Abstract
Background
Acne vulgaris is a common and chronic disease that impacts on physical and psychological perceptions. Cosmeceutical products are widely used as adjunct therapy to standard treatments.
Objective
To evaluate the efficacy of cosmeceutical products comprising glycolic acid, salicylic acid, gluconolactone, and licochalcone A as adjunct therapy to adapalene in mild-to-moderate acne vulgaris.
Materials and methods
A 28-day, double-blind, within-person comparative study was conducted with a total of 25 subjects. Each participant received two products, consisting of (1) a cosmeceutical product mixed with 0.1% adapalene, and (2) 0.1% adapalene, and was asked to apply them separately on each hemi-side once nightly for 28 days. The number of acne lesions, severity of acne vulgaris, physician’s and patient’s global assessment of acne severity, visual analog scale of radiance, skin biophysics, safety assessment, and VISIA® camera system were evaluated. The primary efficacy outcome was to compare the reduction of inflammatory lesions between two treatments at day 7 by using non-inferiority comparison.
Results
The mean differences of inflammatory lesions reduction at day 7 between the two groups was 0.391 (90% CI = 0.253–0.530). The differences between two groups fell within our acceptable margin for the 90% CI. The spot score from VISIA® showed higher statistically significant improvement in the combination side.
Conclusion
The results showed no hindrance of using a cosmeceutical combined with standard treatment. Nevertheless, this cosmeceutical product showed some benefits in reducing complications from acne.
Clinical trial registration
Thai Clinical Trials Registry (primary site), no. TCTR20171031005.
Keywords: acne vulgaris, cosmeceutical, moisturizer, adapalene, retinoids
Introduction
Acne vulgaris is a common and chronic disease, with a prevalence rate of 9.38% (male 8.96%, female 9.81%) worldwide in 2010, resulting in 7.2 years lived with “disability” from the Global Burden of Disease Study 2013.1,2 Acne vulgaris can cause myriads of problems, both physical and psychological, such as post-acne redness, post-inflammatory hyperpigmentation, hypertrophic or atrophic acne scars, as well as influencing quality-of-life.3–6
Topical retinoids are the mainstay in the maintenance phase of treatment and are also recommended to use as monotherapy in mild degree acne or in combination with antimicrobials in more severe disease. Retinoids increase epithelial turnover and have comedolytic and anti-inflammatory actions.7 Nevertheless, “retinoid reactions” such as erythema, burning sensation, erythema, peeling, and retinoid dermatitis are well recognized.8,9
Interestingly, the role of “dermocosmetics” is increasingly important, as many patients and even physicians now resort to them as first-line management in mild-to-moderate acne. This area of acne management is highly controversial, lacking strong evidence to support.10,11 The active ingredients in some products are designed to work on the four main pathogeneses.10,12,13 There were many reports showing beneficial effects when combined with standard treatment, resulting in increased efficacy and decreased side effects,14–16 whereas some reports showed no superiority of cosmeceutical product over standard medication.17,18
As many physicians are reluctant to prescribe skin moisturizer when treating acne patients for fear of worsening the therapeutic outcome. The objective of this study was to determine whether a combination of cosmeceutical products with a retinoid, adapalene, would result in similar improvements in mild-to-moderate acnes compared with retinoid alone (a non-inferiority comparison). Our secondary objective was to test the ability of these active ingredients to reduce the side-effects of retinoids and to decrease complications of acne.
Materials and methods
This was a single center, double blinded split-face comparative study. The study was conducted from June 2017 through September 2017. This study was approved by the Institutional Review Board, Faculty of Medicine, Chulalongkorn University, Thailand, and was registered at Thai Clinical Trials Registry as a primary site, no. TCTR20171031005. This study was conducted in accordance with the Declaration of Helsinki. All participants provided informed consent before starting the study. Individual participant data of the study, after deidentification, is not being shared with others. We do not share any specific data, and no other study-related documents will be made available. Our patients’ data were recorded in a case record form. The case record form and essential documents will be kept in a designated place for 15 years. The data and documents are available if requested by relevant authorities.
Study population
Female and male participants aged between 18–40 years old with clinically diagnosed mild-to-moderate degree facial acne vulgaris according to the Acne Consensus Conference (ACC)19 were recruited. The participants were excluded if they used oral acne treatment within 4 weeks, topical treatment within 2 weeks, or light/laser therapy or oral treatment within 4 weeks. Participants who had any other dermatologic diseases in the study area were not eligible. Female participants with childbearing potential could not be pregnant during the study and were advised to use reliable contraceptive measures throughout the study period.
Treatment regimen
Each participant received two kinds of products, which consisted of (1) active ingredients, namely glycolic acid 7%, salicylic acid 1%, gluconolactone 2.0%, licochalcone A 0.05%, mixed with adapalene 0.1% (Beiersdorf, Germany), and (2) the adapalene 0.1%. Each product was applied once daily on one side of the face at night in a quantity of a half fingertip unit (0.25 grams). All participants were provided with skin cleanser (Eucerin, Beiersdorf, Germany) for twice-daily use. The protocol was continued for 28 days with four visits, which were on days 0 (baseline), 7, 14, and 28. All skin assessments were measured at each visit. Participants were advised not to use new skin care products or other medications during the entire course of the study.
Efficacy assessment
Efficacy for acne vulgaris was measured by counting of the acne lesions, which comprised comedones, inflammatory papules, and nodulocystic lesions, by the investigator (KK). The severity of acne vulgaris was graded as mild, moderate, or severe depending on the ACC.19 Physician’s global assessment (PGA) and patient’s global assessment20 (PaGA) were evaluated for each hemi-side at every visit. Responses for PGA and PaGA were 5-point Likert scales, where 0 refers to clear, residual hyperpigmentation and erythema; a response of 1 means almost clear of skin lesions, a few scattered comedones, and a few small papules; 2 suggests a mild degree, easy to recognize; 3 refers to moderate degree acne, many comedones, papules, and pustules; and a response of 4 suggests severe degree acne, all of the area covered with comedones, numerous papules, and pustules (a few nodules and cysts may also be present).
Efficacies regarding skin brightening and radiance were evaluated on each hemi-side at every visit by subjects, using the visual analog scale (VAS), a subjective measurement, which is a 10 centimeters (100 millimeters) horizontal line. The subjects were asked to mark on the line what they thought represented their score. The minimum score is 0 (no radiance at all) and the maximum score is 100 (most radiant).
Safety assessment
At study visits on days 7, 14, and 28, all subjects evaluated the safety from each treatment. Subjects would score each hemi-side with regards to erythema, burning sensation, and itch sensation (a 4-point Likert Scale; 0 = no, 1 = mild, 2 = moderate, 3 = severe). Similarly, the investigator (KK) also evaluated the safety profile by physical examination at the treated area in each hemi-side, then scored erythema on a 4-Likert scale; 0 = no, 1 = mild, 2 = moderate, 3 = severe.
Skin biophysical properties assessment
Before evaluation at each visit, the subjects would wash the face and rest for 10–15 minutes. The following biophysical properties were measured; first, the water content of the stratum corneum with Corneometer® CM 825 (Courage-Khazaka, Cologne, Germany) and, second, the transepidermal water loss with Tewameter® TM 300 (Courage-Khazaka) at days 0, 7, 14, and 28. The corneometer scores are presented as arbitrary Corneometer® units (0–120) and the Tewameter measured in g/m2/h. These measurements were performed in a temperature- and humidity-controlled room (72.56±6.34°C and 21.58%±1.31% humidity). We located the site to measurement at 3 centimeters lateral to the lateral rim of alar nasi, horizontally. All measurements were done in triplicate, and the mean values were calculated.
Photographic assessment
At all visits, digital photographs were taken by the Visia® camera system (software version 6.4.2, Canfield Scientific, Parsippany, NJ, US). The Visia system is a standardized photographic method with left, center, and right images. The system can detect spots, red areas, as a feature, showing an absolute score in a selected area (“mask” area), as well as auto-mask the same area at other visits. The cheek area in each hemi-side was used to compare the efficacy of the treatment.
To evaluate the spots and red area, the software can measure the “absolute scores”, which are appropriate to use for comparison. They represent the fractional area of each skin feature relative to the area masked. The higher the score, the less problems the subjects have.
Outcome
The primary efficacy outcome was the reduction of inflammatory lesions between two treatments at day 7 by using non-inferiority comparison.
The secondary outcomes were the reduction of inflammatory lesions between two treatments at day 28, and the side-effects of the two treatments, such as burning sensation, erythema, scaling, and acne complication, such as post-acne erythema and post-inflammatory hyperpigmentation. The differences in VISIA spot and red areas between the treatments were considered.
Statistical analyses
The subject’s demographic data, such as age, gender, weight, and height, were reported as mean and SD or percentage. Baseline demographic characteristics were compared between treatment groups using Student’s t-test or chi-squared test. The number of comedones, inflammatory papules, nodulocystic acne, and inflammatory lesions (inflammatory papules add nodulocystic acne) in each hemi-side at each visit were reported as mean and SD, as well as the severity of acne vulgaris. Visual analog radiance score, skin biophysics, and adverse effects were shown as mean and SD.
For primary outcome, we set the non-inferiority margin at –1, assuming that the lower bound of the 90% CI of mean difference of inflammatory lesions between the control and treated groups was not less than (or smaller than) –1 unit. With a power of 80% and a one-sided significant level of 0.05, a sample size of 25 participants was needed after adjusting for a dropout rate at day 28 of 10%.
Comparisons of efficacy assessment, safety assessment, skin biophysical properties assessment, and photographic assessment between a cosmeceutical product mixed with 0.1% adapalene and 0.1% adapalene groups over the follow-up period of 28 days were conducted using a linear mixed-effect model with patients as a random effect. The comparisons of Visia spot count and red area feature count were analyzed by a Poisson regression mixed model.
All data management and analyses were conducted with R statistical software R version 3.4.1,21 with the following packages: lme4, effects, and emmeans. In addition to non-inferiority analysis, all other statistical analysis was conducted with the significant levels of 0.05.
Results
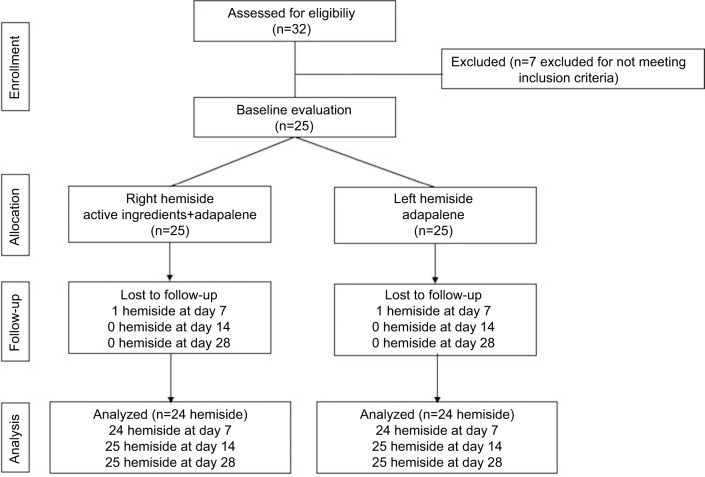
A total number of 32 subjects were screened. Four subjects were excluded due to severe acne vulgaris, and three subjects were not able to follow the study protocol. Consequently, the total number of the subjects was 25 (Figure 1). Baseline characteristics of the study population are shown in Table 1. The average age was 24.32±5.76 years; participants were 80% female and 20% male. The number of acne lesions, severity of acne vulgaris, PGA, PaGA, VAS, and skin biophysics compared each hemi-side are shown in Table 1.
Figure 1.
Flowchart of study population.
Table 1.
Baseline demographic and clinical characteristics
| Patient demographic data (n=25) | Mean (SD) | ||
|---|---|---|---|
| Age (years) | 24.32 (5.76) | ||
| Female (n, %) | 20, 80% | ||
| Adapalene | Active ingredient+Adapalene | ||
| Mean (SD) | Mean (SD) | P-value | |
| Clinical characteristics | |||
| Comedones | 8.72 (7.05) | 8.32 (4.85) | 0.625 |
| Nodulocystic lesions | 0.84 (1.34) | 0.56 (1.08) | 0.258 |
| Total inflamed lesions | 2.96 (2.32) | 3.36 (2.87) | 0.327 |
| Total lesions | 11.48 (7.29) | 11.68 (5.71) | 0.797 |
| ACC grading | 1.56 (0.51) | 1.60 (0.50) | 0.205 |
| PaGA | 2.52 (0.77) | 2.84 (0.80) | 0.073 |
| PGA | 2.32 (0.56) | 2.44 (0.51) | 0.265 |
| VAS radiance | 3.04 (1.81) | 2.68 (1.90) | 0.145 |
| Skin biophysical properties | |||
| Transepidermal water loss (g/m2/h) | 12.55 (5.84) | 13.85 (6.62) | 0.112 |
| Skin hydration (corneometer unit) | 45.17 (9.31) | 45.37 (9.65) | 0.888 |
Abbreviations: ACC, Acne Consensus Conference; PaGA, patient’s global assessment; PGA, physician’s global assessment; VAS, visual analog scale.
Patients’ baseline demographics, clinical characteristic and skin biophysical properties among two treatment sides did not show statistically significant differences.
Efficacy assessment
Comedones
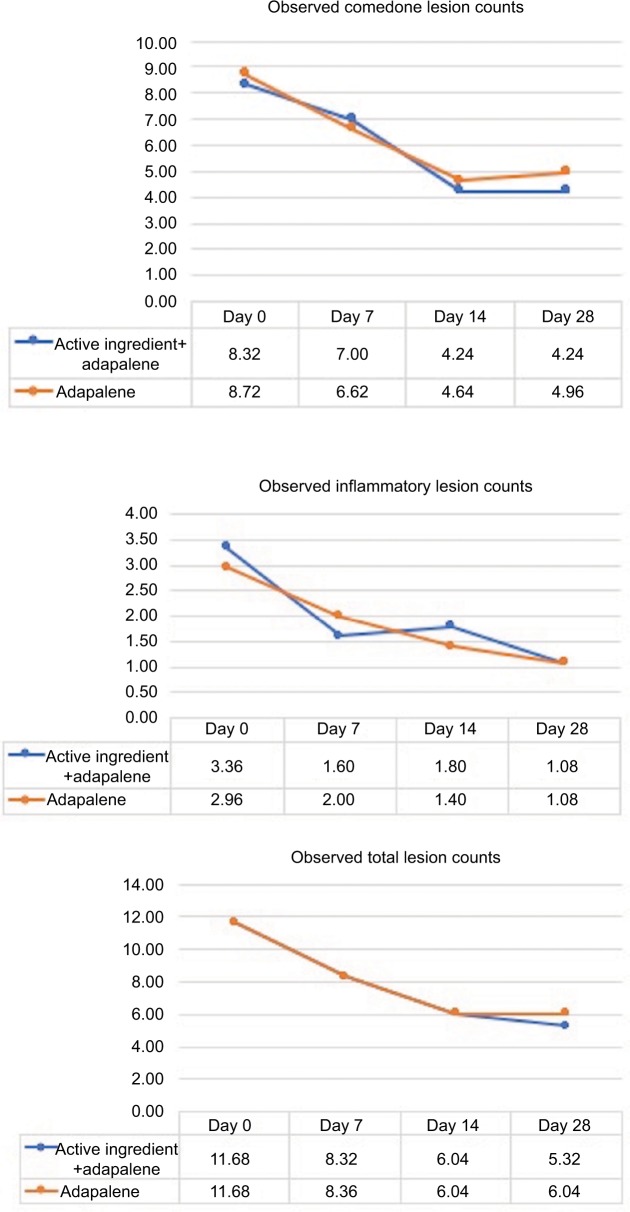
The observed comedones counts at baseline, days 7, 14, and 28 are shown in Figure 2. At days 7 and 14, the number of comedones decreased from baseline, but they were increased at day 28. The comedones counts between the two groups were not statistically significantly different over time.
Figure 2.
Observed comedone, inflammatory lesion, and total lesion counts.
Inflammatory lesions
The observed inflammatory lesions (inflammatory papules and nodulocystic lesion) counts at days 7, 14, and 28 are also shown in Figure 2.
At day 7, inflammatory lesions decreased on the side receiving the active ingredients with adapalene more than the adapalene monotherapy, but they were increased at day 14. However, at day 28, both groups have comparable inflammatory lesion counts. There were no significant changes in inflammatory lesions at all the time points between the two groups.
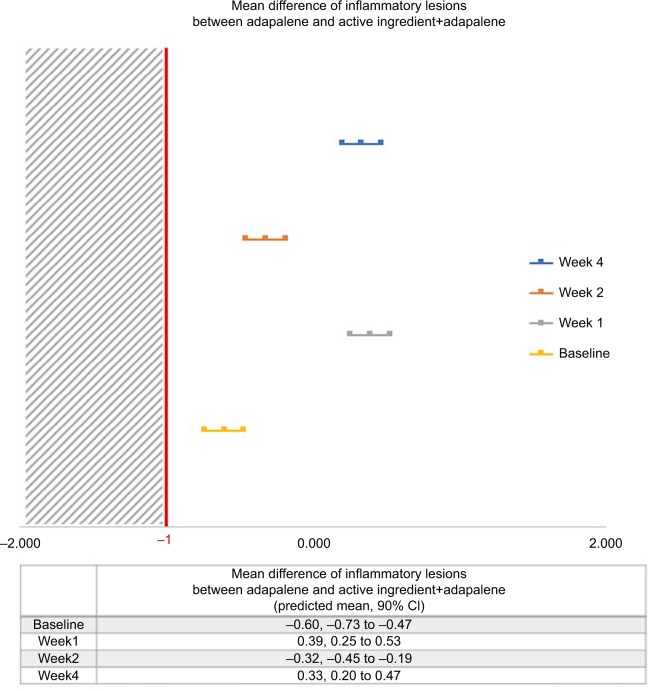
For the primary outcome, the mean differences of inflammatory lesions reduction at day 7 between two groups was 0.391 (90% CI=0.253–0.530; Figure 3). The lower confidence intervals of the mean differences between the two groups for every follow-up time did not exceed the non-inferiority margin of –1 (Figure 3).
Figure 3.
Predicted mean difference of inflammatory lesions between group.
The mean differences of inflammatory lesions reduction between both groups at days 14 and 28 were −0.320 (90% CI=−0.454–0.185) and 0.333 (90% CI=0.222–0.466), respectively. The data are also shown in Figure 3. The treatment result is illustrated in Figure 4.
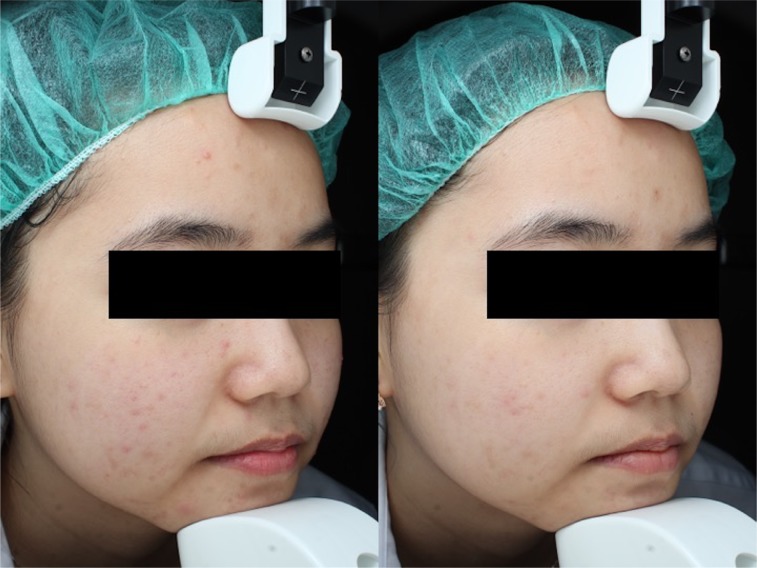
Figure 4.
Inflammatory lesions at baseline and day 28 (at the end of the study) in the active ingredient group.
Severity of acne
The severity of acne evaluated by ACC in both groups were decreased continuously at days 7, 14, and 28 from baseline. In the same way, PGA and PaGA showed that both treatments had similar improvement at days 7, 14, and 28 from baseline (Table 2). However, we could not find statistically significant differences in the severity of acne, PGA, and PaGA among the two groups.
Table 2.
Efficacy and skin biophysical properties
| Adapalene | Active ingredient+adapalene | |
|---|---|---|
|
| ||
| Predicted mean (95% CI) | Predicted mean (95% CI) | |
|
| ||
| Efficacy | ||
|
| ||
| Comedones | ||
| Day 0 | 8.68 (6.57–10.78) | 8.40 (6.30–10.51) |
| Day 7 | 6.82 (4.70–8.95) | 6.55 (4.42–8.68) |
| Day 14 | 4.62 (2.51–6.73) | 4.35 (2.23–6.46) |
| Day 28 | 4.74 (2.63–6.84) | 4.46 (2.36–6.57) |
|
| ||
| Inflammatory lesion | ||
| Day 0 | 2.99 (2.40–3.58) | 3.13 (2.54–3.72) |
| Day 7 | 1.83 (1.23–2.43) | 1.97 (1.37–2.58) |
| Day 14 | 1.49 (0.90–2.08) | 1.63 (1.04–2.22) |
| Day 28 | 1.01 (0.42–1.50) | 1.15 (0.56–1.74) |
|
| ||
| Severity of acne (ACC grading) | ||
| Day 0 | 1.58 (1.41–1.74) | 1.58 (1.42–1.75) |
| Day 7 | 1.42 (1.25–1.59) | 1.42 (1.25–1.59) |
| Day 14 | 1.19 (1.02–1.35) | 1.19 (1.02–1.36) |
| Day 28 | 1.02 (0.85–1.18) | 1.02 (0.86–1.19) |
|
| ||
| Skin biophysical properties | ||
|
| ||
| Transepidermal water loss (g/m2/h) | ||
| Day 0 | 12.32 (10.31–14.33) | 14.07 (12.06–16.09) |
| Day 7 | 11.54 (9.49–13.59) | 13.30 (11.24–15.35) |
| Day 14 | 11.92 (9.91–13.94) | 13.68 (11.65–15.70) |
| Day 28 | 12.40 (10.39–14.41) | 14.15 (12.14–16.17) |
|
| ||
| Skin hydration (corneometer unit) | ||
| Day 0 | 45.11 (42.22–48.00) | 45.43 (42.54–48.32) |
| Day 7 | 53.36 (50.39–56.32) | 53.68 (50.71–56.64) |
| Day 14 | 54.37 (51.48–57.27) | 54.70 (51.78–57.60) |
| Day 28 | 50.21 (47.32–53.09) | 50.53 (47.64–53.42) |
Abbreviation: ACC, Acne Consensus Conference.
Efficacy for brightening and RADIANCE skin
Skin brightening and radiance skin measured through the visual analog scale were increasing from the baseline in both groups. However, we could not find a statistically significant difference between both groups at days 7, 14, and 28.
Safety assessment
At all study visits (days 7, 14, and 28), the safety profile of both groups, defined as the average score of erythema and scaling, were evaluated by subjects and the investigator (KK). Most of the patients reported erythema and a scaling score ≤2, which means none reported severe symptoms. The erythema and scaling score from both patients and physician between the two treatments were not statistically significantly different at days 7, 14, and 28.
Moreover, we also evaluated the worst symptoms from the patients, defined as pruritus and burning sensation, at days 7, 14, and 28. Almost all of the patients reported pruritus sensation as “0=none” on both treatments, which was not significantly different. For the burning sensation, the patients reported a burning sensation on active ingredient with the adapalene side more than the adapalene alone side at days 7, 14, and 28. The mean score for the treatment group was significantly higher than control at day 7 (, 95% CI=0.962–1.507 vs , 95% CI=0.223–0.767), day 14 (, 95% CI=0.767–1.833 vs , 95% CI=0.058–0.582), and day 28 (, 95% CI=0.698–1.222 vs , 95% CI=−0.102–0.422) (Table 3).
Table 3.
Safety assessment and Visia score
| Adapalene | Active ingredient+adapalene | |
|---|---|---|
|
| ||
| Predicted mean (95% CI) | Predicted mean (95% CI) | |
|
| ||
| Safety assessment | ||
|
| ||
| Patient report | ||
| Burning sensation | ||
| Day 0 | 0.00 (−0.26–0.26) | 0.00 (−0.26–0.26) |
| Day 7 | 0.50 (0.22–0.77) | 1.23 (0.96–1.51)a |
| Day 14 | 0.32 (0.06–0.58) | 1.30 (0.77–1.30)a |
| Day 28 | 0.16 (–0.10–0.42) | 0.96 (0.70–1.22)a |
|
| ||
| Erythema | ||
| Day 0 | –0.08 (−0.21–0.05) | –0.08 (−0.05–0.21) |
| Day 7 | 0.16 (0.03–0.29) | 0.31 (0.18–0.44) |
| Day 14 | 0.07 (−0.06–0.19) | 0.22(0.09–0.35) |
| Day 28 | 0.02 (−0.11–0.15) | 0.18 (0.05–0.31) |
|
| ||
| Physician report | ||
| Scaling score | ||
| Day 0 | –0.01 (−0.15––0.13) | 0.01 (−0.13–0.15) |
| Day 7 | 0.13 (−0.01–0.23) | 0.15 (0.01–0.30) |
| Day 14 | 0.12 (−0.03–0.26) | 0.14 (−0.01–0.28) |
| Day 28 | 0.37 (0.23–0.51) | 0.39 (0.25–0.53) |
|
| ||
| Erythema score | ||
| Day 0 | −0.02 (−0.06–0.02) | 0.02 (−0.02–0.06) |
| Day 7 | −0.02 (−0.06–0.03) | 0.02 (−0.03–0.06) |
| Day 14 | 0.01 (−0.05–0.05) | 0.04 (−0.01–0.08) |
| Day 28 | 0.03 (−0.02–0.06) | 0.06 (0.02–0.10) |
|
| ||
| Visia absolute score | ||
|
| ||
| Red area score | ||
| Day 0 | 28.72 (26.82–30.61) | 28.26 (26.36–30.61) |
| Day 7 | 29.91 (28.01–31.81) | 29.46 (27.56–31.36) |
| Day 14 | 30.01 (28.12–31.91) | 29.56 (27.66–31.45) |
| Day 28 | 29.09 (27.12–30.98) | 28.63 (26.74–30.52) |
|
| ||
| Spot score | ||
| Day 0 | 43.23 (39.21–47.25) | 45.58 (41.56–49.60) |
| Day 7 | 45.77 (41.72–49.82) | 48.12 (44.07–52.17)b |
| Day 14 | 44.36 (40.34–48.38) | 46.71 (42.69–50.73)b |
| Day 28 | 45.29 (41.27–49.31) | 47.64 (43.62–51.66)b |
Note:
P-value=0.0020 and
P-value=0.0243. Bold values indicate statistical significance.
Skin biophysical properties assessment
The skin hydration (Corneometer) was increased from baseline in both treatment groups at days 7, 14, and 28, but it showed no statistically significant differences among the two treatment groups. The transepidermal water loss (TEWL) of both treatment groups decreased from baseline at days 7 and 14 and slightly increased at day 28. However, we could not find statistically significant differences between the two treatment groups (Table 2).
Photographic assessment
The Visia “spots” absolute score for treatment group was significantly higher than control at day 7 (, 95% CI=44.070–52.165 vs , 95% CI=41.722–49.817), day 14 (, 95% CI=42.687–50.730 vs , 95% CI=40.339–48.383), and day 28 (, 95% CI=43.617–51.660 vs , 95% CI=41.267–49.313) from baseline (Table 3).
Although the Visia “red area” absolute score in both treatment groups was increased from baseline at days 7 and 14 and slightly decreased at day 28, it showed no statistically significant difference between the groups in any follow-up visits.
Discussion
There are many external factors which affect the result of acne treatment, such as skin care, moisture, cosmeceutical, cleanser, and exposome. The cosmeceutical products have increasing roles in acne treatment and maintenance therapy.
The combination of our study product (active ingredients of glycolic acid, salicylic acid, gluconolactone, and licochalcone A) with adapalene was non-inferior to adapalene monotherapy with respect to inflammatory lesions. Most adverse effects were similar on both sides, although slightly more burning was reported on the combination side.
The results on redness as measured by the Visia system were not different between both sides. Of note, the results on spots as measured by the Visia system were better on the combination side. These measurements could be more efficient that the human eyes and tend to be more objective.
Adapalene, a third generation synthetic retinoid, has high affinity to retinoic acid receptor (RAR) β and γ. It has an anti-inflammatory effect due to it inhibiting the arachidonic acid via the lipoxygenase pathway and decreasing toll-like receptor 2 (TLR-2) expression in epidermal keratinocytes.22–24
Our result showed decreased comedones in both groups. Apart from the adapalene effects, our active ingredients are comprised of glycolic acid 7%, salicylic acid 1%, and gluconolactone 2%. The glycolic acid is an alpha-hydroxy acid (AHA) that can be used in all Fitzpatrick skin types. Of note is that a low concentration (2–5%) of glycolic acid disrupts the intercellular cohesiveness in the stratum corneum, resulting in normal epidermal keratinization. It also increases skin thickness within the epidermis and papillary dermis.25,26 Salicylic acid is a beta-hydroxy acid, which has action to normal keratinization, decreases inflammation, and reduces sebum production with a comedolytic effect. The concentration of salicylic to treat acne is 0.5–5%.11,27 Gluconolactone, an alpha-hydroxy acid, affects keratinization and reduces inflammatory lesions. Moreover, it has less side-effects than benzoyl peroxide.28
Apart from comedones, our study has demonstrated that both treatments could reduce inflammatory lesions from baseline. Licochalcone A, extracted from Glycyrrhiza inflata, has an anti-inflammatory effect with inhibition of prostaglandins synthesis. It has an effect both in in vitro and in vivo studies.29–31 Licochalcone A has benefits on sensitive facial skin, rosacea, atopic dermatitis, seborrheic dermatitis, and acne.32–36
Adapalene has very little skin irritations, ie, no more than white petrolatum.37,38 This can be the explanation of why the difference of skin biophysical properties between the two groups were not observed. Moisturizer or cosmeceutical products should help minimize the side-effects of retinoids.39 Similar to what was demonstrated by Chularojanamontri et al,16 the skin hydration and transepidermal water loss were not statistically significantly different between adapalene plus active formulation and adapalene alone. They demonstrated that the addition of concomitant moisturizer to adapalene does not interfere with therapeutic effect. It was also demonstrated that the combination of adapalene with moisturizer resulted in slightly more pruritus and burning sensation at day 7.40 These observations were in line with our study.
The strengths of our study are its good protocol compliance, with only one patient missing a visit, and consistency of assessment by the same investigators, as well as comprehensive (both subjective and objective) evaluations of the safety profile of treatment. The compounding of moisturizer with adapalene in one tube enhances ease of applications, while ensuring blinding of the study products.
Limitations include the use of adapalene as the main acne treatment. The results may not be applicable to other anti-acne preparations. Only 20% of our subjects were male. Whether both genders would respond similarly is questionable. Also, the total study period was 28 days, some additional benefits may be seen if the follow-up time was longer.
Conclusion
As commonly known, the effects from cosmeceuticals were not obviously to surpass the standard treatment in acne. This study showed that addition of cosmeceuticals to adapalene had no benefits over adapalene alone in the view of treatment. However, it has benefits upon side-effects. Our results showed no hindrance of using a cosmeceutical combined with standard treatment. In future studies, use of other acne medications, the maintenance effect from cosmeceutical products, and longer follow-up times may be justified.
Acknowledgments
We would like to thank members of Skin Unit’s Research Facilities for Academic and Clinical Excellence (SURFACE), Division of Dermatology, Department of Medicine, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, for providing us for the equipment and convenience. We thank Miss Sarutya Tungjairukkarndee, Miss Maythikarn Tameyapradit, and Miss Pitchayanin Tunakh for their kind support. The study was funded by Beiersdorf (Thailand). The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of Disease Study 2010. The Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karimkhani C, Dellavalle RP, Coffeng LE, et al. Global skin disease morbidity and mortality. JAMA Dermatology. 2017;153(5):406–412. doi: 10.1001/jamadermatol.2016.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tasoula E, Gregoriou S, Chalikias J, et al. The impact of acne vulgaris on quality of life and psychic health in young adolescents in Greece. Results of a population survey. An Bras Dermatol. 2012;87(6):862–869. doi: 10.1590/S0365-05962012000600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misery L, Wolkenstein P, Amici JM, et al. Consequences of acne on stress, fatigue, sleep disorders and sexual activity: a population-based study. Acta Derm Venereol. 2015;95(4):485–488. doi: 10.2340/00015555-1998. [DOI] [PubMed] [Google Scholar]
- 5.Fabbrocini G, Annunziata MC, D’Arco V, et al. Acne scars: pathogenesis, classification and treatment. Dermatol Res Pract. 2010;2010(6171):1–13. doi: 10.1155/2010/893080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hazarika N, Archana M. The psychosocial impact of acne vulgaris. Indian J Dermatol. 2016;61(5):515–520. doi: 10.4103/0019-5154.190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e33. doi: 10.1016/j.jaad.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1(4):327–348. doi: 10.2147/ciia.2006.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veraldi S, Barbareschi M, Benardon S, Schianchi R. Short contact therapy of acne with tretinoin. J Dermatolog Treat. 2013;24(5):374–376. doi: 10.3109/09546634.2012.751085. [DOI] [PubMed] [Google Scholar]
- 10.Araviiskaia E, Dréno B. The role of topical dermocosmetics in acne vulgaris. J Eur Acad Dermatol Venereol. 2016;30(6):926–935. doi: 10.1111/jdv.13579. [DOI] [PubMed] [Google Scholar]
- 11.Decker A, Graber EM. Over-the-counter acne treatments: a review. J Clin Aesthet Dermatol. 2012;5(5):32–40. [PMC free article] [PubMed] [Google Scholar]
- 12.Green BA, Yu RJ, Van Scott EJ. Clinical and cosmeceutical uses of hydroxyacids. Clin Dermatol. 2009;27(5):495–501. doi: 10.1016/j.clindermatol.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Draelos ZD. The art and science of new advances in cosmeceuticals. Clin Plast Surg. 2011;38(3):397–407. doi: 10.1016/j.cps.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Abels C, Kaszuba A, Michalak I, Werdier D, Knie U, Kaszuba A. A 10% glycolic acid containing oil-in-water emulsion improves mild acne: a randomized double-blind placebo-controlled trial. J Cosmet Dermatol. 2011;10(3):202–209. doi: 10.1111/j.1473-2165.2011.00572.x. [DOI] [PubMed] [Google Scholar]
- 15.Poli F, Ribet V, Lauze C, Adhoute H, Morinet P. Efficacy and safety of 0.1% retinaldehyde/6% glycolic acid (diacneal) for mild to moderate acne vulgaris. A multicentre, double-blind, randomized, vehicle-controlled trial. Dermatology. 2005;210(Suppl 1):14–21. doi: 10.1159/000081498. [DOI] [PubMed] [Google Scholar]
- 16.Chularojanamontri L, Tuchinda P, Kulthanan K, Varothai S, Winayanuwattikun W. A double-blinded, randomized, vehicle-controlled study to access skin tolerability and efficacy of an anti-inflammatory moisturizer in treatment of acne with 0.1% adapalene gel. J Dermatol Treat. 2016;27(2):140–145. doi: 10.3109/09546634.2015.1079298. [DOI] [PubMed] [Google Scholar]
- 17.Barros BS, Zaenglein AL. The use of cosmeceuticals in acne: help or hoax? Am J Clin Dermatol. 2017;18(2):159–163. doi: 10.1007/s40257-016-0249-6. [DOI] [PubMed] [Google Scholar]
- 18.Lee HE, Ko JY, Kim YH, et al. A double-blind randomized controlled comparison of APDDR-0901, a novel cosmeceutical formulation, and 0.1% adapalene gel in the treatment of mild-to-moderate acne vulgaris. Eur J Dermatol. 2011;21(6):959–965. doi: 10.1684/ejd.2011.1546. [DOI] [PubMed] [Google Scholar]
- 19.Pochi PE, Shalita AR, Strauss JS, et al. Report of the Consensus Conference on Acne Classification. Washington, D.C., March 24 and 25, 1990. J Am Acad Dermatol. 1991;24:495–500. doi: 10.1016/s0190-9622(08)80076-x. [DOI] [PubMed] [Google Scholar]
- 20.Antoniou C, Dessinioti C, Sotiriadis D, et al. A multicenter, randomized, split-face clinical trial evaluating the efficacy and safety of chromophore gel-assisted blue light phototherapy for the treatment of acne. Int J Dermatol. 2016;55(12):1321–1328. doi: 10.1111/ijd.13349. [DOI] [PubMed] [Google Scholar]
- 21.R Core Team . R R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 22.Shroot B. Pharmacodynamics and pharmacokinetics of topical adapalene. J Am Acad Dermatol. 1998;39(2 Pt 3):S17–S24. doi: 10.1016/s0190-9622(98)70440-2. [DOI] [PubMed] [Google Scholar]
- 23.Millikan LE. Adapalene: an update on newer comparative studies between the various retinoids. Int J Dermatol. 2000;39(10):784–788. doi: 10.1046/j.1365-4362.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- 24.Valins W, Amini S, Berman B. The expression of toll-like receptors in dermatological diseases and the therapeutic effect of current and newer topical toll-like receptor modulators. J Clin Aesthet Dermatol. 2010;3:20–29. [PMC free article] [PubMed] [Google Scholar]
- 25.Ditre CM, Griffin TD, Murphy GF, et al. Effects of alpha-hydroxy acids on photoaged skin: a pilot clinical, histologic, and ultrastructural study. J Am Acad Dermatol. 1996;34(2 Pt 1):187–195. doi: 10.1016/s0190-9622(96)80110-1. [DOI] [PubMed] [Google Scholar]
- 26.Fartasch M, Teal J, Menon GK. Mode of action of glycolic acid on human stratum corneum: Ultrastructural and functional evaluation of the epidermal barrier. Arch Dermatol Res. 1997;289(7):404–409. doi: 10.1007/s004030050212. [DOI] [PubMed] [Google Scholar]
- 27.Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455–461. doi: 10.2147/CCID.S84765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt MJ, Barnetson RS. A comparative study of gluconolactone versus benzoyl peroxide in the treatment of acne. Australas J Dermatol. 1992;33(3):131–134. doi: 10.1111/j.1440-0960.1992.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 29.Cui Y, Ao M, Li W, Hu J, Yu L. Anti-inflammatory activity of licochalcone a isolated from Glycyrrhiza inflata. Z Naturforsch C. 2008;63(5–6):361–365. doi: 10.1515/znc-2008-5-609. [DOI] [PubMed] [Google Scholar]
- 30.Funakoshi-Tago M, Nakamura K, Tsuruya R, et al. The fixed structure of Licochalcone A by alpha, beta-unsaturated ketone is necessary for anti-inflammatory activity through the inhibition of NF-kappaB activation. Int Immunopharmacol. 2010;10(5):562–571. doi: 10.1016/j.intimp.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Kolbe L, Immeyer J, Batzer J, et al. Anti-inflammatory efficacy of Licochalcone A: correlation of clinical potency and in vitro effects. Arch Dermatol Res. 2006;298(1):23–30. doi: 10.1007/s00403-006-0654-4. [DOI] [PubMed] [Google Scholar]
- 32.Weber TM, Ceilley RI, Buerger A, et al. Skin tolerance, efficacy, and quality of life of patients with red facial skin using a skin care regimen containing Licochalcone a. J Cosmet Dermatol. 2006;5(3):227–232. doi: 10.1111/j.1473-2165.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 33.Jovanovic Z, Angabini N, Ehlen S, Mokos ZB, Subotic M, Neufang G. Efficacy and tolerability of a cosmetic skin care product with Trans-4-t-butylcyclohexanol and Licochalcone A in subjects with sensitive skin prone to redness and rosacea. J Drugs Dermatol. 2017;16(6):605–610. [PubMed] [Google Scholar]
- 34.Udompataikul M, Srisatwaja W. Comparative trial of moisturizer containing licochalcone a vs. hydrocortisone lotion in the treatment of childhood atopic dermatitis: a pilot study. J Eur Acad Dermatol Venereol. 2011;25(6):660–665. doi: 10.1111/j.1468-3083.2010.03845.x. [DOI] [PubMed] [Google Scholar]
- 35.Wananukul S, Chatproedprai S, Charutragulchai W, Randomized CW. Randomized, double-blind, split-side comparison study of moisturizer containing licochalcone vs. 1% hydrocortisone in the treatment of infantile seborrhoeic dermatitis. J Eur Acad Dermatol Venereol. 2012;26(7):8, 94–897. doi: 10.1111/j.1468-3083.2011.04187.x. [DOI] [PubMed] [Google Scholar]
- 36.Angelova-Fischer I, Rippke F, Fischer TW, Neufang G, Zillikens D. A double-blind, randomized, vehicle-controlled efficacy assessment study of a skin care formulation for improvement of mild to moderately severe acne. J Eur Acad Dermatol Venereol. 2013;27(Suppl 2):6–11. doi: 10.1111/jdv.12168. [DOI] [PubMed] [Google Scholar]
- 37.Greenspan A, Loesche C, Vendetti N, et al. Cumulative irritation comparison of adapalene gel and solution with 2 tazarotene gels and 3 tretinoin formulations. Cutis. 2003;72(1):76–81. [PubMed] [Google Scholar]
- 38.Brand B, Gilbert R, Baker MD, et al. Cumulative irritancy potential of adapalene cream 0.1% compared with adapalene gel 0.1% and several tretinoin formulations. Cutis. 2003;72(6):455–458. [PubMed] [Google Scholar]
- 39.Chularojanamontri L, Tuchinda P, Kulthanan K, Pongparit K. Moisturizers for acne: what are their constituents? J Clin Aesthet Dermatol. 2014;7(5):36–44. [PMC free article] [PubMed] [Google Scholar]
- 40.Hayashi N, Kawashima M. Study of the usefulness of moisturizers on adherence of acne patients treated with adapalene. J Dermatol. 2014;41(7):592–597. doi: 10.1111/1346-8138.12520. [DOI] [PubMed] [Google Scholar]