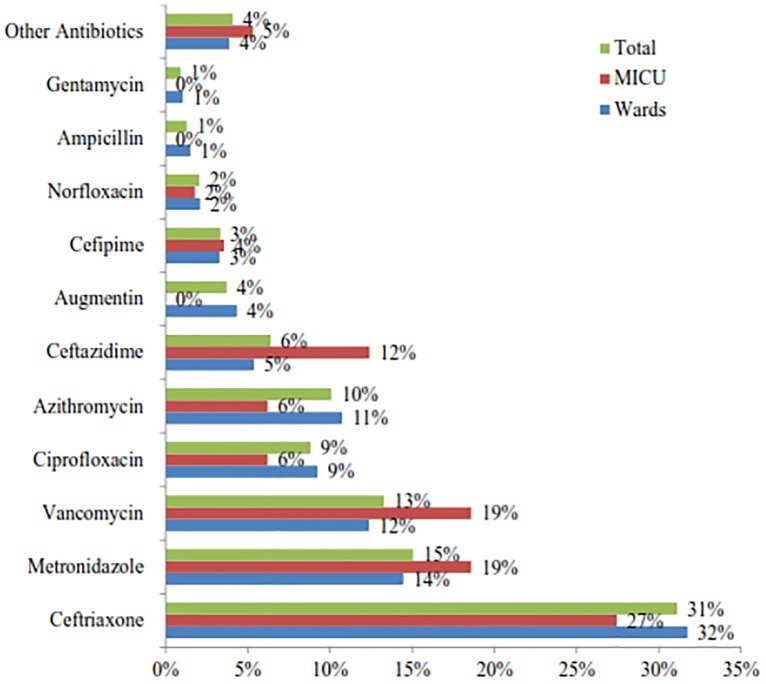
Comparatively (ward vs. ICU); cephalosporin (41% vs. 43%), anti-anaerobic (15% vs. 19%) and glycopeptides (vancomycin only) (12% vs. 19%) were the prevalently used class of drugs across the settings (Fig 2). The most frequently prescribed antibiotics were (ward vs. ICU) ceftriaxone (32% vs. 27%), metronidazole (14% vs. 19%) and vancomycin (12% vs. 19%). When grouped in a class comparatively (ward vs. ICU); cephalosporin (41% vs. 43%), anti-anaerobic (15% vs. 19%) and glycopeptides (vancomycin only) (12% vs. 19%) were the prevalently used class of drugs across the settings (Fig 2).
Fig 2. Types of antibiotics used in hospitalized patients with bacterial infection in the internal medicine ward of TASH in 2014, Addis Ababa, Ethiopia.
Other Antibiotics: Wards: Clindamycin (5), Cloxacillin (4), Gemifloxacin (1), Cefotaxime (2), Cephalexin (1), Crystalline penicillin (1), Doxycycline (3), Meropenem (2), Chloramphenicol (3), Clarithromycin (2), Amoxicillin (2); ICU: Clindamycin (1), Clarithromycin (1), Amikacin (1), Doxycycline (1), Imipenem (1), Cotrimoxazole (1).
Antibiotic metrics
A given patient with a bacterial infection was exposed to 1–7 antibiotic courses with a mean of two or more. On average, a given patient with an infection had about two antibiotics simultaneously for both settings. The number of days that elapsed while the patient was on any antibiotic was 13.5 for the wards and 9.5 for the medical ICU (Table 3).
Table 3. Antibiotic use based on different metrics for hospitalized patients with bacterial infection in the internal medicine ward of TASH in 2014, Addis Ababa, Ethiopia.
| Variable | Wards | ICU | |
|---|---|---|---|
| Agent days, mean ± standard deviation (SD) (range (R)) | 23.2±19.5(2–135) | 18.5±11.9(5–55) | |
| Antibiotic days, mean ± SD (R) | 13.5±19.6(2–51) | 9.5±5.9 (3–30) | |
| Antibiotics course, mean ± SD (R) | 2.4 ±1.1 (1–7) | 2.7±1.4 (1–7) | |
| Maximum no. of antibiotics at a time, mean ± SD (R) | 1.9±0.6 (1–4) | 2.2±0.8(1–5) | |
| Treatment periods | One | 237(84.0%) | 38(92.7%) |
| Two | 39(13.8%) | 2 (4.9%) | |
| Three | 6(2.1%) | 1(2.4%) | |
Changes to initial therapy
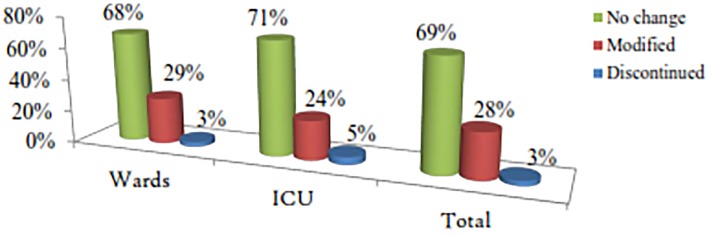
This portion specifically deals with the initial therapy and its adjusted component. Adjustments not related to this were not addressed here. All patients in the ICU and almost all (99.6%) patients in the wards (except one (0.4%)) were started with empiric therapy. The initial therapy was adjusted only in a quarter of admitted patients (Fig 3).
Fig 3. Adjustments to the initial antibiotic therapy for hospitalized patients with systemic bacterial infection in the internal medicine ward of TASH in 2014, Addis Ababa, Ethiopia.
Appropriateness of antibiotic use
About 80% of the ward and 90% of the ICU patients had empiric antibiotics prescribed according to international guidelines. On the other hand, among the total 34 culture reports originated from the wards (Table 1), only 5 of them were available within 72 h of antibiotic initiation. Three of the available reports were negative and thus susceptibility was done for the two positive cultures only. The changes made for these 2 were based on the susceptibility report and thus taken as appropriate. However, no discontinuation of the empiric antibiotic therapy was performed for negative culture reports in all of the wards, thus considered to be inappropriate (Table 4). Although 238 wards admitted patients with an intravenous antibiotic survived to the required date, 20 were not candidates for oral therapy (see the Annex for limiting conditions). Among the 218 candidates, only 15 (6.9%) of them had oral switches (Table 4).
Table 4. Appropriateness of antibiotic use based on the five quality indicators of antibiotic use among hospitalized patients with systemic bacterial infection in the internal medicine ward of TASH in 2014, Addis Ababa, Ethiopia.
| Quality indicators for appropriate antibiotic usage | Appropriate (Frequency(Percentage)) | |
|---|---|---|
| Wards | ICU | |
| Empiric therapy is according to the guidelines | 226 of 281 = 80.4% | 37 of 41 = 90.2 |
| Empiric therapy correctly changed according to culture susceptibility result reported within 72 h |
2 of 2 susceptibility reports = 100% | - |
| Empiric therapy discontinued within 5 antibiotic days due to lack of culture reports | 0 of 3 culture negative reports | - |
| Dose and dosing interval adapted to renal function | 8 of 21 with GFR < 50 mL/min/1.73 m2 = 38.1% |
1 of 6 = 16.7% |
| Intravenous to oral changes made within 5 antibiotic days | 15 of 218 patients who had switches within≤5 antibiotic days = 6.9% |
- |
Hospital outcome indicators
There was high mortality among patients with systemic bacterial infection in the hospital, 27.13% in the wards and 58.5% in the ICU. The LoS (in terms of mean ± standard deviations (range)) for wards and ICU was 18.5 ±12.2 (3–60) and 8.9±4.9 (3–23), respectively. ICU patients also spent 6.3±9.7 (2–41) days in non-ICU internal medicine wards, before or after their admission to the ICU (Table 5).
Table 5. Hospital outcome indicators in hospitalized patients with systemic bacterial infection in the internal medicine ward of TASH in 2014, Addis Ababa, Ethiopia.
| Variables | Wards, n = 282 (Freq, %) | ICU, n = 41(Freq, %) | Total, n = 323 (Freq, %) |
|---|---|---|---|
| Final status of the patient | |||
| Dead | 78(27.7) | 24(58.5) | 102(31.6) |
| Discharged | 204(72.3) | 17(41.5) | 221(68.4) |
| LoS (mean ± SD) | 18.5±12.2(3–60) | 8.9±4.9(3–23) | 17.3±11.9(3–60) |
| LoS outside (for ICU only) | 0 | 6.3±9.7(2–41) | - |
Predictors of hospital outcome
The predictors presented below were for the wards only.
Predictors of mortality
Digestive diseases (AOR = 6.94, 95% CI: (2.24, 21.49), p = 0.001) and different signs and symptoms of disease (AOR = 2.43, 95% CI: (1.30, 4.56), p = 0.005) of the primary admission diagnosis, sepsis (AOR = 2.59, 95% CI: (1.12, 5.99), p = 0.026) among infection diagnosis and vancomycin use (AOR = 2.60, 95% CI: (1.30, 5.21), p = 0.007) were independent positive predictors. Antibiotic days above ten (AOR = 0.37, 95% CI: 0.20, 0.70), p = 0.002) was a negative predictor (Table 6).
Table 6. Binary logistic regression analysis for predictors of mortality for hospitalized patients with systemic bacterial infection in the internal medicine ward of TASH in 2014, Addis Ababa, Ethiopia.
| Variables | Mortality (yes) (%) | COR (95% CI) | AOR (95% CI) |
|---|---|---|---|
| Admissions diagnosis of signs & symptoms (yes) | 29(37.7) | 1.92 (1.20, 3.37)* | 2.43 (1.30, 4.56)** |
| Admissions diagnosis of digestive problems (yes) | 11(61.1) | 4.62 (1.72, 12.40)** | 6.94 (2.24, 21.49)** |
| Admission diagnosis of HIV (yes) | 20(44.4) | 2.47 (1.28, 4.77)** | 1.53 (0.70, 3.37) |
| Sepsis (yes) | 8(44.4) | 3.53 (1.69, 7.34)*** | 2.59 (1.12, 5.99)* |
| Immunosuppressed (yes) | 43(35.5) | 1.98 (1.17, 3.37)** | 1.46 (0.77, 2.77) |
| Vancomycin (yes) | 32(39.5) | 2.20 (1.27, 3.83)** | 2.60 (1.30, 5.21)** |
| Antibiotic days (median) (> 10) (yes) | 31(21.7) | 1.85 (1.086, 3.138)* | 0.37 (0.20, 0.70)** |
*p < 0.05;
**p< 0.01;
***p<0.001;
HIV: human immunodeficiency virus; COR: crud odds ratio; AOR: Adjusted odds ratio; CI: confidence interval
Predictors of prolonged length of stay
The analysis revealed that presence of hospital acquired infection (AOR = 3.01, 95% CI: (1.05, 8.62) p = 0.040), antibiotic days beyond the median (>10 days) (AOR = 4.05, 95% CI: (1.96, 8.37), P = 0.000) and agent days beyond the median (>15 days) (AOR = 2.18, 95% CI: (1.01, 4.68), P = 0.046) were positively associated with prolonged LoS. Whereas presence of meningitis infection was negatively associated with prolonged LoS (AOR = 0.25, 95% CI: (0.07, 0.93), p = 0.039) (Table 7).
Table 7. Binary logistic regression analysis for predictors of prolonged length of stay for hospitalized patients with systemic bacterial infection in the internal medicine ward of TASH in 2014, Addis Ababa, Ethiopia.
| Variables | Prolonged LoS (> 16 days) (yes) (%) | COR (95% CI) | AOR (95% CI) |
|---|---|---|---|
| Aspirational pneumonia (yes) | 7(25.9) | 0.38 (0.16, .93)* | 0.56 (0.21, 1.52) |
| Hospital acquired pneumonia (yes) | 17(81.0) | 5.65 (1.85, 17.26)** | 1.18 (0.28, 4.96) |
| Meningitis (yes) | 4(22.2) | 0.22 (0.06, 0.77)* | 0.25 (0.07, 0.93) |
| Origin of infection | |||
| Unknown | 69(413) | 1.00 | (Reference) |
| Community Acquired | 36(45.0) | 1.16 (0.68, 1.99) | 1.83 (0.93, 3.59) |
| Hospital acquired | 24(68.6) | 3.20 (1.42, 6.74)** | 3.01 (1.05, 8.62) |
| Ceftazidime (yes) | 27(75.0) | 3.74 (1.64, 8.52)** | 2.64 (0.90, 7.73) |
| Vancomycin (yes) | 50(61.7) | 2.00 (1.18, 3.41)* | 0.80 (0.34, 1.89) |
| Ciprofloxacin (yes) | 37(61.7) | 2.16 (1.18, 3.91)* | 1.28 (0.60, 2.75) |
| Antibiotic days (median) (> 10 days) | 104 (72.7) | 5.95 (3.56, 9.96) *** | 4.05 (1.96, 8.37) |
| Agent days (median) (> 15 days) | 19(30.2) | 4.24 (2.58, 6.99) | 2.18 (1.01, 4.68) |
| Adjustment to Empiric therapy | |||
| No change | 74(38.3) | 1.00 | (Reference) |
| Modified | 54(66.7) | 3.22 (1.86. 5.55)*** | 1.07 (0.51, 2.27) |
| Discontinued | 1(12.5) | 0.23 (0.03, 1.91) | 1.99 (0.39, 10.12) |
*p < 0.05;
**p< 0.01,
***p< 0.001;
COR: crud odds ratio; AOR: Adjusted odds ratio; CI: confidence interval
Discussion
Consistent with a review report in low and middle-income countries [16], pneumonia was the most common infection in hospitalized patients. Unlike studies conducted in regional hospitals of Ethiopia where penicillins’ were the number one medications prescribed [17,18], cephalosporins were the most commonly used drugs in our setup. Comparable to previous studies in the hospital [19], empiric therapy was initiated for more than 99.6% of patients in the wards and all patients in the ICU of the present study. The studies conducted in TASH [19], including the current, were in complete disagreement with a study performed in one teaching hospital [20], where empiric therapy was initiated only in 19.4% of the patients.
One of the important issues in stewardship is the need assessment performed in line with hospital outcome indicators [4]. Being one of the outcome indicators, prolonged LoS was enormously associated with higher hospital costs [21]. The mean LoS reported for the medical wards in this study (18.5 days) was about four times higher than reported in Pakistan (4.74 days) [22] and six times of Iran report (3.02 days) [23] for the general patients, and as well as about 2 times higher than reported in Switzerland (9.8 days) [24] for pneumonia cases. The most probable reasons for an extended LoS observed in TASH might be related to the hospital system gaps like waiting for diagnostic or therapeutic procedures, or delay in discharge [23]. Another possible explanation could be due to the presence of MDR bacterial strains that could potentially extend the in-hospital daycare [25]. The present study also showed about a three-fold and four-fold higher ICU mortality than reported in high (20%) and low (15.4%) antibiotic resistance countries, respectively [26]. On the other hand, a study conducted among pneumococcal bacteremia patients in 21 hospitals in 10 countries (including developed and developing countries) reported a mortality rate of 16.9% [27]. This was lower than the ward mortality found in the current study (27.7%). Another study conducted in a Gambian hospital [28] reported an overall mortality of 6% and bacteremia attributed mortality of 8.3%, which is above 3 times lower than reported in this study. All these collectively indicate that the mortality in the current study was incomparably high, seeking an immediate attention.
Since the predictors in the medical ICU did not reach statistical significance, the points in the subsequent discussion were solely for the medical wards, unless otherwise indicated.
Among the primary admission diagnosis, in agreement with different studies [29,30] different signs and symptoms of diseases (AOR = 2.43, 95% CI: (1.30, 4.56), p = 0.005) like hypotension were associated with mortality. Digestive disorders (AOR = 6.94, 95% CI: (2.24, 21.49), p = 0.001) were also associated with mortality. Digestive disorders based on ICD-10 in our study encompass liver-cirrhosis, which might be the most probable reason for death in the current study [31]. Among the infection diagnoses, sepsis had a profound association with mortality both in the univariate (p<0.001) and multivariate (AOR = 2.59, 95% CI: (1.12, 5.99), p = 0.026) models. Accordingly, antimicrobial stewardship programs should make it a prime concern [16,32].
In the current study, patients using vancomycin were more than 2 times more likely to die (39.5%) both in the univariate and multivariate binary logistic models (COR = 2.20, 95% CI: 1.27–3.83, p<0.01; (AOR = 2.60, 95% CI: (1.30, 5.21), p = 0.007). Although no studies were found with a similar methodological approach in support of our evidence, this could possibly be explained by the inappropriate use of the drug in the hospital. Based on our assessment using a quality indicator investigational tool that included dosing for renal function (S1 File), vancomycin misuse had been the first most reason. In addition, one vancomycin use evaluation study conducted in the internal medicine ward of TASH revealed that vancomycin dose was not adjusted or adjusted inadequately in 96.5% of the cases [33]. Another possible explanation may be attributed to the different complications that are inherent in the drug’s pharmacology [34] and the emergence of resistant strains that potentially decrease the drug’s outcome [34,35]. In the current study, patients with prolonged antibiotic days (>10 days) were less likely to die AOR = 0.37, 95% CI: 0.20, 0.70), p = 0.002). Prolonged antibiotic exposure, in fact, is associated with multiple drawbacks like the emergence of antibiotic resistance [21,22]. Several studies attempted to address this concern and compared shorter (one-week) versus longer treatment durations and found no difference in outcome [36,37]. The difference observed between the current and those studies might be methodological, including setting a cut-off date for mortality. Hence, using cut-off date mortality, correlational timing relative to antibiotic initiation and death, and using other advanced methodological options could best reveal this association.
In contrast with an observational study in Italy [26], prolonged LoS did not have an association with any of the primary admission diagnoses. Similar to other studies [21,22], however, hospital-acquired infection (AOR = 3.01, 95% CI: (1.05, 8.62) p = 0.040) was associated with a prolonged hospital stay. In addition, prolonged antibiotic days beyond the median (>10 days) (AOR = 4.05, 95% CI: (1.96, 8.37), P = 0.000) and prolonged agent days (beyond the median >15 days) AOR = 2.18, 95% CI: (1.01, 4.68), P = 0.046) were independently associated with prolonged LoS. This may imply that patients will stay admitted until they finish their medication or the antimicrobial drug treatment will be prolonged until the patient get improved for any other clinical scenarios.
Given the cost of combination therapy, guidelines restrict such treatment approaches for certain group of patients [38] and recommend prompt de-escalation based on the patient’s clinical course, and culture & susceptibility test results [36,37]. Despite this concept and Mettler et al [20] report, though almost all our patients started with broad-spectrum combination empiric therapies, the modification was done only for the quarter of the patients (29% for wards vs. 24% for ICU). Even these modifications did not necessarily indicate streamlining (lowering the estimate) since the majority of the modifications involved the addition of therapy for clinical deterioration, identification of new site of infection, and for culture-positive microbiologic reports [12,38].
Among the 5 quality indicators, only concordance to the guideline and intravenous to oral switch were tested for statistical association and found to be associated neither in the univariate nor in the multivariate model with both outcome indicators. The remaining 3 quality indicators had too low observations to test, with profoundly different denominators.
Since this study was a 3 month long prospective observational, unlike to the previous works, it has made a timely, relevant and comprehensive contribution in uncovering the facts for the prudent use of antibiotics. Being an observational analysis, however, it had limitations. The study was conducted in only one hospital, and practice patterns, patients’ characteristics and microbiology resistance patterns may vary among hospitals, which may limit its generalizability. The use of international guidelines might also under or overestimate the report. Because of the initial different design, there were some partially addressed factors (like the origin of infection and MDR risk status) and the recommendations based upon these may possibly introduce some bias. Although the use of multivariate analysis helped to control a substantial proportion of confounding variables, data related to some important variables like the severity of the illness, the presence of medical devices, previous ICU admission and antibiotic exposure status were not addressed. Therefore, all these might have affected the outcomes.
Conclusion
This observation showed that about half of admitted patients had suspected infection and received antibiotics on an empiric basis. Almost none of the empiric antibiotics were justified based on microbiologic cultures. Whilst pulmonary infections were the most frequent type of infections, cephalosporins were the most commonly prescribed drug class. Presence of digestive disease, different signs, and symptoms of the disease, sepsis, and vancomycin use were positive predictors of mortality. On the other hand, hospital-acquired infection, beyond the median antibiotic days (> 10 days) and agent days (>15 days) were independently associated with prolonged LoS. This suggests that local guidelines or any stewardship activities should give priority to all these issues. Future researchers in the hospital should better address ICU cases separately, focus on modifiable risk factors, use a time-to-event analysis and other advanced methodological designs.
Supporting information
(DOCX)
Acknowledgments
The data collectors of this research and all staff members of the study sites as well as the patients during the data collection period should receive our heartfelt gratefulness. We also thank Addis Ababa University and Tikur Anbesa Specialized Hospital for administrative facilitation provided to do this research.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Addis Ababa University is the source of the funding.
References
- 1.Gottlieb T, Nimmo GR. Antibiotic resistance is an emerging threat to public health: An urgent call to action at the antimicrobial resistance summit 2011. Med J Aust. 2011;194:281–3. [DOI] [PubMed] [Google Scholar]
- 2.Roberts RR, Hota B, Ahmad I, Scott RD II, Foster SD, Abbasi F, et al. Hospital and Societal Costs of Antimicrobial-Resistant Infections in a Chicago Teaching Hospital: Implications for Antibiotic Stewardship. Clin Infect Dis [Internet]. 2009;49(8):1175–84. Available from: https://academic.oup.com/cid/article-lookup/doi/10.1086/605630 [DOI] [PubMed] [Google Scholar]
- 3.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant enterobacteriaceae: Epidemiology and prevention. Clin Infect Dis. 2011;53(1):60–7. 10.1093/cid/cir202 [DOI] [PubMed] [Google Scholar]
- 4.Jenkins TC, Stella SA, Cervantes L, Knepper BC, Sabel AL, Price CS, et al. Targets for Antibiotic and Health Care Resource Stewardship in Inpatient Community-Acquired Pneumonia: A Comparison of Management Practices with National Guideline Recommendations. Infecction. 2013;41(1):135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dellit TH, Owens RC, Mcgowan JE, Gerding DN, Weinstein RA, Burke JP, et al. Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship. Clin Infect Dis Oxford Journals. 2007;44(2):159–77. [DOI] [PubMed] [Google Scholar]
- 6.Lee CR, Cho IH, Jeong BC, Lee SH. Strategies to minimize antibiotic resistance. Int J Environ Res Public Health. 2013;10(9):4274–305. 10.3390/ijerph10094274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macdougall C, Polk RE, Campus V. Antimicrobial Stewardship Programs in Health Care Systems. Clin Microbiol Rev. 2005;18(4):638–56. 10.1128/CMR.18.4.638-656.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michaels K, Mahdavi M, Krug A, Kuper K. Implementation of an Antimicrobial Stewardship Program in a Community Hospital: Results of a Three-Year Analysis. Hosp Pharm [Internet]. 2012;47(8):608–16. Available from: http://archive.hospital-pharmacy.com/doi/abs/10.1310/hpj4708-608 [Google Scholar]
- 9.Yismaw G, Negeri C, Kassu A. A five-year antimicrobial resistance pattern observed in Shigella species isolated from stool samples in Gondar University Hospital, northwest Ethiopia. JHealth Dev. 2006;20(3):194–8. [DOI] [PubMed] [Google Scholar]
- 10.Biadglegne F, Abera B. Antimicrobial resistance of bacterial isolates from urinary tract infections at Felge Hiwot Referral Hospital, Ethiopia. Ethiop J Heal Dev [Internet]. 2010;23(3):1498 Available from: http://www.ajol.info/index.php/ejhd/article/view/53248 [Google Scholar]
- 11.Eshetu S, Bitew A, Tigist G, Abera D, Gizaw S. Multi-Drug Resistance Profile of Bacteria Isolated from Blood Stream Infection at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. EC Microbiol. 2018;14(3):119–26. [Google Scholar]
- 12.Alemkere G, Gilagil G, Gebrehiwot T, Tilahun Z, Mengist HM. Physicians ‘ utilization of microbiologic reports and determinants of their preference to order culture in Tikur Anbessa Specialized Hospital. BMC Res Notes [Internet]. 2018;11(675):1–6. Available from: 10.1186/s13104-018-3782-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beedemariam G, Haile Mariam D, Abebe W, Amogne W, Tenna A, Fenta TG, et al. Opportunities and barriers to implementing antibiotic stewardship in low and middle- income countries: Lessons from a mixed- methods study in a tertiary care hospital in. PLoS One. 2018;13(12):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Bosch CMA, Geerlings SE, Natsch S, Prins JM, Hulscher MEJL. Quality Indicators to Measure Appropriate Antibiotic Use in Hospitalized Adults. Clin Infect Dis [Internet]. 2015;60(2):281–91. Available from: https://academic.oup.com/cid/article-lookup/doi/10.1093/cid/ciu747 [DOI] [PubMed] [Google Scholar]
- 15.Van Den Bosch CM, Hulscher ME, Natsch S, Gyssens IC, Prins JM, Geerlings SE. Development of quality indicators for antimicrobial treatment in adults with sepsis. BMC Infect Dis. 2014;14(345):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zar HJ, Madhi SA, Aston SJ, Gordon SB. Pneumonia in low and middle income countries: Progress and challenges. Thorax. 2013;68(11):1052–6. 10.1136/thoraxjnl-2013-204247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desta Z, Abula T, Gebre-Yohannes A, Worku A. Drug prescribing patterns for outpatients in three hospitals in north-west Ethiopia. JHealth Dev [Internet]. 2002;16(2):183–9. Available from: http://ejhd.org/index.php/ejhd/article/viewFile/766/582 [Google Scholar]
- 18.Getachew E, Aregaw S, Adissie W, And, Agalu A. Antibiotic prescribing pattern in a referral hospital in Ethiopia. African J Pharm Pharmacol [Internet]. 2013;7(38):2657–61. Available from: http://academicjournals.org/journal/AJPP/article-abstract/E3D29D241308 [Google Scholar]
- 19.Sileshi A, Tenna A, Feyissa M, Shibeshi W. Evaluation of ceftriaxone utilization in medical and emergency wards of Tikur Anbessa specialized hospital: A prospective cross-sectional study. BMC Pharmacol Toxicol [Internet]. 2016;17(1):1–10. Available from: 10.1186/s40360-016-0057-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mettler J, Simcock M, Sendi P, Widmer AF, Bingisser R, Battegay M, et al. Empirical use of antibiotics and adjustment of empirical antibiotic therapies in a university hospital: A prospective observational study. BMC Infect Dis. 2007;7(21):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA. Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant gram-negative bacteria. Antimicrob Agents Chemother. 2010;54(1):109–15. 10.1128/AAC.01041-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Usman M, Jamal S, Tabassum S, Zafran M. Length of stay of patients in a tertiary care hospital. Gomal J Med Sci. 2011;9(1):18–21. [Google Scholar]
- 23.Ghods A asghar, Khabiri R, Raeisdana N, Ansari M, Hoshmand Motlagh N, Sadeghi M, et al. Predictors of Inappropriate Hospital Stay: Experience From Iran. Glob J Health Sci [Internet]. 2014;7(3):82–9. Available from: http://www.ccsenet.org/journal/index.php/gjhs/article/view/39192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suter-Widmer I, Christ-Crain M, Zimmerli W, Albrich W, Mueller B, Schuetz P, et al. Predictors for length of hospital stay in patients with community-acquired Pneumonia: Results from a Swiss Multicenter study. BMC Pulm Med. 2012;12(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lye DC, Earnest A, Ling ML, Lee TE, Yong HC, Fisher DA, et al. The impact of multidrug resistance in healthcare-associated and nosocomial Gram-negative bacteraemia on mortality and length of stay: Cohort study. Clin Microbiol Infect [Internet]. 2012;18(5):502–8. Available from: 10.1111/j.1469-0691.2011.03606.x [DOI] [PubMed] [Google Scholar]
- 26.Hanberger H, Antonelli M, Holmbom M, Lipman J, Pickkers P, Leone M, et al. Infections, antibiotic treatment and mortality in patients admitted to ICUs in countries considered to have high levels of antibiotic resistance compared to those with low levels. BMC Infect Dis. 2014;14(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu VL, Chiou CCC, Feldman C, Ortqvist A, Rello J, Morris AJ, et al. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin Infect Dis. 2003;37. [DOI] [PubMed] [Google Scholar]
- 28.Hill PC, Onyeama CO, Ikumapayi UNA, Secka O, Ameyaw S, Simmonds N, et al. Bacteraemia in patients admitted to an urban hospital in West Africa. BMC Infect Dis. 2007;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon SB. Patient outcome in adults with pneumococcal meningitis or bacteraemia admitted to QECH. Malawi Med J. 2003;15(2):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCabe C, Kirchner C, Zhang H, Daley J, Fisman DN. Guideline-concordant therapy and reduced mortality and length of stay in adults with community-acquired pneumonia: Playing by the rules. Arch Intern Med [Internet]. 2009;169(16):1525–31. Available from: http://archinte.jamanetwork.com/article.aspx?doi=10.1001/archinternmed.2009.259 [DOI] [PubMed] [Google Scholar]
- 31.Fernández J, Acevedo J, Castro M, Garcia O, Rodríguez de Lope C, Roca D, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: A prospective study. Hepatology. 2012;55(5):1551–61. 10.1002/hep.25532 [DOI] [PubMed] [Google Scholar]
- 32.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the united states. Crit Care Med. 2013; [DOI] [PubMed] [Google Scholar]
- 33.Zeleke B, Engidawork E. Drug Utilization Evaluation of Vancomycin Among Hospitalized Patients in Internal Medicine Wards of Tikur Anbessa Specialized Hospital. Am J Heal Res. 2015;3(6):333–7. [Google Scholar]
- 34.Moore CL, Lu M, Cheema F, Osaki-Kiyan P, Perri MB, Donabedian S, et al. Prediction of failure in vancomycin-treated methicillin-resistant Staphylococcus aureus bloodstream infection: A clinically useful risk stratification tool. Antimicrob Agents Chemother. 2011;55(10):4581–8. 10.1128/AAC.00115-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minejima E, Lou M, Nieberg P, Wong-Beringer A. Patients presenting to the hospital with MRSA pneumonia: Differentiating characteristics and outcomes with empiric treatment. BMC Infect Dis. 2014;14(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capellier G, Mockly H, Charpentier C, Annane D, Blasco G, Desmettre T, et al. Early-Onset Ventilator-Associated Pneumonia in Adults Randomized Clinical Trial: Comparison of 8 versus 15 Days of Antibiotic Treatment. PLoS One. 2012;7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.el Moussaoui R, de Borgie CAJM, van den Broek P, Hustinx WN, Bresser P, van den Berk GEL, et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. Bmj [Internet]. 2006;332(7554):1355–0. Available from: http://www.bmj.com/cgi/doi/10.1136/bmj.332.7554.1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock, 2012. Intensive Care Med [Internet]. 2013;39(2):165–228. Available from: http://link.springer.com/10.1007/s00134-012-2769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.