Abstract
Oocyte ageing is the most important factor affecting egg quality of several fish species after ovulation. Oxidative stress has been proposed as the initiator of the oocyte ageing process in other vertebrates. To identify the role of oxidative stress and apoptosis on the progress of oocyte ageing in the common carp Cyprinus carpio, changes in the relative mRNA abundance of selected transcripts were examined. The possible alteration in the oxidation status of the oocytes during ageing was also studied. In addition, the activity of antioxidant enzymes during oocyte ageing was evaluated. Oocytes from 6 females were incubated in vivo for 14 hours post-ovulation (HPO) and in vitro for 10 hours post-stripping (HPS) at 20°C before fertilization. Hatching rates were over 65% up to 4–6 HPO, finally dropping to 1.3% at 12–14 HPO.Hatching rates were over 65% up to 4–6 HPO, finally dropping to 1.3% at 12–14 HPO. Hatching rates were more than 70% for the eggs stored in vitro up to 6 HPS and then decreased to 21.3% at 10 HPS. The results demonstrated no significant changes in the relative mRNA levels of oxidative stress-related genes or genes involved in the cell cycle during the progress of oocyte ageing in common carp. Additionally, the amount of TBARS and carbonyls did not change as time elapsed following ovulation. The apoptosis-related genes however, were significantly altered following the prolonged time interval between ovulation and fertilization. The lack of response of both activities of antioxidant enzymes and oxidation products during oocyte ageing strengthens the conclusion that oxidative stress is unlikely to be a main factor determining the progress of oocyte ageing in common carp. However, an increase in the mRNA abundance of apoptosis-related genes demonstrates that apoptotic pathway might be involved in the progress of oocyte ageing.
Introduction
Fertilization success, embryo quality and later life of the offspring are highly dependent on the integrity of the oocyte, which contains important information for orchestrating embryogenesis [1, 2] and for remodelling parental genomes [3, 4]. Oocyte ageing, which refers to the time period between ovulation and fertilization, has been identified as the most important factor affecting egg quality of several fish species after ovulation [5–7]. During ovulation, mature eggs are released from follicle cells into the ovarian or body cavity while they are still in metaphase of the second meiotic division stage [8]. After ovulation, the eggs remain in the ovarian or body cavity until the occurrence of spawning, which is stimulated by environmental factors, or until the eggs are collected by artificial techniques. Delayed spawning in nature, delayed egg stripping in culture conditions and delayed fertilization (after egg collection) lead to oocyte ageing, and finally, egg over-ripening. During oocyte ageing, major morphological, physiological, biochemical, histological, cellular and molecular changes occur inside the eggs [9]. These changes deteriorate the quality of ovulated eggs and lead to a limited fertilization rate [10, 11], increased larval malformation [12–14] and increased ploidy anomalies [15, 16]. The successful egg storage time time period during which eggs remain viable after ovulation and stripping varies from a few minutes to a few weeks depending on the fish species and storage temperature [9].
Oocytes are large cells responsible for embryo development by providing the embryo with transcripts and proteins until the onset of zygotic transcription. Therefore, the dependence of the early stages of embryogenesis on maternal mRNAs and proteins are not surprising [17]. The effect of fish oocyte ageing on several aspects of egg quality has been studied. However, until now, there has been only poor understanding about the processes and underlying mechanism of oocyte ageing in fish, as well as other vertebrates. There are few studies analysing the genomics and transcriptomics of egg quality associated with the oocyte ageing in fish. Different quantities of mRNAs between over-ripened and freshly ovulated rainbow trout Oncorhynchus mykiss eggs have been reported [18–20]. In Atlantic halibut Hippoglossus hippoglossus, poor hatching success was correlated with low transcript levels of specific genes [21]. In rainbow trout eggs, ova ageing resulted in the downregulated expression of specific microRNAs and their target genes mainly involved in cell death and signal transduction, stress response and DNA damage, RNA degradation, and energy and transcription regulation [22].
Studies on other vertebrates have proposed the involvement of oxidative stress as the initiating factor on the progress of oocyte ageing [23–25]. These studies report that oxidative stress can, in turn, trigger many cascades affecting oocyte quality, such as mitochondrial dysfunction, DNA damage, perturbed Ca2+ homeostasis and lipid peroxidation. In general, ageing is associated with increases in levels of endogenous reactive oxygen species (ROS) and decreases in antioxidant defences; leading to a wide range of oxidative damage in cell structures, including lipid peroxidation of membranes, enzyme inactivation, protein oxidation, and DNA damage [26–28]. Alteration of the lipidome, associated with oocyte ageing, was evaluated in a mouse model [29]. The latter study reported that several phospholipid classes were significantly decreased in aged oocytes, which suggests the involvement of oxidative damage in lipid plasma membrane composition and, as a result, unfavourable outcomes of oocyte ageing. The enzymatic antioxidant system can scavenge ROS and therefore decrease the effect of oxidative stress. Very little information is available about how oocyte antioxidant defences change during oocyte ageing. Additionally, a decline in critical cell cycle factors [30] and impaired mitochondrial function [31, 32] have been shown be related to the deleterious effects of oocyte ageing.
Fish are good model animals to evaluate oocyte ageing because they display a vast diversity of reproductive modes, and most species produce a high number of oocytes compared with that of other animals. Due to some ethical concerns and the intrinsic nature of other vertebrates’ oocytes, it is difficult to study the oocyte ageing process. Furthermore, as the demand for assisted reproduction technology is increasing and very few studies in this field are available, a comparative study on the process of oocyte ageing in fish might be beneficial for research on other vertebrates. The present study examines some cellular and molecular changes associated with oocyte ageing in the common carp Cyprinus carpio, focusing on the possible role of oxidative stress on the progress of the time-dependent oocyte over-ripening process. The evaluation was done at the levels of transcriptome and antioxidant enzyme assays, lipid and protein oxidation status as well as the egg phenotype and functional changes during the oocyte ageing. The common carp was selected for the experiment because the ovulation of the eggs occurs simultaneously in each individual female and our previous experience with the oocyte ageing in this species was satisfactory regarding practical approaches [11]. Genes involved in oxidative damage and stress response, mitochondrial function, fertilization, embryo development, transcriptional regulation and cell cycling as well as the ones related to the apoptosis were screened for their mRNA abundance during egg over-ripening. We also investigated the alteration in oxidation status of oocytes during post-ovulatory ageing by measuring thiobarbituric acid reactive substances (TBARS) as a marker of lipid oxidation, and carbonyls, which show the extent of protein oxidation. In addition, the role of oxidative stress in the progress of oocyte ageing was assessed by evaluation the activity of antioxidant enzymes, catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPX) and glutathione reductase GR, were examined during oocyte ageing. The combination of the aforementioned parameters will give a broad picture and understanding of the ongoing mechanisms in the oocyte ageing process. Identifying molecular mechanisms involved in the decline of oocyte quality with the progress of oocyte ageing could have important implications for aspects of basic research and practically applications for aquaculture purposes to prevent or delay oocyte ageing.
Materials and methods
Ethics
The experimental procedures were performed according to the ethical rules of the EU-harmonized Animal Welfare Act of the Czech Republic. The unit is licensed (No. 53100/2013-MZE-17214) according to the Czech National Directive (the Law against Animal Cruelty, No. 246/1992). The methodological protocol of the current study, experimental manipulations and sampling procedures were specifically approved by the expert committee of the Institutional Animal Care and Use Committee (IACUC) of the University of South Bohemia, Czech Republic. Two co-authors of this study involved in the field part of the experiment own the certificates (CZ 01672 and CZ 01660) giving permission to conduct and manage experiments involving animals according to section 15d paragraph 3 of Act no. 246/1992 Coll. The study did not involve endangered or protected species. To examine ovulation and to collect gametes, brood fish were anaesthetized with a 0.03 ml/L clove oil water bath to enhance animal welfare and minimize stress.
The study was carried out at the Genetic Fisheries Center (GFC), Research Centre of Aquaculture and Biodiversity of Hydrocenoses (RIFCH), University of South Bohemia (USB), Vodnany, Czech Republic with geographic coordinates of 49.1479° N, 14.1751° E. The permission to conduct the study was issued by the owner of the site; RIFCH, USB.
Fish
Brood fish were captured from earthen ponds when the average daily water temperature reached 16°C during the breeding season at the Genetic Fisheries Center (GFC), Research Centre of Aquaculture and Biodiversity of Hydrocenoses (RIFCH), University of South Bohemia (USB), Vodnany, Czech Republic with geographic coordinates of 49.1479° N, 14.1751° E. The fish were then transferred to indoor rectangular-shaped tanks (each 5.7 m3 capacity) supplied with water from a recirculating system and maintained under dim conditions (<20 lx), with females and males housed separately. The holding temperature was gradually increased to 20°C over time. After the experiment, broodfish were housed back to the earthen ponds at the GFC, RIFCH, USB for the purpose of using in the future research. The larvae originating in the study also were released to the small earthen ponds center. None of the experimental animals either broodfish or the larvae were sacrificed during this study.
Egg storage in the ovarian fluid and gamete collection
After a 3-day period of acclimation, female brood fish were treated with an intramuscular injection of carp pituitary homogenate CPH (0.3 mg kg-1 body weight). This step was followed by a second injection with CPH (3.5 mg kg-1 body weight) 12 hours later. Male brood fish (4.5 ± 0.4 kg body weight, mean ± SEM) were subjected to a single intramuscular injection of CPH (2 mg kg-1 body weight) that was given simultaneously with the second injection of the females. These procedures were performed according to Horvath and Tamas, 1985 [33]. The females were examined for ovulation every 2 hours, starting 10 hours after the second injection. Six females that ovulated within 2 hours (weighing 4.2 ± 0.2 kg body weight), were selected randomly, marked with coloured tags and used for the experiment. Almost half of the eggs were stripped from each female and used for the in vitro egg storage experiment. The other half of the eggs was retained inside the fish bodies to be used for the in vivo egg storage experiment. These two experiments were performed as described below.
In vivo oocyte ageing
In total, seven batches of eggs from each of the six females were separately stripped within 2- hour intervals and then fertilized during the experimental period of 14 hours after ovulation. An egg batch of 7 g was stripped individually from each female and fertilized immediately (0–2 hours post-ovulation, HPO). The rest of the eggs were left inside the ovarian cavity of each female for the next fertilization time. Thus, eggs were retained inside the fish body before fertilization for 0–2, 2–4, 4–6, 6–8, 8–10, 10–12 and 12–14 HPO.
In vitro oocyte ageing
Seven batches of 7 g egg aliquots were collected from each of the six females and stored in sterile six-well cell culture plates (each well diameter: 3.5 cm). The eggs were stored two layers deep [34] in the ovarian fluid, and no solution, artificial media or extender was added. All plates were individually covered by their own lids and stored in the dark at 20°C in the laboratory incubator for 10 hours. To provide the humidified atmosphere, a few plates were filled with water and placed into the storage chambers [34, 35]. Stored ova were fertilized at 0 (immediately after stripping), 2, 4, 6, 8, 10 and 12 hours post-stripping.
Artificial fertilization and egg development
For each sampling time, egg quality was evaluated by measurement of the eyeing, eyed-egg mortality, hatching and larval malformation rates. Artificial fertilization, incubation and examination of egg developmental success were performed according to Samarin et al. [11].
RNA isolation and reverse transcription
At all HPOs and HPSs, 1 g of the unfertilized eggs was placed in cryotubes, frozen in liquid nitrogen and then stored at -80°C freezer until RNA isolation. Samples were collected in three replicates for each fertilization time.
To prepare total RNA, an equal weight of 20 eggs was taken from each tube and treated with TRIzol reagent (Invitrogen Corporation, Carlsbad, CA) in Precellys24 homogenizer (Bertin Instruments), 5500 rpm for 2×20 sec with a 5 sec interval. RNA was isolated using the PureLink RNA Purification Kit (Invitrogen) according to the manufacturer’s instructions. During RNA isolation, samples were depleted of genomic DNA using a PureLink DNase Kit (Invitrogen) according to the manufacturer’s protocol. The RNA concentration and RNA purity were assessed using a NanoDrop Spectrophotometer (ND-1000, Thermo Scientific).
A concentration of 1000 ng RNA was used to generate cDNA using TaqMan reverse transcription Reagents (Life Technologies). Random hexamers were used to prime the reaction. Reverse transcription was performed at 25°C for 10 min, at 48°C for 1 hour and finally 95°C for 5 min. Control reactions were run without TaqMan reverse transcriptase and used as negative controls in the real-time PCR study.
Real-time PCR analysis
Nucleotide sequences corresponding to common carp mRNA (NCBI) were used for primer design (by Primer3) [36, 37]. Transcript levels of 16 genes, hsp70, cox1, sod, gpx1, cyclinA, cyclinB, jnkA, jnkB, caspase3A, caspase9, bax, bcl2, cathepsinB, cathepsinZ, vasa and igf2, were determined by real-time PCR in duplicate using the LightCycler 480 (Roche Applied Science, Germany). The reaction mix for real-time PCR consisted of 4 μl diluted (1:10) cDNA, 1 μl forward and reverse primer (final concentration of 500 nM, Table 1), and 5 μl SYBR Green-I Master (Roche). All primers were provided by Invitrogen. A standard curve was included for each primer pair to evaluate primer efficiency. All samples were analysed in parallel, and a non-template control with water substituted for cDNA was run for each primer pair. The real-time PCR reaction was run under the following conditions: preincubation at 95°C for 5 minutes, amplification for 45 cycles at 95°C for 15 seconds and 60°C for 1 minutes, melting curve at 95°C for 5 seconds and 65°C for 1 minutes, cooling at 40°C for 10 seconds.
Table 1. Real-Time PCR primer sequences.
| Target gene | Forward primer (5′-3′) | Reverse primer (5′-3′) | Genbank accession no. |
|---|---|---|---|
| hsp 70 | GAGAGGCTGATTGGAGATGC | ACTGCACAACTGGGTCATCA | HQ259767 |
| cytochrome c oxidase I | TCCACGGAGGATCCATTAAA | GGATAGGACGATCCCTGTGA | HQ235998 |
| vasa | AGGCCAGGAAGTTTGCCTAT | TGCAGCCCTTTAACACCTCT | KP661178 |
| Caspase3a | TGATGGCAAAGTATGGCAAA | ATCAAAGACTGGCTGGTTGG | KF055462 |
| Caspase9 | CCTGTGGAGGAGGTGAGAAG | ATGGGAATAGCGTCCATCTG | KC676314 |
| 18s ribosomal RNA | AGTTCCGACCGTAAACGATG | AGACTCGTGGTTTCCCACAC | JQ619778 |
| beta-actin gene | GCAAAGGTTTTGTGCTCCAT | CATGGATACCGCAAGATTCC | JQ619775 |
| Bcl2 | GGGATGCCTTTGTGGAGTTA | TCACTCCTGCCAAGCCTAGT | EU490408 |
| Bcl2 associated X protein (Bax) | GGAGATGAGCTGGATGGAAA | AAGATCTCTCGGGCCACTTT | KJ174685 |
| glutathione peroxidase (Gpx1) | GGAGAAGCTGGAAGTGAACG | TCACCCATCAAGGACACAGA | GQ376155 |
| gapdh | GTGATGCAGGGGCTCAGTAT | CTCTCTTGGCACCACCCTTA | AJ870982 |
| IGF2 | TGCAAAACCCATGAAGTCTG | AAGAGGCCCTCCTGAGATGT | KP663718 |
| cyclin A | TGCATGTCTGTCCTGAGAGG | TCCACTTCCGGAGGATACAC | EU380205 |
| cathepsin Z | GAGAGAAAGGCTGGCTCAGA | GGGTCTCCGTACATGCAGTT | AY949988 |
| cathepsin B | AAAGGACCCAGACTCCCTGT | TTTAAGAGTGGGGCAGTTGG | AB215097 |
| Mn/ SOD | TTATGCAGCTTCACCACAGC | ACATCACCCTTAGCCAGTGC | JF411603 |
| cyclin B | CCAGAAAAGCAGCTGTAGCC | TCTTCCTCAAAGCCTGTCGT | EU293852 |
| jnkA | TCGATGAGAGGGAACACACA | GACCTCGAATGACACCGTTT | JN542470 |
| jnkB | CCAACCTCTGCCAAGTCATT | CCGAGTGGAGGTGTTTTGTT | AB001744 |
The comparative CT method was used for relative quantification of target gene expression levels. To normalize relative gene expressions, three reference genes, glyceraldehyde 3-phosphate dehydrogenase (gapdh), 18S ribosomal RNA (18s) and beta-actin (b-actin), were tested. The mRNA abundance of gapdh proved to be highly stable in common carp eggs collected at different time points after ovulation, and it did not show any significant change in Ct values in the eggs at different HPOs and HPSs. Therefore, gapdh proved the most stable and was used as the reference gene. Relative expression was then calculated according to the equation 2-ΔCt. Sequences of primers used are listed in Table 1.
Antioxidant enzyme activity assays
Preparation of post-mitochondrial supernatant
Samples of fish eggs (approx. 400 mg) were homogenized using a Tissue Lyser II (Qiagen) in 0.1 M K-phosphate buffer (pH 7.4). Homogenates were centrifuged at 15,000 g, 4°C for 15 min in a Micro 200 R. Supernatants were removed, stored at 0–4°C and used for protein determination and enzyme assays. Protein levels were measured spectrophotometrically using bovine serum albumin as a standard [38]. The samples were diluted to a protein content of 10 mg/mL.
Antioxidant enzyme activity
Catalase (CAT) activity was quantified as a decrease in hydrogen peroxide in a 96-well flat-bottomed UV-transparent microtitre plate. CAT activity was assessed spectrophotometrically at 240 nm and was performed following the method of Claiborne, 1985 [39]. Calculations were made using a molar extinction coefficient of 40 M−1 cm−1.
The superoxide dismutase (SOD) activity was determined by the method of Nishikimi et al. [40]. Superoxide dismutase activity was assessed spectrophotometrically at 420 nm and expressed as the nitro blue tetrazolium formation per milligram of protein per minute.
Glutathione peroxidase (GPX) activity was measured using the method of Mohandas et al. [41]. Briefly, incubation mixture (0.3 mL) contained post-mitochondrial supernatant (approx. 0.2 mg of protein), K-phosphate buffer 0.05 M (pH 7.0), in EDTA 1 mM, sodium azide 1 mM and glutathione reductase from baker's yeast (7.5 ml from stock containing 1 U/ml), reduced glutathione 4 mM and NADPH 0.8 mM. The reaction was started via the addition of 0.5 mM of hydrogen peroxide. The oxidation of NADPH was recorded at 340 nm. Calculations were made using the molar extinction coefficient 6220 M−1 cm−1.
The glutathione reductase (GR) activity was measured by the method of Cribb et al. [42]. Briefly, incubation mixture (0.25 mL) contained post-mitochondrial supernatant (approx. 0.3 mg of protein), K-phosphate buffer 0.05 M (pH 7.0), NADPH (0.4 mM), oxidized glutathione (0.4 mM) and diethylenetriaminepentaacetic acid (DTPA) (1 mM). Disappearance of NADPH was measured at 340 nm and calculated as NADPH oxidized formation using the molar extinction coefficient of 6220 M−1 cm−1.
All assays were performed spectrophotometrically in quadruplicate or triplicate using a 96-well microplate reader (Tecan Infinite M200, Germany). Samples were held on ice; measurements were made at 25°C. Variations in absorbance at each reaction well were linear over time (R2>0.8).
Thiobarbituric acid reactive substances
The thiobarbituric acid reactive substances (TBARS) method was used to evaluate oocyte lipid peroxidation according to the method described by Li et al. [43]. After reaction with thiobarbituric acid in darkness for 15–20 hours (overnight) at room temperature (20°C), the reaction complex was detected at a wavelength of 530 nm against the sample blank using a UV-visual plate reader (AF 2200; Austria). The results were expressed as equivalents to malonaldehyde (MDA) in μg/g.
Protein oxidation
Protein oxidation was estimated as carbonyls after incubation with 2,4-dinitrophenylhydrazine (DNPH) in 2 N hydrochloric acid following a slightly modified method from the one described by Oliver et al. [44]. Carbonyl concentration was analysed as DNPH calculated on the basis of absorption of 21.0 mM-1 cm-1 at 370 nm for aliphatic hydrazones. The protein concentration was measured at 280 nm in the same sample and quantified using bovine serum albumin as a standard.
Statistical analysis
The normality of the data was ascertained using SPSS software version 18. Differences between the means of the groups for each measured egg quality parameter (eyeing, hatching, eyed-egg mortality and larval malformation rates) as well as each individual gene, lipid and protein oxidation levels and antioxidant enzyme activity were evaluated using an ANOVA followed by Duncan’s multiple range test. Wilcox test; a non-parametric statistical hypothesis test, was used to compare the in vivo and in vitro egg storage results. P < 0.05 was considered to be significant.
Results
Egg viability
In vivo oocyte ageing
The eyeing and hatching values remained almost constant, approximately 83% and 75%, respectively, for the eggs fertilized up to 4 HPO (Fig 1). Although not significantly different, the eyeing and hatching rates increased to 89% and 82%, respectively, at 2–4 HPO. Thereafter, the values decreased linearly over time and dropped to 6.3% for the eyeing rates and 1.3% for the hatching rates at 12–14 HPO. Eyed-egg mortality and larval malformation rates were not significantly different for the eggs fertilized up to 6 hours after ovulation. The highest eyed-egg mortality and larval malformation were found at 12–14 HPO, 80.9 ± 5.1% and 62.5 ± 11.8%, respectively.
Fig 1. Effects of in vivo oocyte ageing on the eyeing, hatching, eyed-egg mortality and larval malformation rates in common carp (mean ± SD).
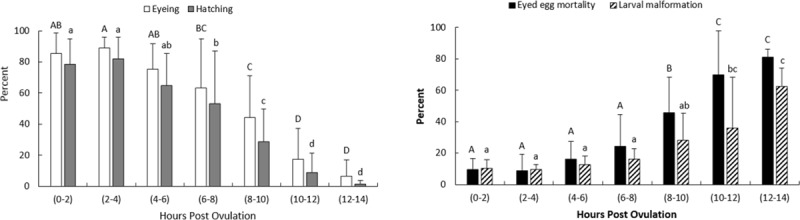
Means sharing a common alphabetical symbol do not differ significantly.
In vitro oocyte ageing
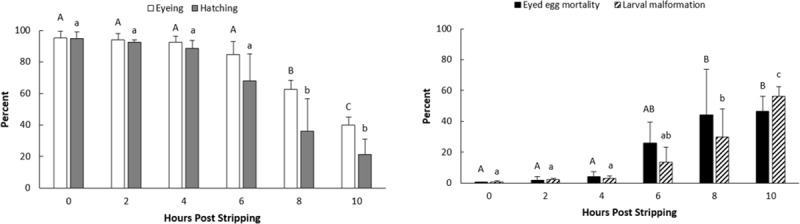
The eyeing and hatching rates were almost constant, approximately 91% and 86%, respectively, up to 6 HPS (Fig 2). After 10 HPS the eyeing and hatching rates were measured to be 40 ± 5.1% and 21.3 ± 9.7%, respectively. The eyed-egg mortality and larval malformation rates also did not show any marked increase during 6 hours of the in vitro egg storage. After 6 HPS, the eyed-egg mortality and larval malformations increased significantly over time so that at 10 HPS, 46% of the eyed-eggs died, and 56% of the hatched larvae were malformed.
Fig 2. Effects of in vitro oocyte ageing on the eyeing, hatching, eyed-egg mortality and larval malformation rates in common carp (mean ± SD).
Relative mRNA abundance of selected transripts
In vivo oocyte ageing
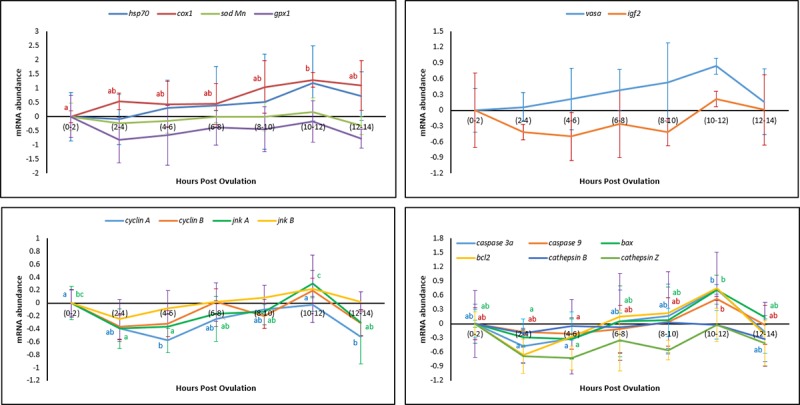
The mRNA levels of 16 selected transcripts were quantified in freshly ovulated eggs and in aged eggs at different HPOs using gapdh as the reference gene. Genes related to the oxidative injury and stress response (hsp70, sod and gpx1) did not show any significant changes in their mRNA abundance during in vivo oocyte ageing (Fig 3). The increased level of cox1, however, was observed at 10–12 HPO. Among the genes related to the cell cycle, the mRNA levels of cyclinA and jnkA increased during oocyte ageing, while cyclinB and jnkB exhibited constant levels. Relative mRNA abundance of the apoptosis related genes (caspase3A, caspase9 and bax) increased during oocyte ageing while the relative abundance of bcl2, cathepsinB and cathepsinZ remained constant during this period. Both vasa and igf2 showed a constant levels during post-ovulatory oocyte ageing.
Fig 3. Effect of in vivo oocyte ageing on the mRNA levels of the selected transcripts in common carp (mean ± SD).
In vitro oocyte ageing
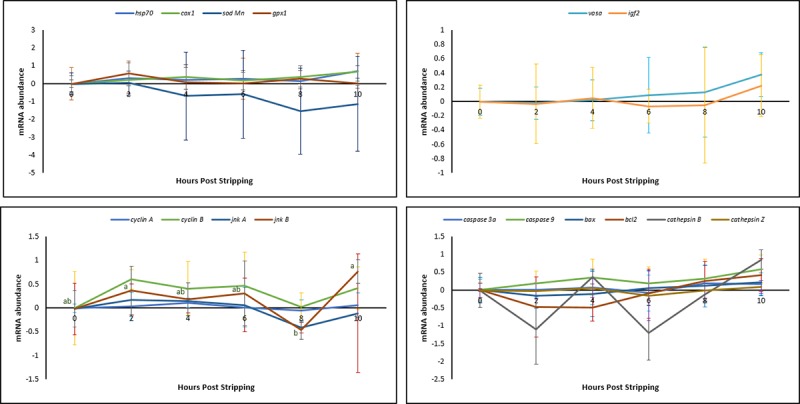
The mRNA profiles of the same selected genes were examined during 10 HPS. Except for jnkB, none of the examined genes showed any significant changes in their mRNA abundance during in vitro oocyte ageing for 10 hours after the stripping (Fig 4).
Fig 4. Effect of in vitro oocyte ageing on the mRNA levels of the selected transcripts in common carp (mean ± SD).
Effect of oocyte ageing on the lipid and protein oxidation of the oocytes
In vivo oocyte ageing
The level of TBARS, an intracellular stress marker, in the oocytes exhibited no significant changes during post-ovulatory ageing, remaining at approximately 1.5 MDA (μg g-1) (Fig 5). Protein oxidation, measured as carbonyl values, also did not show any significant changes through 12–14 HPO (Fig 6).
Fig 5. Effects of in vivo and in vitro oocyte ageing in common carp on TBARS, expressed as malonaldehyde (MDA) (μg g-1) (mean ± SD).
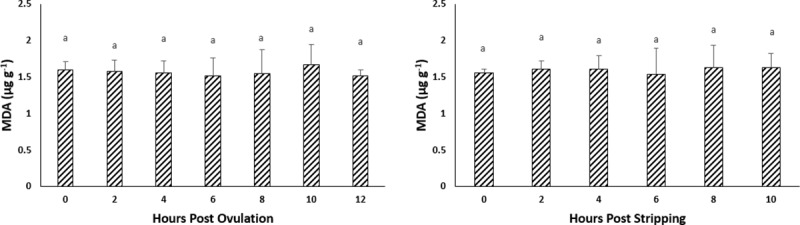
Fig 6. Effects of in vivo and in vitro oocyte ageing in common carp on carbonyls (nmol mg-1) (mean ± SD).
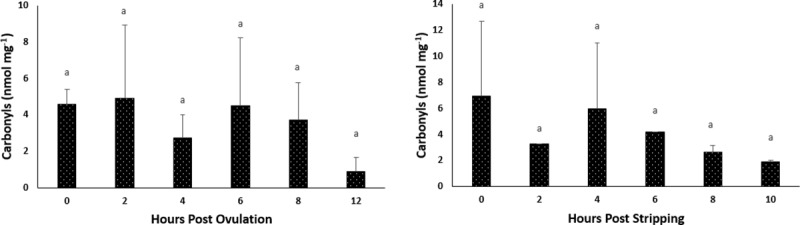
In vitro oocyte ageing
The level of TBARS did not show any significant changes during post-stripping oocyte ageing (Fig 5). Similarly, carbonyl values, also stayed constant through 10 HPS (Fig 6).
Comparing the in vivo and in vitro egg storage results showed no significant changes for any of the examined genes at any of the time points except for the cathepsinZ, cyclinA and jnkA at 4–6 hours.
Effect of oocyte ageing on the activity of antioxidant enzymes
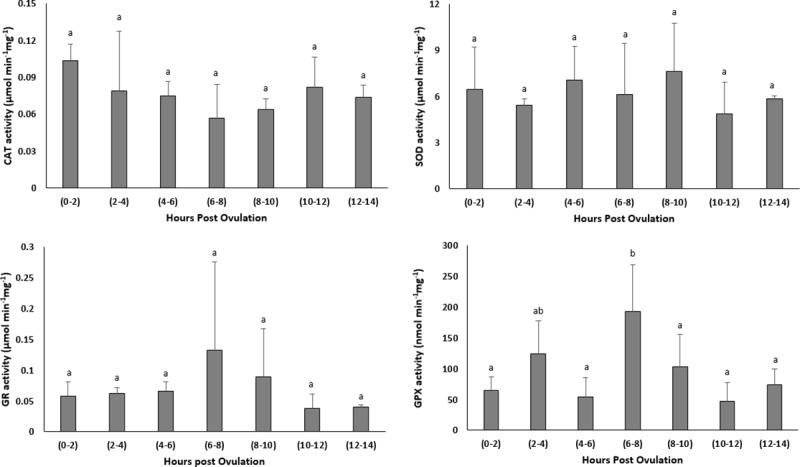
In vivo oocyte ageing
The activity of antioxidant enzymes, CAT, SOD and GR remained stable with prolonged in vivo storage of the oocytes (Fig 7). The activity of GPX however, increased significantly up to 6–8 HPO.
Fig 7. Effects of in vivo oocyte ageing in common carp on the activities of catalase (CAT) (μmol/min/mg), superoxide dismutase (SOD) (μmol/min/mg), glutathione reductase (GR) (μmol/min/mg) and glutathione peroxidase (GPX) (μmol/min/mg) (mean ± SD).
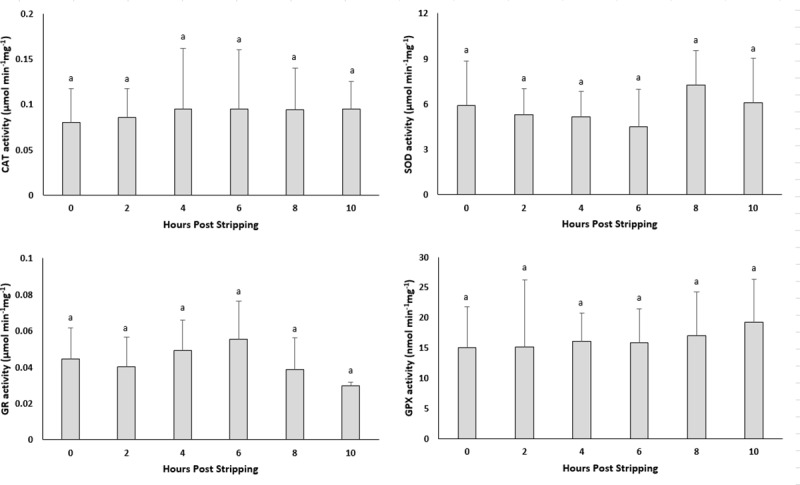
In vitro oocyte ageing
The CAT, SOD, GR and GPX activities did not show any significant changes through post-stripping oocyte ageing (Fig 8).
Fig 8. Effects of in vitro oocyte ageing in common carp on the activities of catalase (CAT) (μmol/min/mg), superoxide dismutase (SOD) (μmol/min/mg), glutathione reductase (GR) (μmol/min/mg) and glutathione peroxidase (GPX) (μmol/min/mg) (mean ± SD).
Discussion
With elapsing time after ovulation, oocytes experience changes that negatively affect the egg quality and successive developmental stages. In the present study, the egg quality remained constant when they were stored up to 4–6 hours in vivo and 6 hours in vitro after ovulation at 20°C. The observed decreasing trend for the eyeing and hatching rates and the increasing trend for the eyed-egg mortality and larval malformation percentages after ovulation is in accordance with the previously reported experiments on the other fish species [10, 16, 18]. However, the successful egg storage time differs from a few minutes to a few weeks and highly depends on the fish species and the storage temperature [9].
It is currently undetermined as to whether a single event acts to trigger a cascade of other factors or if several biochemical and functional changes occur separately to create an aged oocyte [45]. Most authors believe that the onset of ageing in oocytes is associated with an increase in ROS, consequently leading to an increase in oxidative stress [23–25, 45]. The important consequences of oxidative stress in the oocytes are ROS-induced mitochondrial dysfunction, lipid alterations and DNA fragmentation, followed by decreasing ATP production and apoptosis. Esponda and Diaz, 2006 [46] analysed the presence of hsp70 mRNA and protein in samples collected from 20 HPO aged mice oocytes and found that Hsp70 protein is not present in freshly ovulated oocytes; however, this protein appears in the cytoplasm of aged oocytes. They also reported increased hsp70 mRNA levels in the aged oocytes. The intra-cytoplasmic level of glutathione, which has a major role in protecting oocytes from damage by ROS, decreased in aged mouse oocytes [47]. Additionally, the level of lipid peroxidation, which is an indicator of the degree of oxidative stress, increased in the in vivo-aged mouse oocytes [24]. In human embryos, a positive correlation has been reported between the concentration of hydrogen peroxide and the occurrence of apoptosis [48], indicating the initiating role of hydrogen peroxide on ageing. Hence, we examined the idea proposed for other vertebrates of whether oxidative stress affects the progress of oocyte ageing. Our results demonstrated no significant changes in the mRNA levels of oxidative stress-related genes or genes involved in the cell cycle during the progress of oocyte ageing in the common carp, with the exception of cox1. Additionally, as time elapsed following ovulation, the amount of TBARs (which is the main indicator of lipid peroxidation) and the amount of carbonyls (which show the extension of protein oxidation) did not change in the oocytes, indicating no increase in oxidative stress. Our experiments regarding evaluation the activity of antioxidant enzymes during oocyte ageing have also confirmed that oxidative stress is not likely the main initiator in the progress of oocyte ageing. The enzymatic antioxidant system can scavenge ROS and therefore decrease the effect of oxidative stress. If oxidative stress would be the initiator of deleterious effects during post-ovulatory oocyte ageing, then an alteration in antioxidant enzyme activity and oxidation markers should occur following ovulation. Our results indicated no significant changes in the activity of CAT, SOD and GPX during post-ovulatory ageing of common carp oocytes. The up-regulation of cox was also reported in the mouse oocyte with maternal ageing, i.e., the ovarian ageing [32]. On the other hand, cox1, located in the mitochondrial membrane, is the last enzyme in the respiratory electron transport chain. Therefore, any changes in the mRNA expression pattern of cox1 might affect ATP synthesis and promote mitochondrial disfunction. Hamatani et al. [32] observed a decrease in the transcription levels of ATP-related genes in mouse oocytes during maternal ageing.
The relative mRNA levels of vasa in our study showed an upward trend during both in vivo and in vitro oocyte ageing, however, did not differ significantly. This result is in consistent with the results obtained in another recent experiment by our research group indicating that levels of vasa mRNA increase during in vitro oocyte ageing in African catfish Clarias gariepinus [49]. Vasa is a gene involved in the development of primordial germ cells (PGCs), and its activity is required for both differentiation of the germ cells into gametes [50] and the functionality of germ cells [51]. Loss of vasa function in the mouse affects differentiation of the male germ cells, resulting in male sterility and lack of any phenotype [50]. Vasa protein is an essential component of germplasm and represents a poorly understood complex of RNA and proteins that is required for germ cell determination. Null mutation leads to sterility in female mice, resulting from severe defects in oogenesis [52]. On the other hand, Tarin et al. [23] concluded that oocyte ageing was associated with a distorted secondary sex ratio in favour of males. Our preliminary results with zebrafish Danio rerio (vasa GFP transgenic strain), indicated that oocyte ageing significantly affects the number and development of the primordial germ cells (PGCs). Since depletion of PGCs converts the sex differentiation in favour of males in zebrafish [53] and other fish species [54], the oocyte ageing may bias sex ratio in favour of males or increase the probability of the occurrence of completely sterile individuals. The latter idea can be addressed in future studies.
mRNA levels of transcripts involved in the cell cycle was also investigated upon post-ovulatory and post-stripping oocyte ageing. CyclinA and jnkA mRNAs displayed higher abundance in more aged oocytes in vivo, while lower levels in more aged oocytes in vitro. A similar increasing trend in the abundance of the cyclinA1, cyclinA2 and JNK1 has been reported in rainbow trout oocytes aged in vivo [19]. Similarly decreased mRNA levels of two critical cell cycle-related genes, maturation promoting factor (MPF) and mitogen-activated protein kinases (MAPKs), have been reported in oocytes aged in vitro in porcine [30, 55] and murine models [56]. The latter study indicated the role of critical cell cycle factors and cytoplasmic changes in spontaneous activation of the oocyte ageing. The observed opposite trend towards the increased and decreased mRNA levels of the aforementioned genes during ova ageing in vivo and in vitro is of interest for future studies.
Apoptotic cell death is the end point of the oocyte ageing process and occurs through caspase activation [57], increased levels of apoptotic signalling protein Bax, decreased levels of anti-apoptotic protein Bcl-xL [58] and DNA damage [59]. Similarly, in the current study, the genes involved in apoptosis, such as caspase3A, caspase9 and bax, exhibited lower levels at the time of ovulation (0 HPO) than those in over-ripened eggs at 10–12 HPO. This was the same case in the in vitro storage; when the mRNA of the abovementioned genes showed lower abundance at 0 HPS and higher abundance in the more aged oocytes at 10 HPS. By contrast, it has been shown that the level of bax remains unchanged in the mouse oocytes aged in vitro. [60, 61]. At the end of the oocyte ageing time, pro-apoptotic molecules, such as bax, induce the release of cytochrome c, which activates caspases, while anti-apoptotic molecules, such as bcl2, prevent this release [62]. As the expression of the anti-apoptotic protein Bcl2 is decreased during oocyte ageing in mice [61] and pigs [55], the oocytes and developing embryos are more prone to undergo apoptosis [25, 60]. In the present study, the relative mRNA abundance of bcl2 showed no significant changes during both in vivo and in vitro oocyte ageing. Bcl2 is known as a family of proteins regulating cell death by either inducing or inhibiting apoptosis [63, 64]. The observed trend for the relative mRNA abundance of bcl2 in our study might be attributed to the inducing role of the examined gene for apoptosis during ova ageing, while many other genes encoding the Bcl2 protein might have an inhibiting role in the occurrence of apoptosis. Levels of hsp70 and cox1 mRNAs showed the increasing tendency at the beginning phase of oocyte ageing, while increased levels of caspase3A, caspase9 and bax, were obvious at 10–12 HPO and HPS. Consistent with these observations, Lord et al. [25] suggested that oxidative stress in aged oocytes can be considered as an early marker of oocyte ageing before the activation of caspase-3 and before the appearance of the morphological features of oocyte ageing and apoptosis. Although they do not differ statistically, levels of CathepsinB and cathepsinZ mRNAs were both increased during the in vivo and in vitro egg storage, except for the initial decrease of cathepsinZ once the eggs stored in vitro. Upregulation of cathepsinB is associated with cell death [65]. Aegerter et al. [19] also found that in vivo oocyte ageing in rainbow trout is associated with the increased mRNA abundance of cathepsinZ. Lysosomal proteases cathepsinD and cathepsinB act as pro-apoptotic mediators of apoptosis [66]. Therefore, the increased mRNA levels of the cathepsinB and cathepsinZ genes during the oocyte ageing progress might lead to the increased mRNA levels of the apoptotic genes observed in this study.
Our results indicated that the relative mRNA level of igf2 showed a decreasing trend to 6 HPO and HPS and then increased until the complete loss of egg viability occurred. Aegerter et al. [19] also found higher quantities of igf2 mRNAs in more aged rainbow trout oocytes at 14 HPO than that in the freshly ovulated ones. The IGF axis has been shown to play a role in the inhibition of apoptotic cell death [67]. The increased tendency towards the mRNA levels of the igf2 observed in this study therefore, might be considered as a defence mechanism against the occurrence of over-ripening of the eggs and the apoptosis. The same trend was observed for the mRNA abundance changes during in vivo and in vitro oocyte ageing, shows that the oocyte ageing may pass through the same processes either the eggs are aged in vivo or in vitro.
Although examining the mRNA abundance of single target genes could be a good and helpful tool, it is not yet enough to conclude on the possible involvement of oxidative stress in the progress of fish oocyte ageing. In fact, additional analysis such as microarray analysis, total ROS measurement, mitochondrial dysfunction indicators, ATP content of the eggs, etc., are required to fully evaluate the contribution of oxidative stress to the drop of egg quality during postovulatory ageing. Additionally, as there is no clear link between mRNA abundance and protein synthesis in metaphase 2 oocytes, studying the proteome profile changes during the fish oocyte ageing could provide valuable information about the oocyte ageing and its underlying mechanisms. Further analysis of these genes during development in eggs at varying ageing times will be useful and will benefit to the study on fish egg quality.
The epigenetic changes in mouse oocytes have been associated with post-ovulatory ageing [68, 69]. The ageing of oocytes has been shown to significantly alter the methylation pattern of imprinted genes in both mouse oocyte and the developing placenta [69, 70]. This process in turn alters the demethylation events after fertilization [71]. The epigenetic modification related factors (DNA methylation and histone modification) might be involved in defects arising in aged oocytes and the originating embryos. Investigating the epigenetic changes associated with fish oocyte ageing seems to be interesting for the future studies.
The results obtained in our study demonstrate that oxidative stress is not likely the main initiator of the oocyte ageing in common carp. However, complementary tests and analysis are required to clarify the involvement of oxidative injury and stress response in the progress of oocyte ageing. The apoptosis-related genes however, were significantly altered following the prolonged time interval between ovulation and fertilization demonstrating that apoptotic pathway might be involved in the progress of oocyte ageing.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors would like to thank the Elsevier Language Editing Service, for improving the English of the manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files. The data are fully available in the public repository, Open Scientific Framework (OSF: osf.io/myqd3).
Funding Statement
The study was financially supported by the Ministry of Education, Youth and Sports of the Czech Republic - project “CENAKVA” (LM2018099), project Reproductive and Genetic Procedures for Preserving Fish Biodiversity and Aquaculture (CZ.02.1.01/0.0/0.0/16_025/0007370), the Ministry of Agriculture of the Czech Republic: project NAZV (QK1710310) and by NF-CZ07-MOP-3-184-2015 and NF-CZ07-ICP-3-185-2015.
References
- 1.Schultz GA, Heyner S. Gene expression in pre-implantation mammalian embryos. Mutat Res. 1992; 296: 17–31. [DOI] [PubMed] [Google Scholar]
- 2.Minami N, Suzuki T, Tsukamoto S. Zygotic gene activation and maternal factors in mammals. J Reprod Dev. 2007; 53: 707–715. [DOI] [PubMed] [Google Scholar]
- 3.Torres-Padilla ME, Bannister AJ, Hurd PJ, Kouzarides T, Zernicka-Goetz M. Dynamic distribution of the replacement histone variant H3.3 in the mouse oocyte and preimplantation embryos. Int J Dev Biol. 2006; 50: 455–461. 10.1387/ijdb.052073mt [DOI] [PubMed] [Google Scholar]
- 4.Yoshida N, Brahmajisyula M, Shoji S, Amanai M, Perry AC. Epigenetic discrimination by mouse metaphase II oocytes mediated asymmetric chromatin remodelling independently of meiotic exit. Dev Biol. 2007; 301: 464–477. 10.1016/j.ydbio.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 5.Lam TJ, Nagahama Y, Chan K, Hoar WS. Overripe eggs and post-ovulatory corpora lutea in the three spined stickleback. Can J Zool. 1978; 56: 2029–2036. [Google Scholar]
- 6.McEvoy (Barton) L.-A. Ovulatory rhythms and over-ripening of eggs in cultivated turbot, Scophthalmus maximus L. J Fish Biol. 1984; 24: 437–448. [Google Scholar]
- 7.Rime H, Guitton N, Pineau C, Bonnet E, Bobe J, Jalabert B. Post-ovulatory ageing and egg quality: A proteomic analysis of rainbow trout coelomic fluid. Reprod Biol Endocrinol. 2004; 2: 26–36. 10.1186/1477-7827-2-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bobe J, Labbe C. Egg and sperm quality in fish. Gen Comp Endocrinol. 2010; 165: 535–548. 10.1016/j.ygcen.2009.02.011 [DOI] [PubMed] [Google Scholar]
- 9.Samarin AM, Policar T, Lahnsteiner F. Fish oocyte ageing and its effect on egg quality. Rev Fish Sci Aquac. 2015; 23: 302–314. [Google Scholar]
- 10.Lahnsteiner F, Urbanyi B, Horvath A, Weismann T. Biomarkers for egg quality determination in cyprinid fish. Aquaculture. 2001; 195: 331–352. [Google Scholar]
- 11.Samarin AM, Gela D, Bytyutskyy D, Policar T. Determination of the best post-ovulatory stripping time for the common carp, Cyprinus carpio. J Appl Ichthyol. 2015; 31(2): 51–55. [Google Scholar]
- 12.Aegerter S, Jalabert B. Effects of post ovulatory oocyte ageing and temperature on egg quality and on the occurrence of triploid fry in rainbow trout Oncorhynchus mykiss. Aquaculture. 2004; 231: 59–71. [Google Scholar]
- 13.Bonnet E, Fostier A, Bobe J. Characterization of rainbow trout egg quality: A case study using four different breeding protocols, with emphasis on the incidence of embryonic malformations. Theriogenology. 2007; 67: 786–794. 10.1016/j.theriogenology.2006.10.008 [DOI] [PubMed] [Google Scholar]
- 14.Samarin AM, Żarski D, Palińska-Żarska K, Krejszeff S, Blecha M, Kucharczyk D, et al. In vitro storage of unfertilized eggs of the Eurasian perch and its effect on egg viability rates and the occurrence of larval malformations. Animal. 2017; 11(1): 78–83. 10.1017/S1751731116001361 [DOI] [PubMed] [Google Scholar]
- 15.Flajshans M, Kohlmann K, Rab P. Autotriploid tench Tinca tinca (L.) larvae obtained by fertilization of eggs previously subjected to postovulatory ageing in vitro and in vivo. J Fish Biol. 2007; 71: 868–876. [Google Scholar]
- 16.Samarin AM, Blecha M, Uzhytchak M, Bytyutskyy D, Żarski D, Flajshans M, et al. Post-ovulatory and post-stripping oocyte ageing in northern pike, Esox lucius (Linnaeus, 1758), and its effect on egg viability rates and the occurrence of larval malformations and ploidy anomalies. Aquaculture. 2016; 450: 431–438. [Google Scholar]
- 17.Zuccotti M, Merico V, Cecconi S, Redi CA, Garagna S. What does it take to make a developmentally competent mammalian egg? Hum Reprod Update. 2011; 17: 525–540. 10.1093/humupd/dmr009 [DOI] [PubMed] [Google Scholar]
- 18.Aegerter S, Jalabert B, Bobe J. MessengerRNAstockpile of cyclin B, insulin-like growth factor I, insulin-like growth factor II, insulinlike growth factor receptor Ib, and p53 in the rainbow trout oocyte in relation with developmental competence. Mol Reprod Dev. 2004; 67: 127–135. 10.1002/mrd.10384 [DOI] [PubMed] [Google Scholar]
- 19.Aegerter S, Jalabert B, Bobe J. Large scale real-time PCR analysis of mRNA abundance in rainbow trout eggs in relationship with egg quality and post-ovulatory ageing. Mol Reprod Dev. 2005; 72: 377–385. 10.1002/mrd.20361 [DOI] [PubMed] [Google Scholar]
- 20.Bonnet E, Fostier A, Bobe J. Microarray-based analysis of fish egg quality after natural or controlled ovulation. BMC Genomics. 2007; 8: 55 10.1186/1471-2164-8-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mommens M, Fernandes JM, Bizuayehu TT, Bolla SL, Johnston IA, Babiak I. Maternal gene expression in Atlantic halibut (Hippoglossus hippoglossus L.) and its relation to egg quality. BMC Res Notes. 2010; 3: 138 10.1186/1756-0500-3-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma H, Weber GM, Hostuttler MA, Wei H, Wang L, Yao J. MicroRNA expression profiles from eggs of different qualities associated with post-ovulatory ageing in rainbow trout (Oncorhynchus mykiss). BMC Genomics. 2015; 16: 201 10.1186/s12864-015-1400-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarin JJ, Perez-Albala S, Cano A. Consequences on offspring of abnormal function in ageing gametes. Hum Reprod Update. 2000; 6: 532–549. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev. 2003; 66: 143–152. 10.1002/mrd.10341 [DOI] [PubMed] [Google Scholar]
- 25.Lord T, Nixon B, Jones KT, Aitken RJ. Melatonin prevents postovulatory oocyte aging in the mouse and extends the window for optimal fertilization in vitro. Biol Reprod. 2013; 88: 1–9. 10.1095/biolreprod.112.105957 [DOI] [PubMed] [Google Scholar]
- 26.Dean RT, Gieseg S, Davies MJ. Reactive species and their accumulation on radical-damaged proteins. Trends Biochem Sci. 1993; 18: 437–441. [DOI] [PubMed] [Google Scholar]
- 27.Headlam HA, Davies MJ. Markers of protein oxidation: different oxidants give rise to variable yields of bound and released carbonyl products. Free Radic Biol Med. 2004; 36: 1175–1184. 10.1016/j.freeradbiomed.2004.02.017 [DOI] [PubMed] [Google Scholar]
- 28.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007; 47: 143–183. 10.1146/annurev.pharmtox.47.120505.105122 [DOI] [PubMed] [Google Scholar]
- 29.Mok HJ, Shin H, Lee JW, Lee GK, Suh CS, Kim KP, et al. Age-associated lipidome changes in metaphase II mouse oocytes. PLoS ONE. 2016; 11(2): e0148577 10.1371/journal.pone.0148577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kikuchi K, Naito K, Noguchi J, Kaneko H, Tojo H. Maturation/M-phase promoting factor regulates aging of porcine oocytes matured in vitro. Cloning Stem Cells. 2002; 4(3): 211–222. 10.1089/15362300260339494 [DOI] [PubMed] [Google Scholar]
- 31.Tarin JJ, Perez-Albala S, Perez-Hoyos P, Cano A. Postovulatory aging of oocytes decreases reproductive fitness and longevity of offspring. Biol Reprod. 2002; 66: 495–499. [DOI] [PubMed] [Google Scholar]
- 32.Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, et al. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet. 2004; 13(19): 2263–2278. 10.1093/hmg/ddh241 [DOI] [PubMed] [Google Scholar]
- 33.Horvath L, Tamas G. Common Carp, part 1: mass production of eggs and early fry 1st ed Rome: Food and Agriculture Organization of the United Nations; 1985. [Google Scholar]
- 34.Komrakova M, Holtz W. Factors responsible for successful chilled storage of unfertilized rainbow trout (Oncorhynchus mykiss) eggs. Aquaculture. 2009; 286: 156–163. [Google Scholar]
- 35.Babiak I, Dabrowski K. Refrigeration of rainbow trout gametes and embryos. J Exp Zool A Comp Exp Biol. 2003; 300(2): 140–151. 10.1002/jez.a.10319 [DOI] [PubMed] [Google Scholar]
- 36.Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007; 23(10): 1289–1291. 10.1093/bioinformatics/btm091 [DOI] [PubMed] [Google Scholar]
- 37.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012; 40(15): e115 10.1093/nar/gks596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985; 150: 76–85. [DOI] [PubMed] [Google Scholar]
- 39.Claiborne A. Catalase activity In: Greenwall RA, editor. CRC handbook of methods for oxygen radical research. Boca Raton: CRC Press; 1985. pp. 283–284. [Google Scholar]
- 40.Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972; 46: 849–854. [DOI] [PubMed] [Google Scholar]
- 41.Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney—possible implications in analgesic nephropathy. Biochem Pharmacol. 1984; 33: 1801–1807. [DOI] [PubMed] [Google Scholar]
- 42.Cribb AE, Leeder JS, Spielberg SP. Use of a microplate reader in an assay of glutathione-reductase using 5,5'-dithiobis(2-nitrobenzoic acid). Anal Biochem. 1989; 183: 195–196. [DOI] [PubMed] [Google Scholar]
- 43.Li P, Li ZH, Dzyuba B, Hulak M, Rodina M, Linhart O. Evaluating the impacts of osmotic and oxidative stress on common carp (Cyprinus carpio L.) sperm caused by cryopreservation techniques. Biol Reprod. 2010; 83: 852–858. 10.1095/biolreprod.110.085852 [DOI] [PubMed] [Google Scholar]
- 44.Oliver C, Ahn B, Moerman E, Goldstein S, Stadtman E. Age-related changes in oxidized proteins. J Biol Chem. 1987; 262: 5488–5491. [PubMed] [Google Scholar]
- 45.Lord T, Aitken RJ. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction. 2013; 146: 217–227. [DOI] [PubMed] [Google Scholar]
- 46.Esponda P, Diaz H. Age-induced apoptosis and activation of heat shock protein HSP70 in mammalian oocytes. Signaling and Apoptosis. 2006; PP–184a. [Google Scholar]
- 47.Boerjan ML, de Boer P. First cell cycle of zygotes of the mouse derived from oocytes aged postovulation in vivo and fertilized in vivo. Mol Reprod Dev. 1990; 25: 155–163. 10.1002/mrd.1080250208 [DOI] [PubMed] [Google Scholar]
- 48.Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod. 1998; 13: 998–1002. [DOI] [PubMed] [Google Scholar]
- 49.Samarin AM, Sampels S, Policar T, Rodina M, Hematyar N, Samarin AM. mRNA abundance changes during in vitro oocyte ageing in African catfish Clarias gariepinus (Burchell, 1822). Aquac Res. 2018; 49: 1037–1045. [Google Scholar]
- 50.Raz E. The function and regulation of vasa-like genes in germ-cell development. Genome Biol. 2000; 1(3): reviews1017.1–1017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nikolic A, Volarevic V, Armstrong L, Lako M, Stojkovic M. Primordial Germ Cells: Current Knowledge and Perspectives. Stem Cells Int. 2016; 2016: 1741072 10.1155/2016/1741072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saffman E, Lasko P. Germline development in vertebrates and invertebrates. Cell Mol Life Sci. 1999; 55: 1141–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tzung KW, Goto R, Saju JM, Sreenivasan R, Saito T, Arai K, et al. Early depletion of primordial germ cells in zebrafish promotes testis formation. Stem Cell Reports. 2015; 4(1): 61–73. 10.1016/j.stemcr.2014.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu W, Li SZ, Li Z, Wang Y, Li XY, Zhong JX, et al. Complete depletion of primordial germ cells in an All-female fish leads to Sex-biased gene expression alteration and sterile All-male occurrence. BMC Genomics. 2015; 16: 971 10.1186/s12864-015-2130-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma W, Zhang D, Hou Y, Li YH, Sun QY, Sun XF, et al. Reduced expression of MAD2, BCL2, and MAP kinase activity in pig oocytes after in vitro aging are associated with defects in sister chromatid segregation during meiosis II and embryo fragmentation after activation. Biol Reprod. 2005; 72: 373–383. 10.1095/biolreprod.104.030999 [DOI] [PubMed] [Google Scholar]
- 56.Xu Z, Abbott A, Kopf GS, Schultz RM, Ducibella T. Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5-triphosphate sensitivity. Biol Reprod. 1997; 57: 743–750. [DOI] [PubMed] [Google Scholar]
- 57.Takai Y, Matikainen T, Juriscova A, Kim MR, Trbovich AM, Fujita E, et al. Caspase-12 compensates for lack of caspase-2 and caspase-3 in female germ cells. Apoptosis. 2007; 12: 791–800. 10.1007/s10495-006-0022-z [DOI] [PubMed] [Google Scholar]
- 58.Perez GI, Juriscova A, Matikainen T, Morityama T, Kim MR, Takai Y, et al. A central role for ceramide in the age-related acceleration of apoptosis in the female germline. FASEB J. 2005; 19: 860–862. 10.1096/fj.04-2903fje [DOI] [PubMed] [Google Scholar]
- 59.Fujino Y, Ozaki K, Yamamasu S, Ito F, Matsuoka I, Hayashi E, et al. DNA fragmentation of oocytes in aged mice. Hum Reprod. 1996; 11: 1480–1483. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi T, Igarashi H, Amita M, Hara S, Matsuo K, Kurachi H. Molecular mechanism of poor embryo development in postovulatory aged oocytes: Mini review. J Obstet Gynaecol Res. 2013; 39: 1431–1439. 10.1111/jog.12111 [DOI] [PubMed] [Google Scholar]
- 61.Gordo AC, Rodrigues P, Kurokawa M. Intracellular calcium oscillations signal apoptosis rather than activation in in vitro aged mouse eggs. Biol Reprod. 2002; 66: 1828–1837. [DOI] [PubMed] [Google Scholar]
- 62.Li M, Baumeister P, Roy B, Phan T, Foti D, Luo S, et al. ATF6 as a transcription activator of the endoplasmic reticulum stress element: thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Mol Cell Biol. 2000; 20: 5096–5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cleary ML, Smith SD, Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986; 47 (1): 19–28. [DOI] [PubMed] [Google Scholar]
- 64.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984; 226 (4678): 1097–1099. [DOI] [PubMed] [Google Scholar]
- 65.Houseweart MK, Pennacchio LA, Vilaythong A, Peters C, Noebels JL, Myers RM. Cathepsin B but not cathepsins L or S contributes to the pathogenesis of Unverricht-Lundborg progressive myoclonus epilepsy (EPM1). J Neurobiol. 2003; 56(4): 315–27. 10.1002/neu.10253 [DOI] [PubMed] [Google Scholar]
- 66.Kågedal K, Johansson U, Ollinger K. The lysosomal protease cathepsin D mediates apoptosis induced by oxidative stress. FASEB J. 2001; 15: 1592–1594. [DOI] [PubMed] [Google Scholar]
- 67.Butt AJ, Firth SM, Baxter RC. The IGF axis and programmed cell death. Immunol Cell Biol. 1999; 77: 256–262. 10.1046/j.1440-1711.1999.00822.x [DOI] [PubMed] [Google Scholar]
- 68.Huang JC, Yan LY, Lei ZL, Miao YL, Shi LH, Yang JW, et al. Changes in histone acetylation during postovulatory aging of mouse oocyte. Biol Reprod. 2007; 77: 666–670. 10.1095/biolreprod.107.062703 [DOI] [PubMed] [Google Scholar]
- 69.Liang XW, Zhu JQ, Miao YL, Liu JH, Wei L, Lu SS, et al. Loss of methylation imprint of Snrpn in postovulatory aging mouse oocyte. Biochem Biophys Res Commun. 2008; 371(1): 16–21. 10.1016/j.bbrc.2008.03.105 [DOI] [PubMed] [Google Scholar]
- 70.Liang XW, Ge ZI, Wei L, Guo L, Han ZM, Schatten H, et al. The effects of postovulatory aging of mouse oocytes on methylation and expression of imprinted genes at mid-term gestation. Mol Hum Reprod. 2011; 17: 562–567. 10.1093/molehr/gar018 [DOI] [PubMed] [Google Scholar]
- 71.Mayer W, Nivelelai A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature. 2000; 403: 501–502. 10.1038/35000654 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files. The data are fully available in the public repository, Open Scientific Framework (OSF: osf.io/myqd3).


