Abstract
Background:
Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) is an innovative technique for intraperitoneal drug delivery. This study investigates the efficacy of the occupational health safety measures taken to prevent exposition of healthcare workers to the toxic chemotherapy aerosol.
Methods:
Air samples were taken at the working place of the surgeon and of the anesthetist during 2 PIPAC procedures and analyzed for content of platinum by inductively coupled plasma mass spectrometry (ICP-MS). Airborne particles were quantified in real time. Biological monitoring was performed in two surgeons after 50 PIPAC by examining blood samples for possible traces of platinum. Analysis was performed by an independent company.
Results:
Safety measures included tightly closed abdomen, operating room (OR), ventilation meeting requirements of ISO norm 14644–1 class 5, closed aerosol waste system and remote control of PIPAC administration. No traces of platinum were found in the air of the OR (detection limit of 0.0001 mg/filter). No specific rise in particle concentration was detected in the air during the PIPAC procedure, patient closure and removal of the sterile drapes. Blood samples of the surgeons showed no traces of platinum.
Conclusions:
After implementation of adequate safety measures, no signs of environmental contamination or biological exposure of the surgeons were detected during PIPAC.
Keywords: cancer, chemotherapy, occupational health, peritoneum
Introduction
Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) is a new treatment option in patients with peritoneal carcinomatosis (PC). PIPAC has provided encouraging results in the treatment of PC from ovarian, colon and gastric cancer [1–3], and PIPAC may also be used in combination with systemic chemotherapy in selected patients [4].
Employing a new technique for intraperitoneal chemotherapy delivery must be accompanied by relevant safety studies – not only regarding the patients but also addressing the involved health care personnel. To date, one study from the inventor and German pioneer PIPAC center has documented that PIPAC is safe in terms of occupational health aspects [5], but independent confirmation is needed. And since the PIPAC treatment is still undergoing early clinical investigation and further development, focus on the occupational health aspects of this procedure is highly relevant.
In the ideal setting intraperitoneal chemotherapy should be safely delivered without any exposure risk for the involved health care workers in the operating room (OR). Health care worker exposure to cytostatic drugs during PIPAC may result from dermal/ocular contact or inhalation. The carcinogenicity of oxaliplatin is unknown, while data on cisplatin carcinogenicity are more consistent, and oxaliplatin is both chemically and pharmacologically related to cisplatin. Doxorubicin is hazardous to human health by provoking mucosal inflammation, leucopenia, and dilative cardiomyopathy. Additionally, it induces DNA mutation and is carcinogenic to humans [5].
The aim of this study was to measure the presence of airborne platinum particles in the OR during PIPAC and to detect potential traces of cisplatin in the blood of the surgeons.
Materials and methods
This environmental safety study was performed as part of a prospective implementation study on 35 patients receiving PIPAC (“Implementation and evaluation of PIPAC for the treatment of patients with peritoneal carcinomatosis – a feasibility study”). The study complies with the Helsinki Declaration and was approved by The Regional Committees on Health Research Ethics for Southern Denmark (Project-ID: S-20140211, www.clinicaltrials.gov NCT02320448), and all participants – both patients and health care workers – gave informed consent for participation in the study.
The PIPAC procedure
The two surgeons (MG and MBM) performing the PIPAC procedures were certified by the German PIPAC group (Professor Marc Reymond) prior to the first PIPAC procedure. Following translation, adaptation and implementation of a complete clinical PIPAC procedure manual, supplemented by OR simulation tests, the first procedures were performed under the supervision of an international PIPAC expert. Following steps were taken to minimize the risk of health care workers: only certified PIPAC surgeons were involved, adherence to a pre-defined procedure-specific manual was mandatory and preparation of the chemotherapy was performed at the hospital pharmacy. Proper personal protective equipment was essential, including covering of the floor beneath the micropump, protective barrier garments, glasses, surgical aerosol masks and two pairs of gloves.
PIPAC has been previously described [6–8], but in brief, PIPAC is as a laparoscopy controlled administration of pressurized intraperitoneal chemotherapy. PIPAC is repeated every 4–6 weeks, using oxaliplatin 92 mg/m2 body surface in 150 mL dextrose for patients with PC from colorectal cancer and a combination of cisplatin 7.5 mg/m2 body surface in 150 mL saline and doxorubicin 1.5 mg/m2 body surface in 50 mL for patients with PC of any other origin. Two balloon safety trocars (5 and 12 mm, Applied Medical, Dusseldorf, Germany) are inserted through the abdominal wall, and an intraabdominal pressure of 12 mmHg is obtained by insufflation of normothermic CO2. After evacuation of ascites and mapping of the peritoneum according to Sugarbaker’s Peritoneal Cancer Index (PCI) [9], multiple peritoneal biopsies are performed and the biopsy sites are marked by metal clips, in order to detect histological regression. Before chemotherapy administration, three important precautions minimize the risk of chemotherapy exposure for health care workers. 1) The OR ventilation should comply with the requirements of ISO norm 14644–1 class 5, 2) tightness of the abdomen is documented by flow of maximum 0.1 L CO2/min, and 3) the administration is remote controlled, hence all personnel leave the OR during administration and absorption of chemotherapy and warning panels are present on the OR entrance doors. A micropump (CapnoPen®, Capnomed, Villingendorf, Germany) is connected via a high-pressure line to a standard intravenous high-pressure injector (MEDRAD® Salient Dual Contrast Injector, Bayer HealthCare, Leverkusen, Germany) and inserted through the 12 mm trocar. At a flow-rate of 30 mL/min, with a maximum pressure of 200 PSI, chemotherapy is administered within 5–10 min. After another 25 min of simple diffusion, the chemotherapy aerosol is evacuated via a closed line, through two sequential micro particle filters into the air waste system of the hospital. PIPAC is performed in an OR with continuous air conditioning (room temperature: 20°C, excess pressure: 12 Pascal, relative humidity: 55 %), higher air pressure than the surrounding areas, and doors are closed during the procedures.
Measurement of airborne particles
It was decided that the measurement of airborne concentrations of antineoplastic drugs (i. e. cisplatin) should not be performed before the PIPAC procedures were considered routine in our institution. Thus, at least 50 PIPAC procedures should be performed without major technical or patient-related problems before these measurements were performed.
Analysis of the OR air was performed in two consecutive PIPAC patients treated the same day, both for the third time. The first patient was treated with cisplatin and doxorubicin due to PC from pancreatic cancer. The second patient was treated with oxaliplatin due to PC from colon cancer. The airborne measurements were supplemented by blood samples from the surgeons in order to reveal potential traces of cisplatin exposure.
The particle measurements were performed by a national independent organization (Water and Environment, Life Science, Danish Technological Institute, Aarhus, Denmark). The specialist (PBP) performed both particle measurements and subsequent data analysis without blinding. Two locations for the airborne particle measurements were defined, one at the surgeon position and one at the anesthetist position (Figure 1). Zeroing of the instruments and measurement of background levels were carried out before the patient arrived in the OR. Instrumentation for measuring online particle number concentration (P-Trak, model 8525 from TSI [Minnesota, USA], and Scanning Mobility Particle Sizer, nanoscan SMPS, model 3910 from TSI), and online particle mass concentration (DustTrak DRX, model 8533 from TSI) were placed on two trolleys in order to be able to get the instrument close to the patient while enforcing the sterile conditions at the OR table. Pre-weighed 37 mm cellulose filters in cassettes were set up for dust collection on each trolley. The filter cassette was attached to a pump with 1.9 L/min flow. The detection limit for dust was 0.02 mg/filter.
Figure 1:
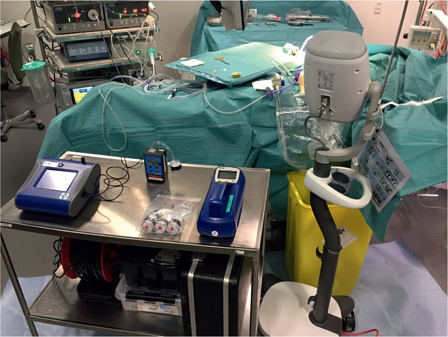
Analysis of the air in the OR during PIPAC.
Metal table with instruments for air analysis during PIPAC at the surgeon’s site.
Analysis for metals collected on the cellulose filters was performed on a Perkin-Elmer (Waltham, Massachusetts, USA) ICP-MS ELAN 5000 and 6100 with FIAS 400 and with an auto-sampler. Samples and blanks were analyzed for content of platinum by inductively coupled plasma mass spectrometry (ICP-MS) with collision cell technology in kinetic energy discrimination mode and with Helium as collision gas. The metals Germanium, Rhodium and Rhenium were used as internal standards. Quantification by ICP-MS was carried out with traceable external standards of the elements. The calibrations were verified with independent traceable control samples. The detection limit for platinum was 0.0001 mg/filter.
Results
Measurement of airborne particles
The measured particle number concentrations in the operation room were low (compared to, e. g., an office), and they remained relatively stable during the whole measurement period in both “patient 1” and “patient 2”. For a short period of time during surgery (using electrosurgical device) in patient 1, a rise in particle number concentration was detected and visible smoke was observed (Table 1 and Figure 2). Particle number concentration rose to approximately 4,400 particles/cm3 at the surgeon position and 3,100 particles/cm3 at the anesthetist position. After that, the particle number concentration dropped steadily until “background level” was reached again.
Table 1:
Particle concentration in the air of the OR during PIPAC.
| Particle number conc. [#/cm3] | Particle mass conc. [mg/m3] | |||
|---|---|---|---|---|
| Avg. | Max. | Avg. | Max. | |
| Patient 1 | ||||
| Position 1 – surgeon | 660 | 4,400 | 0.002 | 0.027 |
| Position 2 – anesthetist | 590 | 3,100 | ||
| Patient 2 | ||||
| Position 1 – surgeon | 580 | 670 | ||
| Position 2 – anesthetist | 660 | 790 | 0.001 | 0.026 |
The table shows the measured particle data when treating “patient 1” and “patient 2”. Number concentration is shown as particle number per cubic centimeter. Mass concentration is shown as milligram per cubic meter. Average and maximum values are shown. Number concentration measurements were performed at both position 1 (surgeon) and 2 (anesthetist) simultaneously, while mass concentration is measured at one position at the time.
Figure 2:
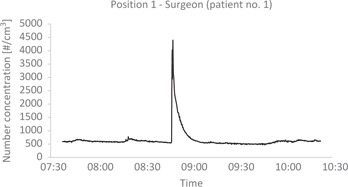
Particle number concentration in the air of the OR during PIPAC.
Particle number concentration shown as number of particles per cubic centimeter as function of time. The figure shows very low number concentrations during most of the period dealing with “patient 1”. One relative high peak was seen at the time surgery was performed.
Particle mass concentration was also very low during the whole period of measurement (Table 1). Spikes were seen in the graphs indicating different events where the staff moved around in the room (Figure 3). No specific rise in either particle concentration was detected during the PIPAC procedure, patient closure and removal of the sterile drapes. Weighing of the filters indicated no collected dust. Analysis by ICP-MS of possible collected platinum dust on filters showed no traces of platinum.
Figure 3:
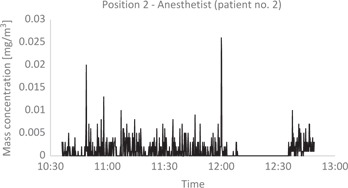
Particle mass concentration in the air of the OR during PIPAC.
Particle mass concentration measured in milligram per cubic meter as function of time. The mass concentrations were low during the whole measurement dealing with “patient 2”. The various peaks indicate different events where staff moved around in the OR. The staff were not in the OR from 11:59 to 12:34 during PIPAC treatment, which made the dust settle.
In two instances in a series of 86 consecutive PIPAC procedures, accidental leakage of the tubing system into the plastic cover occurred due to a combination of human error and poor compliance between the plastic locks on the pump and the high-pressure line.
Blood samples
Blood samples from the two surgeons showed no traces of cisplatin.
Discussion
Apart from the data generated by the German inventors of the PIPAC procedure [5], there are relatively few studies regarding the potential risk of contamination of the OR personnel and environment as a consequence of this procedure. In theory, and despite strict procedure manuals and precautions, the combination of toxic substances and high pressure constitutes a potential risk of exposure. The aim of this study was to measure the presence of airborne platinum particles in the OR during PIPAC and to detect potential traces of cisplatin in the blood of the surgeons. Both OR air analysis and blood tests were designed to detect platinum/cisplatin, as platinum-based chemotherapy is used in all PIPAC procedures.
The main findings of this study were a low number of particles and mass concentrations in the air of the OR during the entire procedure in both patients, and that the filters and blood tests showed no traces of platinum. A short peak in the particle number concentrations during the use of electrosurgical device was expected (i. e. visible smoke), and indirectly indicating the sensitivity of the measuring setup.
Our findings independently confirm the results from the German PIPAC group, where an almost identical PIPAC setup and toxicology analysis (cellulose nitrate filter diameter of 50 mm, flow of 22.5 m3/h, detection limit 0.3 μg/sample) were also unable to detect any signs of contamination [5]. Furthermore, the negative results from the blood tests, independently confirm data from the German group, where blood tests revealed no traces of cisplatin or doxorubicin after 500 PIPAC procedures on five physicians performing PIPAC [10].
We believe that the handling of platinum (and other (diluted) chemotherapeutic agents) within a closed system in a controlled OR environment are the most important factors leading to the present results [11–13]. However, it is interesting to note that using ICP-MS like in the present study, a French group evaluated platinum contamination in an open setting (i. e. HIPEC): No elevated values were detected in the urine, and the air filters were negative, whereas the surgical gloves were heavily contaminated [14]. The latter problem is probably negligible based on the closed system used for PIPAC, but careful disposal of surgical gloves, gowns and drapes into a leak-proof rigid container labeled “cytotoxic agents” [15] is still mandatory in the PIPAC manual. Theoretically, the risk of chemotherapy exposure by OR staff may depend on whether the HIPEC is performed with an open or closed technique [15]. However, the risk of chemotherapy particles in the OR air is probably limited. Even during open HIPEC no study to date has detected chemotherapy particles in the OR [15, 16]. In theory, vaporization of the cytotoxic substance during the PIPAC procedure may increase the risk of inhalation as compared to HIPEC, but so far no data can confirm this.
During the first 86 PIPAC procedures in 31 patients (18 of the patients were treated with cisplatin plus doxorubicin and 13 patients with oxaliplatin) we experienced two cases, where chemotherapy leaked into the plastic cover going from the pump to the patient. The leakages were caused by a combination of human error and poor compliance between the plastic locks on the pump and the high-pressure line. These leakages did not harm the healthcare personnel and there was no environmental contamination since a double safety was installed (a protection sheet was placed beforehand around the tubing to prevent spillage of chemotherapy in case of disconnection). Obviously, critical incidents have to be expected even when adequate preventive measures have been implemented and the events above underline the need for redundant safety measures to guarantee safety even in the case of accidental events. Such critical incidents have to be adequately documented and reported. In the particular case, in order to prevent possible spillage of chemotherapy due to human errors, the tubing system was modified on the basis of the report. Hospitals are now provided by the manufacturer with sterile tubing systems sealed together with the nebulizer, which virtually excludes accidental disconnection.
The German PIPAC group has provided relevant patient safety data [17], and with the present procedure setup and precautions, PIPAC must also be considered a safe procedure as seen from the perspective of the OR personnel. However, since the PIPAC procedure is under continuous development and improvement [18, 19], safety issues regarding both patients and OR personnel must also be monitored in the future.
The low number of procedures and measurements may represent a limitation to the conclusion of the present study, but since our data are in agreement with safety data from other PIPAC studies [5, 10], we find that this study validates the occupational health safety profile of the PIPAC procedure.
Conclusions
The present study confirms previous data on PIPAC occupational health and safety aspects. No platinum was detected in the OR air or in the blood from the surgeons. Thus, when following a strict safety protocol, PIPAC may be performed without any risk of chemotherapy exposure.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
- Tempfer CB, Winnekendonk G, Solass W, Horvat R, Giger-Pabst U, Zieren J, et al. Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: A phase 2 study. Gynecol Oncol 2015;137:223–8. [DOI] [PubMed]
- Demtroder C, Solass W, Zieren J, Strumberg D, Giger-Pabst U, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Colorectal Dis 2016;18:364–71. [DOI] [PubMed]
- Nadiradze G, Giger-Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg 2016;20:367–73. [DOI] [PMC free article] [PubMed]
- Robella M, Vaira M, De Simone M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World J Surg Oncol 2016;14:128. [DOI] [PMC free article] [PubMed]
- Solass W, Giger-Pabst U, Zieren J, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC): occupational health and safety aspects. Ann Surg Oncol 2013;20:3504–11. [DOI] [PMC free article] [PubMed]
- Solass W, Hetzel A, Nadiradze G, Sagynaliev E, Reymond MA. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc 2012;26:1849–55. [DOI] [PubMed]
- Solass W, Kerb R, Murdter T, Giger-Pabst U, Strumberg D, Tempfer C, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 2014;21:553–9. [DOI] [PMC free article] [PubMed]
- Tempfer CB, Celik I, Solass W, Buerkle B, Pabst UG, Zieren J, et al. Activity of pressurized intraperitoneal aerosol chemotherapy (PIPAC) with cisplatin and doxorubicin in women with recurrent, platinum-resistant ovarian cancer: preliminary clinical experience. Gynecol Oncol 2014;132:307–11. [DOI] [PubMed]
- Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol 1998;14:254–61. [DOI] [PubMed]
- Reymond MA. PIPAC pressurized intraperitoneal aerosol chemotherapy – cancer under pressure. Berlin: Walter de Gruyter, 2014:226 p.
- Nygren O, Gustavsson B, Strom L, Eriksson R, Jarneborn L, Friberg A. Exposure to anti-cancer drugs during preparation and administration. Investigations of an open and a closed system. J Environ Monit 2002;4:739–42. [DOI] [PubMed]
- Yoshida J, Tei G, Mochizuki C, Masu Y, Koda S, Kumagai S. Use of a closed system device to reduce occupational contamination and exposure to antineoplastic drugs in the hospital work environment. Ann Occup Hyg 2009;53:153–60. [DOI] [PubMed]
- Vyas N, Yiannakis D, Turner A, Sewell GJ. Occupational exposure to anti-cancer drugs: A review of effects of new technology. J Oncol Pharm Pract 2014;20:278–87. [DOI] [PubMed]
- Konate A, Poupon J, Villa A, Garnier R, Hasni-Pichard H, Mezzaroba D, et al. Evaluation of environmental contamination by platinum and exposure risks for healthcare workers during a heated intraperitoneal perioperative chemotherapy (HIPEC) procedure. J Surg Oncol 2011;103:6–9. [DOI] [PubMed]
- Gonzalez-Moreno S, Gonzalez-Bayon L, Ortega-Perez G. Hyperthermic intraperitoneal chemotherapy: methodology and safety considerations. Surg Oncol Clin North Am 2012;21:543–57. [DOI] [PubMed]
- Naslund Andreasson S, Anundi H, Thoren SB, Ehrsson H, Mahteme H. Is platinum present in blood and urine from treatment givers during hyperthermic intraperitoneal chemotherapy? J Oncol 2010;2010:649719. [DOI] [PMC free article] [PubMed]
- Blanco A, Giger-Pabst U, Solass W, Zieren J, Reymond MA. Renal and hepatic toxicities after pressurized intraperitoneal aerosol chemotherapy (PIPAC). Ann Surg Oncol 2013;20:2311–16. [DOI] [PMC free article] [PubMed]
- Jung do H, Son SY, Oo AM, Park YS, Shin DJ, Ahn SH, et al. Feasibility of hyperthermic pressurized intraperitoneal aerosol chemotherapy in a porcine model. Surg Endosc 2016;30:4258–64. [DOI] [PubMed]
- Kakchekeeva T, Demtroder C, Herath NI, Griffiths D, Torkington J, Solass W, et al. In vivo feasibility of electrostatic precipitation as an adjunct to pressurized intraperitoneal aerosol chemotherapy (ePIPAC). Ann Surg Oncol 2016. Feb 2. [Epub ahead of print]. doi: 10.1245/s10434-016-5108-4. [DOI] [PMC free article] [PubMed]


