Abstract
We evaluated the survival rates and medical expenditure in patients with lung cancer using a nationwide claims database in South Korea. A retrospective observational cohort study design was used, and 2,919 lung cancer patients and their matched controls were included. Medical expenditures were analyzed with the Kaplan-Meier sample average method, and patients were categorized into 4 groups by operation and primary treatment method (i.e. Patients with operation: OP = surgery, OP+CTx/RTx = surgery with anti-cancer drugs or radiotherapy; Patients without operation: CTx/RTx = anti-cancer drugs or radiotherapy, Supportive treatment). The 5-year medical expenditure per case was highest in the OP+CTx/RTx group ($36,013), followed by the CTx/RTx ($23,134), OP ($22,686), and supportive treatment group ($3,700). Lung cancer-related anti-cancer drug therapy was the major cost driver, with an average 53% share across all patients. Generalized linear regression revealed that monthly medical expenditure in lung cancer patients, after adjustment for follow-up month, was approximately 3.1–4.3 times higher than that in the control group (cost ratio for OP = 3.116, OP+CTx/RTx = 3.566, CTx/RTx = 4.340, supportive treatment = 4.157). The monthly medical expenditure at end of life was estimated at $2,139 for all decedents, and approximately a quarter of patients had received chemotherapy in the last 3 months. In conclusion, this study presented the quantified treatment costs of lung cancer on various aspects compared with matched controls according to the treatment of choice. In this study, patients with operation incurred lower lifetime treatment costs than patients with CTx/RTx or supportive treatment, indicating that the economic burden of lung cancer was affected by treatment method. Further studies including both cancer stage and treatment modality are needed to confirm these results and to provide more information on the economic burden according to disease severity.
Introduction
Global lung cancer deaths were estimated at 1.7 million in 2015, contributing to approximately 20% of all cancer-related deaths [1, 2]. As is the case worldwide, lung cancer is the leading cause of cancer deaths in Korea, accounting for 23% of all cancer deaths even though its prevalence share is relatively low at 4.3% of all cancers [3]. This is related to the high mortality rate and delayed diagnosis of lung cancer. In addition, the mortality rate is affected substantially by age, ethnicity, and socioeconomic circumstances [4, 5]. The 5-year net survival rates varied from 2% in Libya to 30% in Japan for patients diagnosed in 2005–2009 [6], and those in Korea were estimated at 16.2% and 25.1% for patients diagnosed in 2001–2005 and 2010–2014, respectively [3].
Recently, expectations have been rising that overall survival rates for lung cancer could increase with the recent emergence of various biological agents for treatment of lung cancer [7, 8]. However, the treatment costs for new biologics are very high if they are prescribed without any restrictions, and their cost-effectiveness should be evaluated to assess the priorities of target patients and to set up the treatment budget [9]. In particular, the economic burden of lung cancer is already very high. In 2004, lung cancer was associated with highest treatment cost at $4.2 billion, making up approximately 20% of the total treatment costs among cancer patients using Medicare in the United States [10], and imposing the greatest burden among all cancers in European countries [11]. Therefore, it is important to analyze current costs for lung cancer and evaluate the areas in which improvement is needed in order to efficiently manage treatment costs in the future.
Currently, various sources are available to analyze the cost of illness, such as claims data, patient records in medical institutes, and survey data [12]. In Korea, most of the population is covered under the national health insurance (NHI) scheme [13]; nationwide sample cohort data sets extracted from the whole claims database are very useful data sources for cost analysis. In a cost study by Shin et al. (2012) [14], they used the NHI claims data for medical expenditure analysis of the major 6 cancers diagnosed in 2006. According to the study, lung cancer patients spent approximately $20,000 for medical costs covered by NHI for 5 years after diagnosis, and the expenditure was highest in regional stage patients [14]. The study broadly covered 6 cancers; however, there is still an unmet need to target lung cancer patients only and to obtain more specific and wider information on the treatment cost for lung cancer. In addition, the outcome of a cost study for lung cancer may also be significantly affected by disease stages, surgical conditions, end-stage care costs, and overall survival [15, 16], and a cost study should consider those aspects. It is also necessary to evaluate whether the cost analysis includes all items or only the diagnostic-specific items, or whether attributable costs or the net difference due to target diseases should be considered in comparison with a control group [12].
Therefore, this study was conducted to assess the following items associated with lung cancer costs in Korea, considering the impact of disease stage by using primary treatment pattern and comparison with a control group; (1) the amount and components of medical expenditure for 5 years after lung cancer diagnosis; (2) the cost ratio between lung cancer patients and matched controls; and (3) the medical expenditure at end of life. This study may help inform cost data needed for public health policy decisions focused on providing cost-effective prevention and treatment of lung cancer.
Methods
Database
The data source for this study was the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS). The NHIS health system covers the entire national population, including social medical insurance (97%) or Medicaid (3%), and provided biennial health screening services for all insured people over 40 years. The NHIS-HEALS database was established by random selection of 10% of patients receiving a medical check-up (40–79 years old) in 2002 and 2003 and followed them until 2015. The database included the results of health screening and data on beneficiary qualifications, including income level, diagnosis, clinical information, and death record. The diagnostic information was recorded using the International Codes of Disease 10th Edition (ICD-10).
Study design and participant selection
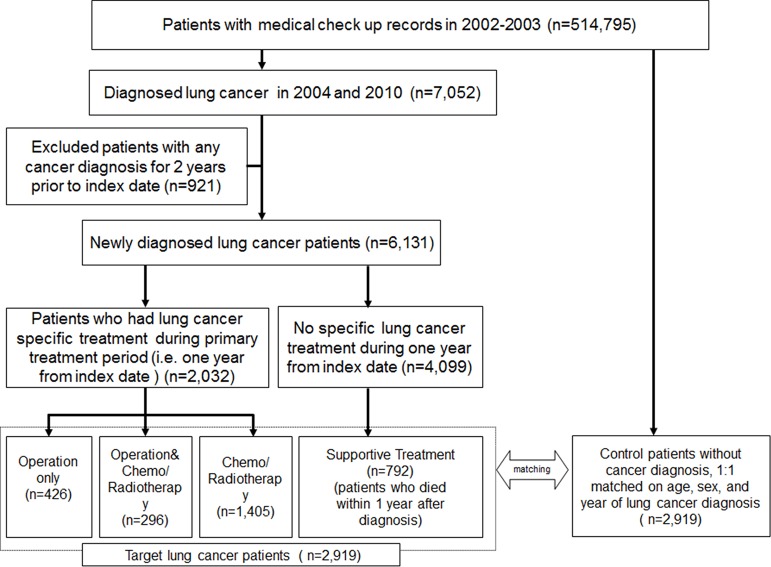
The study was designed as a retrospective observational cohort study to investigate the survival and medical expenditure according to disease stage and primary treatment pattern in patients with lung cancer in comparison with matched controls. A total of 2,919 lung cancer patients were included for this, and the detailed methods to define the target patients and control group were as follows (Fig 1). The population (n = 7,052) with newly diagnosed lung cancer was classified as patients with ICD-10 codes of C33 and C34 between January 2004 and December 2010 to reserve 5-year follow-up periods. The index date for each patient was defined as the first date of lung cancer diagnosis. To rule out the effect of other cancers, we restricted the study population to those who had no diagnostic record of other cancers for 2 years prior to the index date (n = 921). We defined the first year after diagnosis as the primary treatment period and categorized patient groups by treatment (i.e. OP = surgery; OP+CTx/RTx = surgery with anti-cancer drugs or radiotherapy; CTx/RTx = anti-cancer drugs or radiotherapy). During the primary treatment period, there were 4,099 patients who had no record of lung cancer treatment. Of these patients, if death occurred within 1 year after diagnosis, they were allocated to the supportive treatment group (n = 792); the other patients were considered false-positive cases and were excluded. The selected cases were classified into patients with operation treated by OP or OP+Ctx/Rtx and patients without operation treated by CTx/Rtx or supportive treatment. Control patients were selected from the entire population of the database excluding patients with any kind of cancer, with a 1:1 match for age, sex, and year of lung cancer diagnosis using an exacting matching algorithm. The control was given the same index date as the corresponding lung cancer patient.
Fig 1. Patient selection scheme.
Characteristics of study population
Patient characteristics, such as age, sex, comorbidities, income level, and smoking status, were investigated for 1 year prior to the index date. A Charlson comorbidity index (CCI) indicating comorbidity status was constructed by diagnosis codes from the 1 year before the index date according to a previous paper [17]. Income level was categorized into 5 groups (Medicaid & NHI self-employed/employee subscriber low, NHI self-employed subscriber medium, NHI self-employed subscriber high, NHI employee subscriber medium, and NHI employee subscriber high) based on the insurance amount that the beneficiary qualified for. Information on smoking status was collected using questionnaires at the health status check-up. Smoking status was grouped into ever or current smoker, never-smoker, and missing because of a limitation of the database. The definition of surgery, anticancer therapy (chemotherapy or target therapy) related to lung cancer, and radiotherapy in this study is summarized in supporting information (S1 Table. Definition of Lung cancer specific treatment)
Medical expenditure for 5 years and end of life
We calculated the total medical expenditure of lung cancer patients according to the primary treatment pattern. Total expenditure was evaluated for 5 years, because all patients were followed for at least 5 years or until death. To reduce the bias associated with early death or loss to follow-up before 5 years, we used the Kaplan-Meier sample average (KMSA) estimator method [18, 19]. This calculation provides a nonparametric estimate of the average expenditures for patients with variable lengths of follow-up evaluation. The KMSA estimator is calculated using the following formula: where ‘t’ represents the post-index-date month, S(t) is the survival probability, and Ct is the mean actual cost in period t among patients who survived in month t. In this study, we divided the time interval into months. In addition, the distribution of medical cost by treatment category was analyzed for 1 or 5 years from the index date using the same method used for estimating the total medical expenditure. Major treatment was categorized as the use of pharmaceuticals, surgery, radiotherapy, and other treatments. Pharmaceuticals were categorized as anti-cancer chemotherapy, anti-cancer target therapy, and others.
We defined end of life as the last 3 months prior to the death date. The patients who died during the study period and those who survived more than 3 months after the index date were selected and their medical expenditure was analyzed for the cost of end of life.
In this study, we considered only direct medical expenditure; indirect expenditures (i.e. caregiver expenditures, transportation fee, and productivity loss) were not calculated. Total medical expenditure consists of physician visits, medical procedures, and pharmaceutical expenditures covered by NHI, including insurer reimbursement and patient’s co-payments. Pharmaceutical expenditure includes medication cost and dispensing fees from pharmacists.
Statistical analysis
The numeric variables were reported as the mean and standard deviation (SD), and frequency and percentage were used for reporting categorical variables. Survival rate was estimated using the Kaplan-Meier method. Estimated expenditure was represented as the mean expenditure adjusted based on the Consumer Price Index for medical care in 2017 and converted into US dollars based on the exchange rate of 2017 (1$ = 1130.48 Korean Won). To assess the impact of lung cancer according to primary treatment method on medical expenditure, generalized linear regression model (GLM) adjusted for age, sex, CCI score, and income level were used. For adjustments of non-normal distribution of medical expenditure, log link and gamma distributions were selected in the GLM. In addition, we reanalyzed an identical GLM after considering the follow-up month as the offset variable to compare the time effect on medical expenditure. SAS enterprise guide 7.1 (SAS Institute Inc., Cary, NC, USA) and R studio version 1.0.136 (R studio, Inc.) was used to perform all analyses.
Ethics approval and informed consent
The NHIS-HEALS database was retrospectively established in an anonymous format, and the informed consent requirement was waived. The study protocol was approved by the institutional review board of Kyungpook National University (approval number: KNU 2018–0021).
Results
Characteristics of the study population
The characteristics of newly diagnosed lung cancer patients are presented in Table 1. The distribution of patients with OP, OP+CTx/RTx, CTx/RTx, and supportive treatment was 14.6%, 10.1%, 48.1%, and 27.1%, respectively. The mean age of all patients was 67.1 (SD: 9.2) years, and 78.1% were male. While the OP+CTx/RTx group was younger in age, the supportive treatment group had a greater number of older people than the other groups (proportion of patients aged 80 or over was 21% vs. 1–4%). Approximately half (51.1%) the patients were ever or current smokers and the average CCI score was 2.3 (SD: 1.3). The CTx/RTx group had the highest proportion of ever or current smokers (54.8%). The distribution of income level was similar in all groups, except for the supportive treatment group. The supportive treatment group had a greater proportion of patients with a low-income level compared to the other treatment groups.
Table 1. Characteristics of the study population.
| Number of patients (%) | Patients with operation | Patients without operation | Total Cases | Total Controls | ||
|---|---|---|---|---|---|---|
| OP | OP+CTx/RTx | CTx/RTx | Supportive treatment | |||
| Total | 426(14.6) | 296(10.1) | 1405(48.1) | 792(27.1) | 2919(100.0) | 2919(100.0) |
| Males | 313(73.5) | 221(74.7) | 1135(80.8) | 611(77.2) | 2280(78.1) | 2280(78.1) |
| Age at diagnosis | ||||||
| Median (interquartile range) | 64.5(13.0) | 62.0(12.0) | 67.0(12.0) | 74.0(10.0) | 68.0(13.0) | 68.0(13.0) |
| Mean (SD) | 63.7(8.9) | 62.1(8.3) | 65.8(8.6) | 73.2(7.7) | 67.1(9.2) | 67.1(9.2) |
| 40–60 (%) | 127(29.8) | 114(38.5) | 315(22.4) | 43(5.4) | 599(20.5) | 599(20.5) |
| 60–80 (%) | 290(68.1) | 180(60.8) | 1033(73.5) | 580(73.2) | 2083(71.4) | 2083(71.4) |
| 80+ (%) | 9(2.1) | 2(0.7) | 57(4.1) | 169(21.3) | 237(8.1) | 237(8.1) |
| Year at diagnosis | ||||||
| 2004 | 58(13.6) | 30(10.1) | 171(12.2) | 132(16.7) | 391(13.4) | 391(13.4) |
| 2005 | 46(10.8) | 34(11.5) | 195(13.9) | 105(13.3) | 380(13.0) | 380(13.0) |
| 2006 | 44(10.3) | 38(12.8) | 197(14.0) | 105(13.3) | 384(13.2) | 384(13.2) |
| 2007 | 59(13.9) | 54(18.2) | 210(15.0) | 109(13.8) | 432(14.8) | 432(14.8) |
| 2008 | 75(17.6) | 50(16.9) | 195(13.9) | 124(15.7) | 444(15.2) | 444(15.2) |
| 2009 | 72(16.9) | 43(14.5) | 204(14.5) | 98(12.4) | 417(14.3) | 417(14.3) |
| 2010 | 72(16.9) | 47(15.9) | 233(16.6) | 119(15.0) | 471(16.1) | 471(16.1) |
| Smoking statusa | ||||||
| Missing | 5(1.2) | 2(0.7) | 22(1.6) | 17(2.2) | 46(1.6) | 33(1.1) |
| Non-smoker | 216(50.7) | 148(50.0) | 613(43.6) | 404(51.0) | 1381(47.3) | 1801(61.7) |
| Smokerb | 205(48.1) | 146(49.3) | 770(54.8) | 371(46.8) | 1492(51.1) | 1085(37.2) |
| Charlson Comorbidity Index(CCI) score | ||||||
| Median (interquartile range) | 2.0(2.0) | 2.0(2.0) | 2.0(2.0) | 2.0(3.0) | 2.0(2.0) | 2.0(2.0) |
| Mean (SD) | 2.3(1.3) | 2.1(1.2) | 2.3(1.3) | 2.6(1.4) | 2.3(1.3) | 2.1(1.3) |
| 1 | 156(36.6) | 122(41.2) | 520(37.0) | 219(27.7) | 1017(34.8) | 1264(43.3) |
| 2 | 116(27.2) | 81(27.4) | 398(28.3) | 227(28.3) | 822(28.2) | 799(27.4) |
| 3 | 76(17.8) | 55(18.6) | 224(15.9) | 146(18.4) | 501(172) | 394(13.5) |
| 4 | 40(9.4) | 20(6.8) | 134(9.5) | 88(11.1) | 282(9.7) | 235(8.1) |
| 5 | 38(8.9) | 18(6.1) | 129(9.2) | 112(14.1) | 297(10.2) | 227(7.8) |
| Income levelc | ||||||
| 1 | 51(12.0) | 40(13.5) | 233(16.6) | 195(24.6) | 519(17.8) | 523(17.9) |
| 2 | 64(15.0) | 46(15.5) | 255(18.2) | 163(20.6) | 528(18.1) | 468(16.0) |
| 3 | 54(12.7) | 44(14.9) | 150(10.7) | 62(7.8) | 310(10.6) | 359(12.3) |
| 4 | 96(22.5) | 70(23.7) | 360(25.6) | 151(19.1) | 677(23.2) | 653(22.4) |
| 5 | 161(37.8) | 96(32.4) | 407(29.0) | 221(27.9) | 885(30.3) | 916(31.4) |
OP = surgery; OP+CTx/RTx = surgery with anti-cancer drugs or radiotherapy; CTx/RTx = anti-cancer drugs or radiotherapy
a The most recent health screening results based on the time of diagnosis
b Current smoker or ex-smoker
c 1: Medicaid & National Health Insurance (NHI) self-employed/employee subscriber Low; 2: NHI self-employed subscriber Medium; 3: NHI self-employed subscriber High; 4: NHI employee subscriber Medium; 5: NHI employee subscriber High
Survival and medical expenditure for 5 years
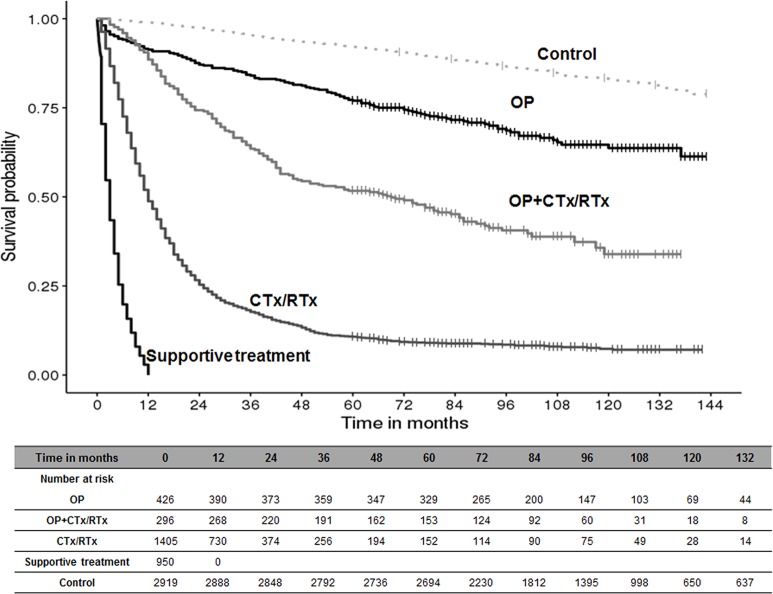
The survival rate of patients receiving different types of primary treatment over 10 years is shown in Fig 2. After diagnosis, the 5-year survival rate was 77.0%, 51.7%, and 10.7% in the OP, OP+CTx/RTx, and CTx/RTx groups, respectively. Patients in the OP group had a significantly higher survival rate than those in the other groups, and the median survival time had not yet been reached during the follow-up period. In the supportive treatment group, the survival rate declined sharply and approximately 50% patients died within 3 months. The average total medical expenditure estimate over 5 years for the lung cancer patients was $19,054 per case (Table 2). The 5-year medical expenditure per case in the OP+CTx/RTx group was the highest ($36,013), followed by the CTx/RTx group ($23,134), the OP group ($22,686), and the supportive treatment group ($3,700). Approximately 53% of the 5-year medical expenditure in all patients was associated with lung cancer-related anti-cancer therapy (Table 3). The distribution of medical expenditure was quite different according to primary treatment during the first year. The distribution of anti-cancer therapy in the CTx/RTx group was higher than all other groups in the first year, as expected. The OP group and supportive treatment group did not use anti-cancer therapy in the first year according to the definition; however, if they survived beyond 1 year, they gradually received anti-cancer therapy (15.4% for the OP group over 5 years). Surgery costs in the OP group accounted for approximately 29.6% of costs in the first year and for 13.7% of the 5-year total cost.
Fig 2. Survival rate by primary treatment pattern.
OP = surgery; OP+CTx/RTx = surgery with anti-cancer drugs or radiotherapy; CTx/RTx = anti-cancer drugs or radiotherapy.
Table 2. Total medical expenditure for 5 years in lung cancer patients.
| Period | Survival rate (%) | Average cumulative cost ($) | ||
|---|---|---|---|---|
| Total cases (n = 2,919) | 1 Year | 45.8% | 11,273 | |
| 2 Year | 32.4% | 14,608 | ||
| 3 Year | 27.2% | 16,573 | ||
| 4 Year | 23.8% | 18,045 | ||
| 5 Year | 21.7% | 19,054 | ||
| Patients with operation (n = 722) | OP (n = 426) | 1 Year | 91.3% | 10,286 |
| 2 Year | 87.1% | 13,708 | ||
| 3 Year | 84.0% | 16,573 | ||
| 4 Year | 81.2% | 19,590 | ||
| 5 Year | 77.0% | 22,686 | ||
| OP+CTx/RTx (n = 296) | 1 Year | 88.5% | 17,560 | |
| 2 Year | 74.3% | 23,989 | ||
| 3 Year | 63.5% | 29,491 | ||
| 4 Year | 54.4% | 33,386 | ||
| 5 Year | 51.7% | 36,013 | ||
| Patients without operation (n = 2,197) | CTx/RTx (n = 1,405) | 1 Year | 48.8% | 14,805 |
| 2 Year | 25.3% | 19,219 | ||
| 3 Year | 17.7% | 21,233 | ||
| 4 Year | 13.3% | 22,534 | ||
| 5 Year | 10.7% | 23,134 | ||
| Supportive treatment (n = 792) | 1 Year | 0.0% | 3,700 | |
| Total controls (n = 2,919) | 1 Year | 98.9% | 1,374 | |
| 2 Year | 97.5% | 2,955 | ||
| 3 Year | 95.3% | 4,731 | ||
| 4 Year | 93.5% | 6,564 | ||
| 5 Year | 92.1% | 8,438 | ||
OP = surgery; OP+CTx/RTx = surgery with anti-cancer drugs or radiotherapy; CTx/RTx = anti-cancer drugs or radiotherapy
Table 3. The distribution of medical expenditure by treatment for 1 year or 5 years.
| Distribution (%) | Patients with operation | Patients without operation | Total cases | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OP | OP+CTx/RTx | CTx/RTx | Supportive treatment | ||||||
| 1 year | 5 year | 1 year | 5 year | 1 year | 5 year | 1 year | 1 year | 5 year | |
| Pharmaceuticals | 20.3% | 41.0% | 62.0% | 64.9% | 77.2% | 76.1% | 30.8% | 68.3% | 0.0% |
| Anti-cancer chemotherapy | 0.0% | 13.2% | 50.8% | 44.4% | 63.0% | 56.0% | 0.0% | 46.4% | 0.0% |
| Anti-cancer target therapy | 0.0% | 2.2% | 1.1% | 6.6% | 3.3% | 7.5% | 0.0% | 6.7% | 0.0% |
| Others | 20.3% | 25.6% | 10.0% | 13.9% | 10.9% | 12.6% | 30.8% | 15.2% | 0.0% |
| Surgery | 29.6% | 13.7% | 10.8% | 6.5% | 0.0% | 0.0% | 0.0% | 2.7% | 0.0% |
| Radiotherapy | 0.0% | 0.7% | 2.3% | 1.9% | 2.6% | 2.2% | 0.0% | 1.9% | 0.0% |
| Other treatment | 50.1% | 44.6% | 24.9% | 26.7% | 20.2% | 21.6% | 69.2% | 27.1% | 0.0% |
OP = surgery; OP+CTx/RTx = surgery with anti-cancer drugs or radiotherapy; CTx/RTx = anti-cancer drugs or radiotherapy
Impact of lung cancer and primary treatment on medical expenditure
The results of the GLM analysis are presented in Table 4. Apart from the supportive treatment group, lung cancer patients had greater medical expenditure per patient than the control group, and the magnitude of the impact on cost for each treatment group was consistent with the 5-year estimated medical expenditure (cost ratio [CR] for OP = 1.857, CR for OP+CTx/RTx = 2.621, CR for CTx/RTx = 1.464, CR for supportive treatment = 0.266). The monthly medical expenditure in all lung cancer patients based on GLM adjusted for the follow-up month was 3.7 times higher than that in the control group (95%CI: 3.5–4.0). Patients in the CTx/Rtx or supportive treatment group had the highest monthly medical expenditure, was approximately 4.2–4.3 times higher than that of the control group. The OP group had relatively lower monthly medical expenditure per patient than the CTx/RTx group (CR = 3.116 for OP; 95% CI 2.842–3.417 vs. CR = 4.340 for CTx/RTx; 95% CI 3.990–4.720).
Table 4. Results of generalized linear regression analysis on medical expenditure.
| Variable | Total medical expenditure per study perioda | Medical expenditure per monthb | |||||
|---|---|---|---|---|---|---|---|
| Cost ratio | 95% LCL | 95% UCL | Cost ratio | 95% LCL | 95% UCL | ||
| Control (Reference) | 1.000 | 1.000 | |||||
| Lung cancer (all) | 1.395 | 1.312 | 1.483 | 3.739 | 3.483 | 4.012 | |
| Control (Reference) | 1.000 | 1.000 | |||||
| Patients with operation | OP | 1.857 | 1.704 | 2.025 | 3.116 | 2.842 | 3.417 |
| OP+CTx/RTx | 2.621 | 2.359 | 2.914 | 3.566 | 3.193 | 3.983 | |
| Patients without operation | CTx/RTx | 1.464 | 1.364 | 1.572 | 4.340 | 3.990 | 4.720 |
| Supportive treatment | 0.266 | 0.245 | 0.289 | 4.157 | 3.786 | 4.564 | |
LCL: lower confidence limits; UCL: upper confidence limits; OP = surgery; OP+CTx/RTx = surgery with anti-cancer drugs or radiotherapy; CTx/RTx = anti-cancer drugs or radiotherapy
a Effects are shown as adjusted for sex, age, smoking status, CCI score, and income level
b Effects are shown as adjusted for sex, age, smoking status, CCI score, income level, and length of follow-up month
Medical expenditure at end of life
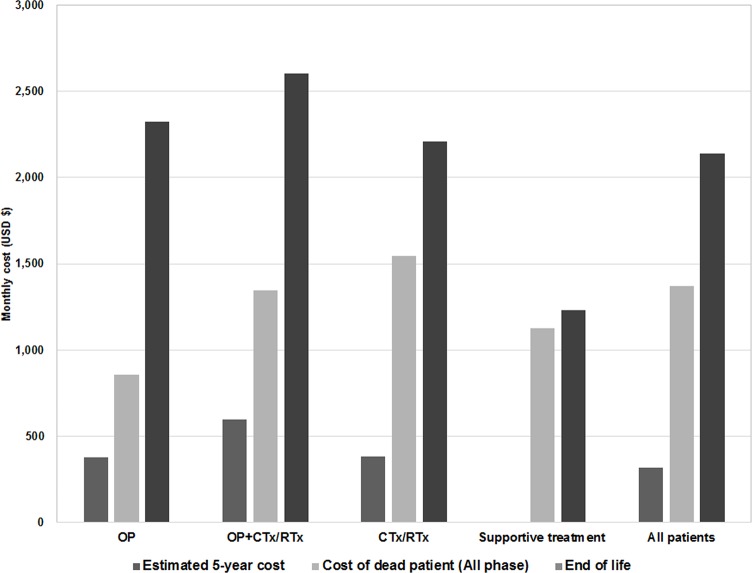
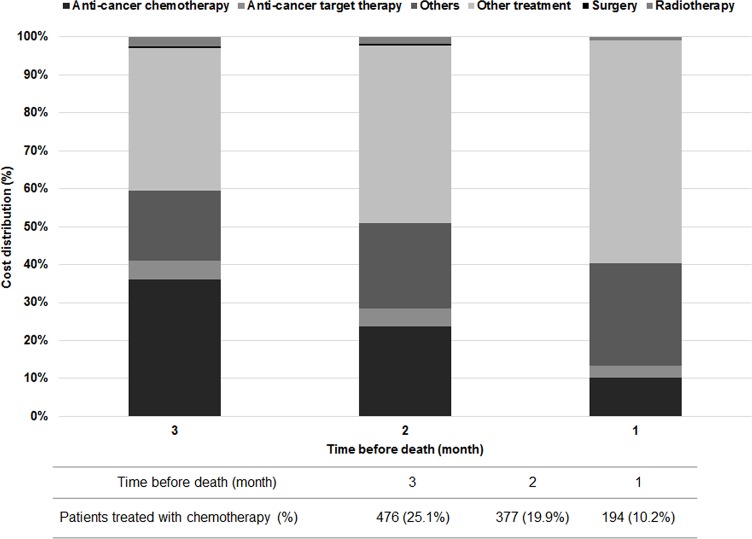
The monthly medical expenditure at end of life was $2,139 for all decedents, and it tended to be much higher than the monthly medical expenditure for overall 5-year cost in all phase patients ($318) (Fig 3). Although the proportion of anti-cancer treatment related to lung cancer in medical expenditure decreased near time of death, it was the greatest cost driver in the end stage. While the proportion of other treatments was gradually increased, that of anti-cancer treatment tended to decrease because the number of anti-cancer therapy users also decreased. The number of people receiving anti-cancer treatment related to lung cancer was 476 patients (25.1%), 377 patients (19.9%), and 194 patients (10.2%) in the last 3 months (Fig 4).
Fig 3. Monthly medical expenditure for end of life by treatment pattern.
OP = surgery; OP+CTx/RTx = surgery with anti-cancer drugs or radiotherapy; CTx/RTx = anti-cancer drugs or radiotherapy.
Fig 4. Distribution of medical expenditure by treatment method for end of life.
Discussion
In this study, we found that lung cancer patients had approximately 4 times higher monthly medical expenditure compared to matched controls during their lifetime. The shorter the life span, the higher the monthly average cost, and it was confirmed that operation significantly affect medical expenditure, as well as survival. Additionally, various cost details for lung cancer were obtained, such as the proportion of drug costs, expenditure differences by treatment pattern and phase, and increased costs and proportions of chemotherapy at the end of life.
The KMSA method was used to analyze costs in this study because the estimates closest to the actual values could be analyzed by including censored data with this method [20]. The total cumulative medical expenditure for 1 year and 5 years was estimated to be approximately $10,000 and $20,000 per person, respectively. The expenditure for 1 year after diagnosis corresponds to approximately 30% of South Korea's GDP per capita of $29,891 in 2017. The absolute costs, as calculated in this study, appear to be lower than those described in studies conducted in other countries. A study analyzing the costs of 1,210 non-small cell lung cancer (NSCLC) patients using claims data from a private health insurance company in the USA showed that the average monthly cost after a diagnosis of lung cancer was $16,577 (SD: 33,350). Approximately 85% of the medical expenditure incurred over 5 years in Korea was spent in 1 month in the USA [16]. In a study comparing the costs of lung cancer among 3 European countries, higher costs compared to Korea were also identified, as the average treatment costs for 2 years were €17,777, €25,063, and €32,500 for the United Kingdom, France, and Germany, respectively [21].
However, each study on the cost of illness has different demographic characteristics, cost scope, and observation period depending on the analysis method and the database used, making it difficult to compare results directly. In addition, the absolute value of lung cancer treatment cost can be greatly influenced by the socioeconomic environment, such as the nation's medical settings, income level, and government subsidization. Thus, we conducted a cost comparison between lung cancer patients and matched controls using GLM analysis and determined the cost ratio between groups. The average monthly costs and total costs were compared as the total cost varies considerably depending on the survival time, and the monthly costs tend to be higher in patients with shorter life spans according to the GLM analysis.
In this study, patients were divided into 4 groups by using primary treatment pattern based on the National Comprehensive Cancer Network guidelines [22]. Patients in the OP group, who can be described as having early stage disease, had a relatively higher expenditure in the initial phase mainly due to surgery; however, the monthly average costs in this group were the lowest among the 4 subgroups, mainly owing to the longer survival period. However, this result may not be applicable if the reimbursement conditions are very different to those in South Korea or the medical cost of surgery is very high. The OP+CTx/RTx group, which is estimated to contain patients with stage 1B–3A, showed the highest expenditure. This group was assumed to have received adjuvant or neo-adjuvant therapy in addition to surgery, and anti-chemotherapy treatments could have increased the total medical expenditure. The CTx/RTx group had the highest cost per month of survival, costing approximately 1.4 times more than the lowest cost group of OP. In a study by Schwarzkopf et al. (2015), the average cost per year survived for the CTx/RTx group was approximately 1.7 times higher than other treatment groups, indicating a similar trend to this study [23]. Since survival is a good endpoint for the effectiveness of cancer treatments, medical costs per month survived could be an indicator of cost-effectiveness. Early diagnosis and surgery of lung cancer has a significant impact on increased survival [24, 25], and the results of this study indirectly demonstrate that early diagnosis is a cost-effective strategy for the management of lung cancer. Our study showed that 27.1% of all participants were not actively treated for lung cancer. Other studies also included groups of patients who received supportive care only, such as 20.5% of patients in a German study involving 17,478 patients with lung cancer[23] and 27.7% of squamous NSCLC patients in a USA study [26]. The median survival of these patients was only a few months; 3 months (Korea), 0.9 months (Germany), and 2 months (USA). It is thought that most patients lost treatment time because of the delayed diagnosis of lung cancer [27], and the treatment had to be stopped because of old age in patients aged 80 years or more (approximately 17% of this study). The relatively high proportion of patients in the supportive treatment group also supports the need for early diagnosis not only to increase the life span but also to achieve cost-effective management of lung cancer.
Drug costs were the highest expenditure component in all subgroups except for the expenditure in the first year of the OP group. These results were consistent with the those of a previous study by Shin et al. (2016) in which 72.6% of total costs were used for drugs [14]. Although there was no expenditure for drug therapy in the first year after diagnosis in the OP group, the drug costs increased to 13.2% in the total 5-year expenditures. This is most likely associated with chemotherapy, which might be initiated in the event of recurrences after surgery. Frequent recurrences increase the cost of chemotherapy and threaten survival, and therefore, a major aim is to prevent recurrences after surgery [28, 29]. The exact proportion of costs resulting from recurrences could not be analyzed in this study, and a detailed analysis of recurrence-related costs should be considered for future studies. Meanwhile, gefitinib was introduced in Korea in 2004, but its usage was limited to second line treatment until 2010; thus, the proportion of patients receiving targeted therapeutic agents might be small in our analysis. Reimbursement coverage was extended for first line therapy in patients with epidermal growth factor receptor mutation from 2011, and new biologic agents were introduced more recently. Therefore, it is expected that there will be a further increase in the cost of drug therapies from 2011. Studies using real-world data considering their costs and effects on survival will be needed to ensure that these expensive agents are affordable and cost-effective compared with conventional agents.
The cost of end of life for patients with cancer is reported to be higher than that of patients without cancer [30]. In this study, the medical expenditure at end of life was approximately 2 times or 7 times higher than the monthly average of the whole observation period for decedents or all patients, respectively. The costs were higher in the CTx/RTx and supportive treatment groups. This might be related to the short survival period and chemotherapies that were implemented until the end of life. This study found that 25.1% of decedents received chemotherapy during the last 3 months of life, and 10.2% of patients received chemotherapy in the last month before their death (Fig 3). The guidelines by the American Society of Clinical Oncology do not recommend chemotherapy for patients with end stage disease and poor performance status [31]. Furthermore, a recent study reported that even patients with a good performance status had poorer quality of life than those who did not receive chemotherapy (odds ratio: 0.34, 95% CI: 0.17, 0.75) [32]. Thus, the use of chemo-agents should be carefully considered as the quality of life and palliative care of the patient must be taken into account at the end of life [33, 34].
This study has several limitations. First, the details on disease stage (i.e. stage I, stage II, stage III, or stage IV) which is the most important prognostic factor for survival were not included owing to lack of information. Instead, patients were categorized by treatment pattern which was determined by several factors including disease stage. Therefore, the study results should be interpreted with caution, because the economic burden of lung cancer differs according to the stage of lung cancer rather than treatment pattern. Second, lung cancer was defined based on ICD-10 codes in the database and there were potential uncertainties in the diagnosis. In addition, NSCLC and SCLC, which have quite different costs and survival rates, could not be separated. Approximately 80–85% of lung cancers in Koran patients are NSCLC [35]; therefore, the results of this study may be more generalizable to NSCLC. Third, the data source for this study only included patients aged 40 years or older with medical records. The age of the target patients may not affect the analysis results significantly as the proportion of lung cancer patients under 40 years is less than 1.5% [36]; however, the medical check-up history could have a small impact on survival outcomes [37]. Nevertheless, we do not believe that selection bias distorted the overall results, and the results of GLM in comparison with controls could compensate for this bias. Forth, cost items might be allocated incorrectly because there was the possibility of misclassification when they were claimed from medical institutes. Lastly, the results of this study did not include patients’ out-of-pocket costs, which were not covered by government insurance and non-medical costs, such as patient care or transportation costs. In Korea, the out-of-pocket costs were assumed to be 15–20% of the total insurance cost for cancer patients [38]. Based on the research by Park et al., lung cancer showed the highest share of out-of-pocket costs among cancers, and direct non-medical costs accounted for approximately 30% of total direct medical expenditure for caregivers, transportation, and complementary and alternative medicine [39]. Considering all these costs, the actual total cost of lung cancer is estimated to be 1.5 times (approximately $30,000) higher than that estimated in this study.
Many studies have been published on the cost of lung cancer; however, few studies compared results between lung cancer patients and matched controls. Our research investigated both survival and cost outcomes and increased costs of lung cancer compared with controls. We aimed to increase the comparability and utility of study results by presenting the cost ratio for both total costs and the average cost per month of survival between target patients and controls. Therefore, these results are applicable not only for Korea but also for other countries.
In conclusion, this study presented various cost details for lung cancer, and confirmed that the economic burden of lung cancer patients was significantly higher than that of matched controls; in addition, it was affected by treatment methods, which was dependent on multiple factors such as stage of the cancer, and overall health. Patients with operation who might be estimated to be at an earlier stage, survived longer, and their lifetime medical expenditures were lower than patients with CTx/RTx or supportive treatment. However, further studies using clinical information would be required to determine the economic burden of lung cancer according to disease stage. In particular, lung cancer screening is to be implemented urgently for high-risk patients considering the high proportion of untreated patients in this study. The societal and economic burden of lung cancer is very high, and therefore, an in-depth study on mortality and costs could support the identification of unmet public health needs and contribute to relieving socioeconomic burdens from cancer.
Supporting information
(DOCX)
Acknowledgments
We thank the Korea National Research Foundation for supporting this research (NRF-2018R1D1A3B07047356). We also thank the National Health Insurance Service for providing data for this study (NHIS-2018-2-101).
Data Availability
Data are available from the Korean National Health Insurance Sharing Service (KNHISS). KNHISS does not allow researchers to provide data personally or share publicly and therefore, the authors cannot provide the data. However, all researchers can access the data in the same manner as the authors upon completing the online data request form. Access to NHIS-NHC data can be achieved from the website of NHISS (https://nhiss.nhis.or.kr) after agreeing to follow the specified research ethics and completing the application process (http://nhiss.nhis.or.kr/bd/ab/bdaba021eng.do). After receiving approval, the researchers can receive the data with a certain fee. The authors did not have any special access privileges to the database.
Funding Statement
HYP received the grant (NRF-2018R1D1A3B07047356) funded by the National Research Foundation of Korea (https://www.nrf.re.kr). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA oncology. 2017;3(4):524–48. Epub 2016/12/06. 10.1001/jamaoncol.2016.5688 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer by World Health Organization. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx Accessed in 1 July 2018. 2012.
- 3.Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2014. Cancer research and treatment: official journal of Korean Cancer Association. 2017;49(2):292–305. Epub 2017/03/11. 10.4143/crt.2017.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Advances in experimental medicine and biology. 2016;893:1–19. Epub 2015/12/17. 10.1007/978-3-319-24223-1_1 . [DOI] [PubMed] [Google Scholar]
- 5.Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: geographical distribution and secular trends. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2008;3(8):819–31. Epub 2008/08/02. 10.1097/JTO.0b013e31818020eb . [DOI] [PubMed] [Google Scholar]
- 6.Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor Characteristics. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2016;11(10):1653–71. Epub 2016/07/02. 10.1016/j.jtho.2016.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manegold C, Adjei A, Bussolino F, Cappuzzo F, Crino L, Dziadziuszko R, et al. Novel active agents in patients with advanced NSCLC without driver mutations who have progressed after first-line chemotherapy. ESMO open. 2016;1(6):e000118 Epub 2018/02/13. 10.1136/esmoopen-2016-000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malhotra J, Jabbour SK, Aisner J. Current state of immunotherapy for non-small cell lung cancer. Translational lung cancer research. 2017;6(2):196–211. Epub 2017/05/23. 10.21037/tlcr.2017.03.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng X, Liu Y, Zhang J, Teng F, Xing L, Yu J. PD-1/PD-L1 checkpoint blockades in non-small cell lung cancer: New development and challenges. Cancer letters. 2017;405:29–37. Epub 2017/07/10. 10.1016/j.canlet.2017.06.033 . [DOI] [PubMed] [Google Scholar]
- 10.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, et al. Cost of care for elderly cancer patients in the United States. Journal of the National Cancer Institute. 2008;100(9):630–41. Epub 2008/05/01. 10.1093/jnci/djn103 . [DOI] [PubMed] [Google Scholar]
- 11.Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. The Lancet Oncology. 2013;14(12):1165–74. 10.1016/S1470-2045(13)70442-X [DOI] [PubMed] [Google Scholar]
- 12.Onukwugha E, McRae J, Kravetz A, Varga S, Khairnar R, Mullins CD. Cost-of-Illness Studies: An Updated Review of Current Methods. PharmacoEconomics. 2016;34(1):43–58. Epub 2015/09/20. 10.1007/s40273-015-0325-4 . [DOI] [PubMed] [Google Scholar]
- 13.Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health policy and planning. 2009;24(1):63–71. Epub 2008/11/14. 10.1093/heapol/czn037 . [DOI] [PubMed] [Google Scholar]
- 14.Shin J-Y, Kim SY, Lee K-S, Lee S-I, Ko Y, Choi Y-S, et al. Costs During the First Five Years Following Cancer Diagnosis in Korea. Asian Pacific Journal of Cancer Prevention. 2012;13(8):3767–72. 10.7314/apjcp.2012.13.8.3767 [DOI] [PubMed] [Google Scholar]
- 15.Cipriano LE, Romanus D, Earle CC, Neville BA, Halpern EF, Gazelle GS, et al. Lung cancer treatment costs, including patient responsibility, by disease stage and treatment modality, 1992 to 2003. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2011;14(1):41–52. Epub 2011/01/08. 10.1016/j.jval.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gildea TR, DaCosta Byfield S, Hogarth DK, Wilson DS, Quinn CC. A retrospective analysis of delays in the diagnosis of lung cancer and associated costs. ClinicoEconomics and outcomes research: CEOR. 2017;9:261–9. 10.2147/CEOR.S132259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care. 2005;43(11):1130–9. . [DOI] [PubMed] [Google Scholar]
- 18.Lin DY, Feuer EJ, Etzioni R, Wax Y. Estimating medical costs from incomplete follow-up data. Biometrics. 1997;53(2):419–34. Epub 1997/06/01. . [PubMed] [Google Scholar]
- 19.Lang K, Lines LM, Lee DW, Korn JR, Earle CC, Menzin J. Lifetime and treatment-phase costs associated with colorectal cancer: evidence from SEER-Medicare data. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2009;7(2):198–204. Epub 2008/10/14. 10.1016/j.cgh.2008.08.034 . [DOI] [PubMed] [Google Scholar]
- 20.Etzioni R, Urban N, Baker M. Estimating the costs attributable to a disease with application to ovarian cancer. Journal of clinical epidemiology. 1996;49(1):95–103. Epub 1996/01/01. . [DOI] [PubMed] [Google Scholar]
- 21.McGuire A, Martin M, Lenz C, Sollano JA. Treatment cost of non-small cell lung cancer in three European countries: comparisons across France, Germany, and England using administrative databases. Journal of medical economics. 2015;18(7):525–32. Epub 2015/03/25. 10.3111/13696998.2015.1032974 . [DOI] [PubMed] [Google Scholar]
- 22.Ettinger DS, Aisner DL, Wood DE, Akerley W, Bauman J, Chang JY, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. Journal of the National Comprehensive Cancer Network: JNCCN. 2018;16(7):807–21. Epub 2018/07/15. 10.6004/jnccn.2018.0062 . [DOI] [PubMed] [Google Scholar]
- 23.Schwarzkopf L, Wacker M, Holle R, Leidl R, Gunster C, Adler JB, et al. Cost-components of lung cancer care within the first three years after initial diagnosis in context of different treatment regimens. Lung cancer. 2015;90(2):274–80. 10.1016/j.lungcan.2015.09.005 . [DOI] [PubMed] [Google Scholar]
- 24.Miller DL, Mayfield WR, Luu TD, Helms GA, Muster AR, Beckler VJ, et al. Community-Based Multidisciplinary Computed Tomography Screening Program Improves Lung Cancer Survival. The Annals of thoracic surgery. 2016;101(5):1864–9. Epub 2016/02/16. 10.1016/j.athoracsur.2015.11.001 . [DOI] [PubMed] [Google Scholar]
- 25.Flehinger BJ, Kimmel M, Melamed MR. The effect of surgical treatment on survival from early lung cancer. Implications for screening. Chest. 1992;101(4):1013–8. Epub 1992/04/01. . [DOI] [PubMed] [Google Scholar]
- 26.Davis KL, Goyal RK, Able SL, Brown J, Li L, Kaye JA. Real-world treatment patterns and costs in a US Medicare population with metastatic squamous non-small cell lung cancer. Lung cancer. 2015;87(2):176–85. 10.1016/j.lungcan.2014.11.002 . [DOI] [PubMed] [Google Scholar]
- 27.Blakely T, Atkinson J, Kvizhinadze G, Wilson N, Davies A, Clarke P. Patterns of cancer care costs in a country with detailed individual data. Medical care. 2015;53(4):302–9. Epub 2015/03/10. 10.1097/MLR.0000000000000330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tremblay L, Deslauriers J. What is the most practical, optimal, and cost effective method for performing follow-up after lung cancer surgery, and by whom should it be done? Thoracic surgery clinics. 2013;23(3):429–36. Epub 2013/08/13. 10.1016/j.thorsurg.2013.05.010 . [DOI] [PubMed] [Google Scholar]
- 29.Vansteenkiste J, De Ruysscher D, Eberhardt WE, Lim E, Senan S, Felip E, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology. 2013;24 Suppl 6:vi89–98. 10.1093/annonc/mdt241 . [DOI] [PubMed] [Google Scholar]
- 30.Langton JM, Reeve R, Srasuebkul P, Haas M, Viney R, Currow D, et al. Health service use and costs in the last 6 months of life in elderly decedents with a history of cancer: a comprehensive analysis from a health payer perspective. British journal of cancer. 2016;114(11):1293–302. Epub 2016/04/27. 10.1038/bjc.2016.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnipper LE, Smith TJ, Raghavan D, Blayney DW, Ganz PA, Mulvey TM, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(14):1715–24. Epub 2012/04/12. 10.1200/jco.2012.42.8375 . [DOI] [PubMed] [Google Scholar]
- 32.Prigerson HG, Bao Y, Shah MA, Paulk ME, LeBlanc TW, Schneider BJ, et al. Chemotherapy Use, Performance Status, and Quality of Life at the End of Life. JAMA oncology. 2015;1(6):778–84. Epub 2015/07/24. 10.1001/jamaoncol.2015.2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi Y, Keam B, Kim TM, Lee SH, Kim DW, Heo DS. Cancer Treatment near the End-of-Life Becomes More Aggressive: Changes in Trend during 10 Years at a Single Institute. Cancer research and treatment: official journal of Korean Cancer Association. 2015;47(4):555–63. 10.4143/crt.2014.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hung YN, Liu TW, Wen FH, Chou WC, Tang ST. Escalating Health Care Expenditures in Cancer Decedents' Last Year of Life: A Decade of Evidence from a Retrospective Population-Based Cohort Study in Taiwan. The oncologist. 2017;22(4):460–9. Epub 2017/02/25. 10.1634/theoncologist.2016-0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.In KH, Kwon YS, Oh IJ, Kim KS, Jung MH, Lee KH, et al. Lung cancer patients who are asymptomatic at diagnosis show favorable prognosis: a korean Lung Cancer Registry Study. Lung cancer. 2009;64(2):232–7. Epub 2008/09/24. 10.1016/j.lungcan.2008.08.005 . [DOI] [PubMed] [Google Scholar]
- 36.National Cancer Information Center. Cancer incidence according to age group. https://www.cancer.go.kr/lay1/S1T639C642/contents.do Accessed in 1 August 2018.
- 37.Rasmussen SR, Thomsen JL, Kilsmark J, Hvenegaard A, Engberg M, Lauritzen T, et al. Preventive health screenings and health consultations in primary care increase life expectancy without increasing costs. Scandinavian journal of public health. 2007;35(4):365–72. Epub 2007/09/06. 10.1080/14034940701219642 . [DOI] [PubMed] [Google Scholar]
- 38.Statistics Korea. National health insurance coverage. http://www.index.go.kr/potal/main/EachDtlPageDetail.do?idx_cd=2763. Accessed in July 2018.
- 39.Park YS, Kim SH, Park SK, Park BJ, Kim YT, Lee SM, et al. Costs for 5-year lung cancer survivors in a tertiary care hospital in South Korea. Lung cancer. 2010;68(2):299–304. Epub 2009/08/04. 10.1016/j.lungcan.2009.06.016 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Data are available from the Korean National Health Insurance Sharing Service (KNHISS). KNHISS does not allow researchers to provide data personally or share publicly and therefore, the authors cannot provide the data. However, all researchers can access the data in the same manner as the authors upon completing the online data request form. Access to NHIS-NHC data can be achieved from the website of NHISS (https://nhiss.nhis.or.kr) after agreeing to follow the specified research ethics and completing the application process (http://nhiss.nhis.or.kr/bd/ab/bdaba021eng.do). After receiving approval, the researchers can receive the data with a certain fee. The authors did not have any special access privileges to the database.