Abstract
Background
The impact of menopause is a consequence of social, physical and mental changes; hormonal changes play an important role in inducing an increased risk of developing depressive symptoms. It is essential to treat mood and vasomotor symptoms and to prevent their onset to promote an improvement in the quality of life, both in terms of clinical and psychological conditions.
Objective
This observational study aims to compare paroxetine and vortioxetine in a sample of patients affected by postmenopausal depression attending the Anxiety and Depression Clinic in terms of: efficacy in determining clinical remission (HDRS ≤ 7) and tolerability; improvement of autonomic and cognitive symptoms.
Methods
39 female outpatients with a diagnosis of Postmenopausal Depression (according to DSM-5 criteria) were evaluated as the routine clinical practice through the following scales: Hamilton Depression Rating Scale (HDRS); Menopause Rating Scale (MRS); Montreal Cognitive Assessment (MoCA); Antidepressant Side-Effect Checklist (ASEC); data from/of baseline, after 8 weeks and 12 weeks were recorded.
Results
Both antidepressants resulted to be effective in clinical remission (HDRS ≤ 7) without statistical differences between the two groups (p = 0.3), although paroxetine showed a faster remission than vortioxetine (p = 0.01). Autonomic symptoms showed a higher improvement in the vortioxetine group (p = 0.002). Paroxetine group referred insomnia and sexual problems while patients taking vortioxetine referred diarrhoea and palpitations. Data show a superiority of cognitive performance in the Paroxetine group (p = 0.005), contrary to what stated in literature.
Conclusions
Data are related to a small sample retrospectively assessed trough a 6-month observation period. Thus, the preliminary results need further research to be confirmed.
Keywords: paroxetine, vortioxetine, post-menopausal depression, autonomic symptoms, cognitive impairment
Introduction
The impact of menopause in women is a consequence of social, physical and mental changes. Although menopause represents a condition linked to physiological aging, it is evident that the consequent physical and mental alterations produce a radical change in women’s life. About 75% of women manifest typical signs and symptoms, which constitute the “climacteric syndrome”; these symptoms seem to be extremely variable from individual to individual and influenced by social, cultural, environmental and psychological factors.1,2 The most common symptoms of the postmenopausal transitions are autonomic symptoms (hot flashes, sweating, palpitations, vertigo, tachycardia), sexual sphere disorders (reduction of libido and vaginal dryness) and psycho-emotional symptoms, such as insomnia, anxiety, depression, asthenia, emotional lability, irritability and apathy.3–7
The onset of psychological symptoms during the menopause transition seems to be related to psychological factors (as the loss of the procreative role and the transformation of external image) and to neurotransmitter alterations linked to the modification of hormone levels. Several studies8,9 describe the neuro-modulation effects of estrogens on serotonergic and noradrenergic systems as a cause of amines dysregulation. Alongside with these, cognitive symptoms are also highlighted, such as loss of concentration and memory; in fact, estrogens modulate synaptic plasticity and neuroprotection and their chronic deficiency reduces the neuronal repair capacity, the number of dendritic spines and the synthesis, deposit and release of neurotransmitters.10–12
Depression peak incidence is estimated between 55 and 74 years,13 and its prevalence is twice in women than in males.14 In modern society, the increase in life expectancy has meant that about a third of women’s life is spent in menopause. Therefore, studies on correlation between menopause and depression acquire primary importance.15 Numerous evidences show that menopause represents a window of vulnerability for the onset of Major Depressive Disorder, both in women with a previous affective disorder, and in women without psychiatric background.16–18
Risk factors for menopausal depression are represented by previous episodes of depression, history of premenstrual syndrome and iatrogenic menopause, but also psychological and social factors such as: negative attitude towards menopause, reduction of social relationships and changes in lifestyle.19–21 Moreover, the presence of major menopausal symptoms, as hot flashes, night sweats and insomnia, represent a confounding factor since they could overlap depression symptoms themselves.22–26
For these reasons, it is widely shared that treating postmenopausal symptoms is essential to gain an improvement in women’s life quality, prevent the onset of Major Depressive Disorder and reduce the related financial and social costs.27,28
The efficacy of antidepressants, particularly Selective Serotonin Reuptake Inhibitors (SSRIs) and Serotonin and Norepinephrine Reuptake Inhibitors (SNRIs), on anxiety and depressive symptoms, but also on vasomotor and cognitive symptoms, is widely documented in professional literature.29–31 Among antidepressants, Paroxetine has proven to be the most effective drug to treat vasomotor and cognitive symptoms in postmenopausal transition, as well as the first non-hormone therapy for vasomotor symptoms approved by the Food and Drug Administration in 2013.32–36
Also in 2013, FDA approved the use of Vortioxetine in the treatment of depressive disorders. Vortioxetine is a so-called serotonin modulator and stimulator with a proven effectiveness both on mood and cognitive symptoms. Compared to the other SSRIs, Vortioxetine seems to present a better tolerability profile, especially for effects poorly tolerated by menopausal women such as loss of libido, weight gain and withdrawal symptoms.37–42
While many authors agree on the antidepressant efficacy of Vortioxetine, there are few studies in literature dealing with the efficacy on menopausal symptoms; preliminary data showed statistically significant efficacy on hot flashes, anxiety, and cognitive complaints43 but the topic needs further analysis. In light of these considerations, this study aims to compare Paroxetine and Vortioxetine in terms of efficacy in: determining clinical remission of affective symptoms (HDRS ≤ 7) and tolerability; improving autonomic symptoms and cognitive impairment.
Materials and Methods
Study Design and Population
In this preliminary observational naturalistic study, 39 female patients in menopausal transition attending the Anxiety and Depression Clinic of the Psychiatry Unit of Varese between April 2017 and January 2018 have been evaluated. Outpatients had to fulfil the following inclusion criteria: age between 45 and 65 years; history of amenorrhea for at least 12 months; presence of depressive symptomatology clinically verified by psychiatric specialist examination; score at the Hamilton Depression Rating Scale (HDRS) ≥ 12; have a prescription of paroxetine or vortioxetine, as a switch from other antidepressants or as a new therapy. Patients’ data were made anonymous obscuring sensitive data in the research to protect the recognizability of the patients. As data were made anonymous and unidentifiable, the Provincial Health Ethical Review Board (Ethics Committee of Insubria – Azienda Socio Sanitaria Territoriale Sette Laghi, Varese, Italy) consulted prior to the beginning of the study, has confirmed that, as it was a retrospective study, it did not need authorization from the Board.
Data collected referred at three evaluation times defined: baseline, T0 (before the start of the treatment), T1 (after 8 weeks), T2 (after 12 weeks). At baseline socio-demographic and clinical data of patients were collected. Socio-demographic variables considered were: age; nationality; current residence; education; working condition; marital status and cohabitation; number of children. Physiological data as age of onset of menopause, any hormone replacement therapy in progress, smoking and alcohol habits, were also collected. Psycho-pathological anamnesis included: investigation of current and previous medical illnesses and therapies; familiarity for depressive disorders or previous depressive episodes; current or previous pharmacological or psychotherapeutic interventions, with particular attention to antidepressant and anxiolytic drugs. The date of antidepressant introduction was considered as baseline. During the therapy the clinicians routinely administer different assessment scales at the time of new therapy introduction and after 8 and 12 weeks of treatment, considering those visits as follow-up. The assessment scales considered are the following: Hamilton Depression Rating Scale (HDRS or HAM-D) for the evaluation of depressive and anxious symptoms; Menopause Rating Scale (MRS) to analyse the typical symptomology of menopause, with particular reference to autonomic symptoms; Montreal Cognitive Assessment (MoCA) to evaluate cognitive performance; Antidepressant Side-Effect Checklist (ASEC), in order to define the tolerability profile of the drug used. The last one was considered for T1 and T2, whereas the others were considered also for the baseline.
Statistical Analysis
Socio-demographic data was described by calculating absolute and relative frequencies for dichotomous or categorical variables, and mean and standard deviation for age at recruitment and at menopause. Patients’ characteristics in the two groups were compared using the Chi-square test, for the categorical variables, and the T-test for the comparison between the age averages. All collected scales were described with mean and standard deviation. T-test was applied to highlight any differences between the mean values in the two groups at each visit. A variance analysis for repeated measures was used to evaluate the effect of the two treatments over time and the possible existence of a different effect over time. The analyses were made both considering the total score of the scales and the sub-scales, if applicable.
Given the number of the sample, the parametric model appears to be sufficiently robust even in case of deviation from the normal distribution, for this reason no transformation in ranks of the scores of the scales was adopted.
The number of treatment-emergent adverse events (TEAE)44 has been evaluated with ASEC; T1 and T2 have been described by reporting the absolute and relative distribution of the severity scale. All tests are considered at a significance level of 0.05. Given the pilot nature of the study no adjustment for multiple tests was adopted. The analyses were carried out with the Statistical Analysis System (SAS) version 9.4 software.
Results
Sociodemographic and Clinical Characteristics
Of the 39 women evaluated, 24 are treated with paroxetine (Par) and 15 with vortioxetine (Vor). All participating women are Italian. Socio-demographic and clinical characteristics of the sample are showed in Table 1. The mean baseline HDRS total score (T0) was 15.5 (SD ± 3.1) in the Par group and 16.2 (SD ± 3.2) in the Vor group, indicating a mild/moderate severity of depressive disease in both groups. Factors such as working conditions, concomitant medical conditions or care of family members with particular problems, which can in many cases negatively impact on the psychophysical stability, are resulted fully comparable in the two groups. The average age of onset of menopause was 47 years in the Par group and 48 years in Vor; most patients were naturally menopausal women in both groups, as shown in Table 1. Globally, the majority of women have reached menopause in a physiological way and only a minority in the Par group is treated with hormone replacement therapy, a condition that does not lead the two groups to differ significantly. No patient presents alcohol or substance abuse and smoking habits are uniform between the two groups. Concerning the psychopathological anamnesis of the two groups it is shown that 80% of women in the Vor group presented a familiar predisposition to depression, compared to 62.5% in the Par group, a difference that appears to be significant (p = 0.02). A previous depressive episode has been referred by 50% of women in the Par group and by 80% of patients in the Vor group. 100% of women in the Vor group had taken an antidepressant therapy in the past versus 25% of patients in the Par group, while the use of benzodiazepines (BDZs), or other psychotropic drugs (as olanzapine and quetiapine) and psychotherapy is homogeneous in the two populations.
Table 1. Socio-demographic and Clinical Characteristics.
| PAR (N = 24) | VOR (N = 15) | p-VALUE* | |
|---|---|---|---|
| Age Average (SD) | 54.8 (5.3) | 54.8 (5-3) | NS |
| Education n (%) | |||
| Degree | 0 (0.0) | 3 (20.0) | |
| High School | 18 (75.0) | 12 (80.0) | 0.02 |
| Middle School | 3 (12.5) | 0 (0.0) | |
| Primary School | 3 (12.5) | 0 (0.0) | |
| Marital status n (%) | |||
| Maiden | 3 (12.5) | 3 (20.0) | |
| Married/living together | 12 (50.0) | 12 (80.0) | 0.02 |
| Widow | 9 (37.5) | 0 (0.0) | |
| Occupation n (%) | |||
| Occasional | 3 (12.5) | 0 (0.0) | NS |
| Stable | 9 (37.5) | 9 (60.0) | |
| Unemployed | 6 (25.0) | 0 (0.0) | |
| Housewife | 3 (12.5) | 3 (20.0) | |
| Retired | 3 (12.5) | 3 (20.0) | |
| Cohabitation n (%) | |||
| Alone | 9 (37.5) | 0 (0.0) | 0.01 |
| With other members | 15 (62.5) | 15 (100.0) | |
| Descendants n (%) | |||
| 0 | 12 (50.0) | 0 (0.0) | |
| 1 | 3 (12.5) | 9 (60.0) | 0.0002 |
| 2 | 6 (25.0) | 6 (40.0) | |
| 3 | 3 (12.5) | 0 (0.0) | |
| Age at menopause Average (SD) | 47.9 (2.9) | 48.2 (3.7) | NS |
| Cause of menopause n (%) | |||
| Physiological | 18 (25.0) | 12 (80.0) | NS |
| Induced | 6 (75.0) | 3 (20.0) | |
| Hormone replacement therapy n (%) | 3 (12.5) | 0 (0.0) | NS |
| Other medical conditions n (%) | 12 (50.0) | 12 (80.0) | NS |
| Medical treatments n (%) | 6 (25.0) | 6 (40.0) | NS |
| Smoke n (%) | |||
| No | 15 (62.5) | 9 (60.0) | NS |
| Ex-smoker | 3 (12.5) | 3 (20.0) | |
| Current smoker | 6 (25.0) | 3 (20.0) | |
| Familiarity for depressive disorders n (%) | 15 (62.5) | 3 (20.0) | 0.02 |
| Previous depressive episodes n (%) | 12 (50.0) | 12 (80.0) | NS |
| Previous ADs use n (%) | 6 (25.0) | 15 (100.0) | <0.0001 |
| Switch from others ADs n (%) | 3 (12.5) | 15 (100.0) | <0.0001 |
| Treatment with BDZs n (%) | |||
| No | 15 (62.5) | 12 (80.0) | NS |
| Introduced | 6 (25.0) | 3 (20.0) | |
| Already in place | 3 (12.5) | 0 (0.0) | |
| Others psychotropic drugs n (%) | |||
| No | 18 (75.0) | 12 (80.0) | NS |
| Introduced | 3 (12.5) | 3 (20.0) | |
| Already in place | 3 (12.5) | 0 (0.0) | |
| Psychotherapy in place n (%) | 3 (12.5) | 3 (20.0) | NS |
*p-value relative to t-test for the comparison between averages and to Chi-square or Fisher’s exact test.
NS: non-significant; ADs: antidepressants; BDZs: benzodiazepines.
Efficacy Results
Regarding the scores of the rating scales at follow-up, a statistically significant difference in the HDRS scores emerged, as shown in Table 2 and in Figure 1. Although both molecules have shown an efficacy in the reduction of both depressive and anxiety symptoms, a statistically significant difference in the control of both symptoms emerged (p = 0.004), showing a major improvement in the Par group.
Table 2. Total Score Averages of Rating Scales.
| PAR | VOR | DIFFERENCE | p-VALUE | |
|---|---|---|---|---|
| HDRS (n = 30) | ||||
| T0 Average (SD) | 15.7 (3.6) | 17.0 (3.0) | −1.3 | 0.30 |
| T1 | 4.7 (2.7) | 7.0 (2.6) | −2.3 | 0.03 |
| T2 | 1.5 (1.7) | 5.3 (3.6) | −3.8 | 0.004 |
| MRS (n = 30) | ||||
| T0 Average (SD) | 13.5 (9.4) | 25.8 (4.5) | −12.3 | <0.0001 |
| T1 | 10.3 (9.6) | 23.5 (8.8) | −13.2 | 0.0007 |
| T2 | 10.8 (12.3) | 18.8 (11.1) | −7.9 | 0.08 |
| MoCA (n = 30) | ||||
| T0 Average (SD) | 24.8 (1.7) | 23.8 (1.5) | 1.08 | 0.09 |
| T1 | 27 (2.3) | 26 (1.3) | 1.0 | 0.18 |
| T2 | 28.2 (0.9) | 25.5 (2.6) | 2.7 | 0.005 |
SD: Standard deviation.
Figure 1.
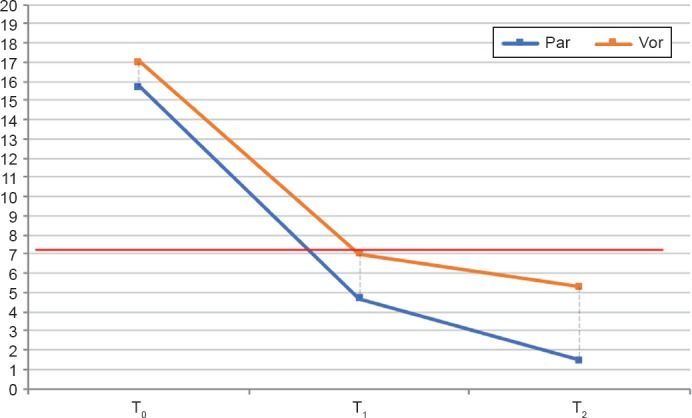
Time Trend of the Total HDRS Scores in the Two Groups (n = 30)
p value (T1): <0.05. p value (T2): <0.01.
Menopausal Symptoms
Concerning menopausal symptoms, the average scores, standard deviations and results of the repeated measures analysis, investigated through MRS scale, are illustrated in Table 2. Figure 2 shows that both treatments are effective in reducing menopausal symptoms over the months, with a significant superiority for vortioxetine even if the Vor group, starts from a higher severity score (p < 0.001).
Figure 2.
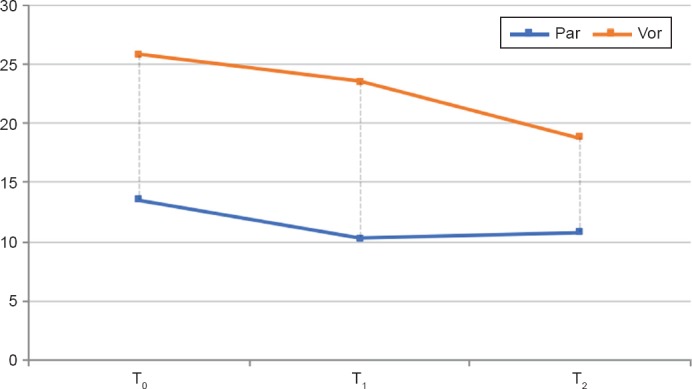
Performance Over Time of the Total MRS Score in the Two Groups
Tolerability
The prevalence of TEAEs has been compared through the ASEC scale scores. Both drugs show a good tolerability profile and no patient discontinued therapy due to the onset of serious side effects. Only three patients (out of the total enrolled group) taking paroxetine referred intolerable gastrointestinal disturbances, with a consequent dose reduction by clinicians to 10 mg/die. Regarding the main TEAEs described in literature, nausea was reported as initially very intense in the first week, but gradually attenuating and disappearing in the following months in the Par group; on the contrary nausea was complained as becoming even more severe in the Vor group (p = 0.002). In the first 8 weeks of treatment in the Par group are reported somnolence, frequent yawnings and from a moderate to severe increase of appetite. The first two effect were characterized by a significant decrease both in terms of frequency and intensity in the following 4 weeks, while weight gain remained significantly high, more pronounced compared to treatment with vortioxetine (p = 0.001). As shown in Figures 3 and 4, a moderate-severe weight gain was referred at T2 by the entire population, described by the patient as extremely difficult to tolerate. In the Vor group, during the first treatment period, the reduction of appetite was detected with moderate intensity, further attenuated at T2. A mild-moderate xerostomia was complained with high frequency in both T1 populations; this effect has been reduced in the Par group but has persisted in the Vor population. Insomnia in the Par group and diarrhoea in the Vor group were respectively reported at T1 but they have been reduced at T2. Headache was detected in both group (16.7% in Par group and 25% in Vor group); this effect was resulted as persisting over time. A very common side effect is constipation, also of high intensity (16.7% in Par group and 25% in Vor group). In the Vor group was detected as tending to decrease over time (0% at T2), while in the Par group has often been maintained, requiring a pharmacological remedy (16.7%). As regard as sexual sphere disorder, libido decrease was described in the Par group, 16.7% of patient reported light intensity and 16.7% moderate intensity symptoms at T1; 33.3% of patient reported light symptoms at T2 while no one reported more symptoms of moderate intensity. In the Vor group, instead, no sexual disfunction was detected at T1 while 25% of patients reported light intensity symptoms at T2. A moderate-severe dizziness and a mild-moderate confusion are also described in both groups, but without statistical significance.
Figure 3.
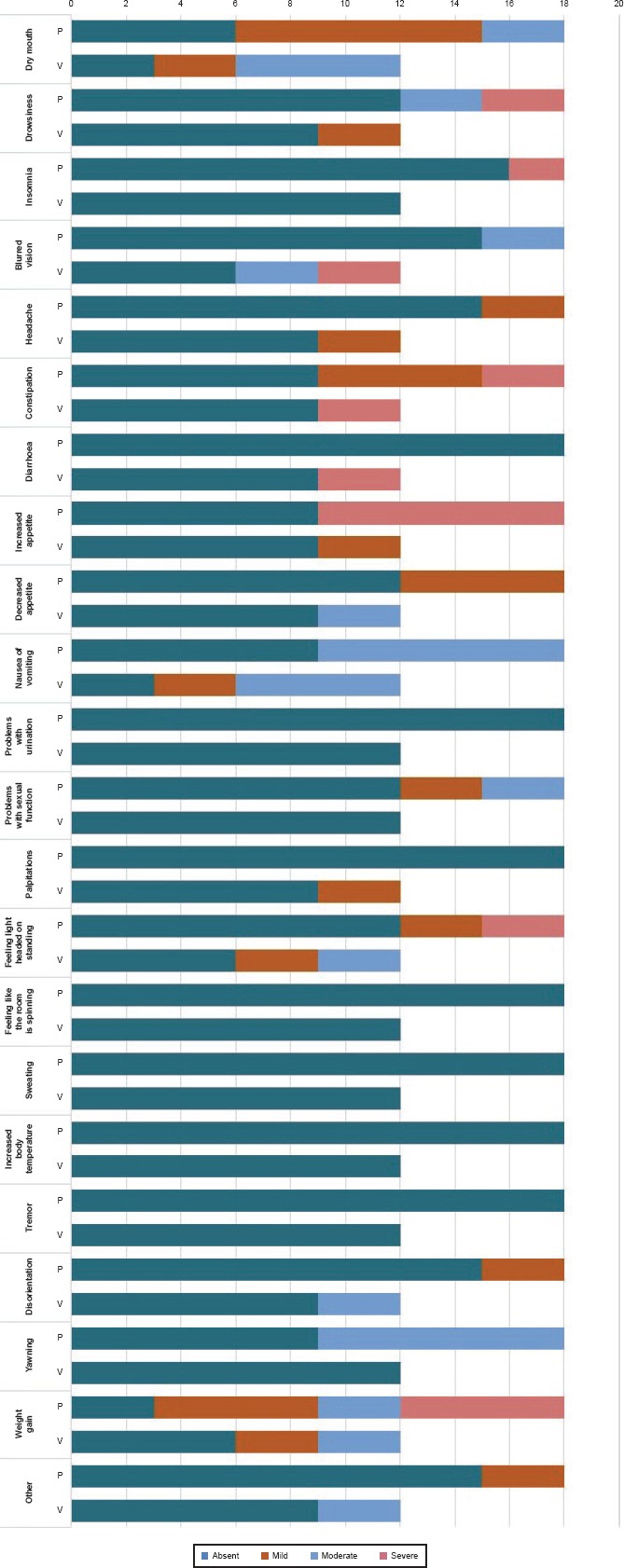
Side Effects in the Two Groups Compared to T1 (n = 30)
Figure 4.
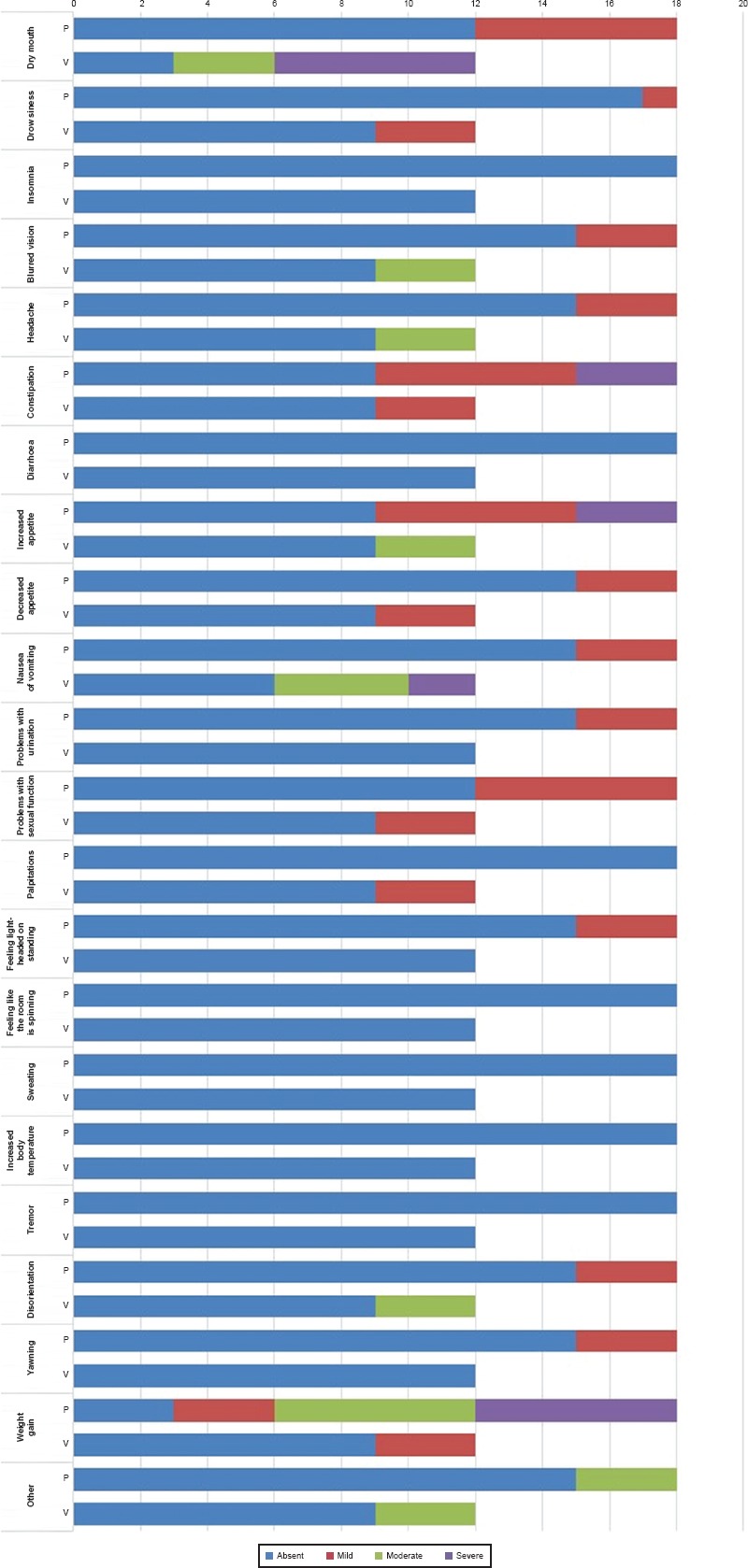
Side Effects in the Two Groups Compared to T2 (n = 30)
Cognitive Impairment
Regarding cognitive impairment, MoCa scale total score shows a homogeneity of the baseline and T1 scores in the two groups, while a significant difference appeared between the two treatments at T2, with better cognitive performance for the Paroxetine group (p value = 0.005), as shown in Table 2.
Discussion and Conclusions
This preliminary study aimed to compare two antidepressants, paroxetine, a well-known molecule commonly used in clinical practice, and vortioxetine, still little used, especially outside of psychiatric context, in terms of efficacy in remission of depressive and anxiety symptoms and tolerability in a population of post-menopausal women diagnosed with depressive disorder. To this purpose, the scores of four clinical scales (HDRS, MRS, MoCA, ASEC), administered over 12 weeks, to 24 patients taking paroxetine and to 15 patients receiving vortioxetine, have been evaluated.
The two groups were globally resulted uniform for both demographic and clinical characteristics. An exception is represented by the psychopharmacological anamnesis: in the Par population 12.5% of patients had used antidepressants in the past, while in the Vor group, all patients had already used an antidepressant; this datum is unanimous with the literature, since vortioxetine is still little used as a first line treatment.45–48 Treatment efficacy was defined on the reduction of the HDRS score over time, considering the remission of the depressive symptoms to the achievement of a score less than or equal to 7. Both treatments were effective in reducing depressive symptomatology over time, showing a significant decrease, although paroxetine showed a more rapid and statistically significant reduction compared to vortioxetine. The efficacy of Paroxetine on anxiety symptoms, both in monotherapy and in combination with benzodiazepines or atypical antipsychotics is widely described in the literature;49–51 conversely data available on vortioxetine are few but seem to be optimistic;52,53 our results support a comparable efficacy of both molecules in reducing these symptoms, although paroxetine reconfirms its superiority over time in getting remission.
Vor group women presented a higher MRS average total score, corresponding to a higher severity of menopausal symptoms. As reported by many authors, both these aspects could have a significant impact on the quality of life’s perception.54,55 Although the final total average scores do not prove a definite remission of menopausal symptoms, the results, in terms of effectiveness in improving symptoms over time, seem to confirm what is already known for paroxetine and confirm for vortioxetine what emerged from preliminary studies.56 Furthermore, vortioxetine seems to demonstrate superior efficacy and a significantly longer time course compared to paroxetine (p value = 0.0007).
With regard to MocA, the results highlighted the effectiveness of both molecules in improving cognitive abilities at the same time. However, vortioxetine seems to present a limitation in this improvement, while paroxetine shows a long-term efficacy over time.
Dealing with TEAEs we could affirm that these are comparable in the two treatments and are likely to depend on individual susceptibility. This data is consistent to the literature confirming that antidepressants tolerability is very variable, and patient’s response depends on individual variability for at least 40%. It can take several months (even years) of clinical trial and error before an effective tolerable antidepressant is found for an individual patient.57
Strenghts and Limitations
The study presents some limitations, as the small sample size due to the short period of observation, and the study design. A larger sample would widen the inclusion and exclusion criteria in order to reduce the variables’ number. It would also be appropriate to re-evaluate some of the tools used, choosing a questionnaire for anxiety. The choice of an adequate scale is, in fact, important in terms of using it as a screening tool or even in common clinical practice.58–60 It could be interesting to investigate the patient’s resilience at T0, in order to identify any differences before the treatment start and in subsequent follow-up to highlight a possible role of therapies on individual adaptability.61,62 As the Vor group starts from a higher score than the Par group, it would be useful to study a larger sample size in order to see if this difference remains, once the confounding variables have been eliminated.
Acknowledgments
None.
References
- 1.Callegari C, Buttarelli M, Cromi A, Diurni M, Salvaggio F, Bolis PF. Female psychopathologic profile during menopausal transition: a preliminary study. Maturitas. 2007;56(4):447–451. doi: 10.1016/j.maturitas.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Callegari C, Buttarelli M, Cromi A, Diurni M, Salvaggio F, Bolis PF. Incidence of depressive symptoms in women attending a menopause clinic: A preliminary study [Incidenza di sintomatologia depressive in donne in cura presso una clinica per la menopausa: uno studio preliminare] Minerva Psichiatrica. 2007;48(1):11–19. [Google Scholar]
- 3.Borkoles E, Reynolds N, Thompson DR, Ski CF, Stojanovska L, Polman RC. The role of depressive symptomatology in peri- and post-menopause. Maturitas. 2015;81(2):306–310. doi: 10.1016/j.maturitas.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Brown L, Bryant C, Brown VM, Bei B, Judd FK. Self-compassion weakens the association between hot flushes and night sweats and daily life functioning and depression. Maturitas. 2014;78(4):298–303. doi: 10.1016/j.maturitas.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Nappi RE, Albani F, Santamaria V, Tonani S, Magri F, Martini E, Chiovato L, Polatti F. Hormonal and psycho-relational aspects of sexual function during menopausal transition and at early menopause. Maturitas. 2010;67(1):78–83. doi: 10.1016/j.maturitas.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 6.de Kruif M, Spijker AT, Molendijk ML. Depression during the perimenopause: A meta-analysis. J Affect Disord. 2016;206:174–180. doi: 10.1016/j.jad.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 7.Mulhall S, Andel R, Anstey KJ. Variation in symptoms of depression and anxiety in midlife women by menopausal status. Maturitas. 2018;108:7–12. doi: 10.1016/j.maturitas.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Freeman EW, Sammel MD, Boorman DW, Zhang R. Longitudinal pattern of depressive symptoms around natural menopause. JAMA Psychiatry. 2014;71(1):36–43. doi: 10.1001/jamapsychiatry.2013.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung SJ, Shin A, Kang D. Menarche age, menopause age and other reproductive factors in association with post-menopausal onset depression: Results from Health Examinees Study (HEXA) J Affect Disord. 2015;187:127–135. doi: 10.1016/j.jad.2015.08.047. [DOI] [PubMed] [Google Scholar]
- 10.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72(5):381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begliuomini S, Casarosa E, Pluchino N, Lenzi E, Centofanti M, Freschi L, Pieri M, Genazzani AD, Luisi S, Genazzani AR. Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. Hum Reprod. 2007;22(4):995–1002. doi: 10.1093/humrep/del479. [DOI] [PubMed] [Google Scholar]
- 12.Vegeto E, Benedusi V, Maggi A. Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front Neuroendocrinol. 2008;29(4):507–519. doi: 10.1016/j.yfrne.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llaneza P, García-Portilla MP, Llaneza-Suárez D, Armott B, Pérez-López FR. Depressive disorders and the menopause transition. Maturitas. 2012;71(2):120–130. doi: 10.1016/j.maturitas.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Jung SJ, Shin A, Kang D. Hormone-related factors and post-menopausal onset depression: results from KNHANES (2010–2012) J Affect Disord. 2015;175:176–183. doi: 10.1016/j.jad.2014.12.061. [DOI] [PubMed] [Google Scholar]
- 15.Birkhäuser M. Depression, menopause and estrogens: is there a correlation? Maturitas. 2002;41(Suppl 1):S3–S8. doi: 10.1016/s0378-5122(02)00009-9. [DOI] [PubMed] [Google Scholar]
- 16.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 17.Judd FK, Hickey M, Bryant C. Depression and midlife: are we overpathologising the menopause? J Affect Disord. 2012;136(3):199–211. doi: 10.1016/j.jad.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Mulhall S, Andel R, Anstey KJ. Variation in symptoms of depression and anxiety in midlife women by menopausal status. Maturitas. 2018;108:7–12. doi: 10.1016/j.maturitas.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 20.Clayton AH, Ninan PT. Depression or menopause? Presentation and management of major depressive disorder in perimenopausal and postmenopausal women. Prim Care Companion J Clin Psychiatry. 2010;12(1) doi: 10.4088/PCC.08r00747blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbs Z, Lee S, Kulkarni J. What factors determine whether a woman becomes depressed during the perimenopause? Arch Womens Ment Health. 2012;15(5):323–332. doi: 10.1007/s00737-012-0304-0. [DOI] [PubMed] [Google Scholar]
- 22.Soares CN. Can depression be a menopause-associated risk? BMC Med. 2010;8:79. doi: 10.1186/1741-7015-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed SD, Ludman EJ, Newton KM, Grothaus LC, LaCroix AZ, Nekhlyudov L, Spangler L, Jordan L, Ehrlich K, Bush T. Depressive symptoms and menopausal burden in the midlife. Maturitas. 2009;62(3):306–310. doi: 10.1016/j.maturitas.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worsley R, Bell R, Kulkarni J, Davis SR. The association between vasomotor symptoms and depression during perimenopause: a systematic review. Maturitas. 2014;77(2):111–117. doi: 10.1016/j.maturitas.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Natari RB, Clavarino AM, McGuire TM, Dingle KD, Hollingworth SA. The bidirectional relationship between vasomotor symptoms and depression across the menopausal transition: a systematic review of longitudinal studies. Menopause. 2018;25(1):109–120. doi: 10.1097/GME.0000000000000949. [DOI] [PubMed] [Google Scholar]
- 26.Muharam R, Setiawan MW, Ikhsan M, Rizkinya HE, Sumapraja K. Depression and its link to other symptoms in menopausal transition. Middle East Fertil Soc J. 2018;23(1):27–30. [Google Scholar]
- 27.Cuadros JL, Fernández-Alonso AM, Cuadros-Celorrio AM, Fernández-Luzón N, Guadix-Peinado MJ, del Cid-Martín N, Chedraui P, Pérez-López FR. Perceived stress, insomnia and related factors in women around the menopause. Maturitas. 2012;72(4):367–372. doi: 10.1016/j.maturitas.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Afshari P, Sedighe M, Mitra T, Mahbobeh K, Mohammadhosain H. Prevalence of Depression in Postmenopausal Women. Jundishapur J Chronic Dis Care. 2015;4(3) [Google Scholar]
- 29.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202–216. doi: 10.1097/01.AOG.0000441353.20693.78. [DOI] [PubMed] [Google Scholar]
- 30.Pehrson AL, Leiser SC, Gulinello M, Dale E, Li Y, Waller JA, Sanchez C. Treatment of cognitive dysfunction in major depressive disorder – a review of the preclinical evidence for efficacy of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors and the multimodal-acting antidepressant vortioxetine. Eur J Pharmacol. 2015;753:19–31. doi: 10.1016/j.ejphar.2014.07.044. [DOI] [PubMed] [Google Scholar]
- 31.Davari-Tanha F, Soleymani-Farsani M, Asadi M, Shariat M, Shirazi M, Hadizadeh H. Comparison of citalopram and venlafaxine’s role in treating sleep disturbances in menopausal women, a randomized, double-blind, placebo-controlled trial. Arch Gynecol Obstet. 2016;293(5):1007–1013. doi: 10.1007/s00404-015-3900-1. [DOI] [PubMed] [Google Scholar]
- 32.Weber L, Thacker HL. Paroxetine: a first for selective serotonin reuptake inhibitors – a new use: approved for vasomotor symptoms in postmenopausal women. Womens Health (Lond) 2014;10(2):147–154. doi: 10.2217/whe.14.3. [DOI] [PubMed] [Google Scholar]
- 33.American College of Obstetricians and Gynecologists. Management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202–216. doi: 10.1097/01.AOG.0000441353.20693.78. [DOI] [PubMed] [Google Scholar]
- 34.Carris N, Kutner S, Reilly-Rogers S. New pharmacological therapies for vasomotor symptom management: focus on bazedoxifene/conjugated estrogens and paroxetine mesylate. Ann Pharmacother. 2014;48(10):1343–1349. doi: 10.1177/1060028014543099. [DOI] [PubMed] [Google Scholar]
- 35.Wei D, Chen Y, Wu C, Wu Q, Yao L, Wang Q, Wang XQ, Yang KH. Effect and safety of paroxetine for vasomotor symptoms: systematic review and meta-analysis. BJOG. 2016;123(11):1735–1743. doi: 10.1111/1471-0528.13951. [DOI] [PubMed] [Google Scholar]
- 36.Ielmini M, Poloni N, Caselli I, Bianchi L, Diurni M, Vender S, Callegari C. Efficacy and Tolerability of Two Different Kinds of Titration of Paroxetine Hydrocloride Solution: an Observational Study. Psychopharmacol Bull. 2018;48(3):33–41. [PMC free article] [PubMed] [Google Scholar]
- 37.Katona CL, Katona CP. New generation multi–modal antidepressants: focus on vortioxetine for major depressive disorder. Neuropsychiatr Dis Treat. 2014;10:349–354. doi: 10.2147/NDT.S39544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Bartolomeis A, Fagiolini A, Maina G. Vortioxetina nel trattamento della depressione maggiore. Riv Psichiatr. 2016;51(6):215–230. doi: 10.1708/2596.26720. [DOI] [PubMed] [Google Scholar]
- 39.Nomikos GG, Tomori D, Zhong W, Affinito J, Palo W. Efficacy, safety, and tolerability of vortioxetine for the treatment of major depressive disorder in patients aged 55 years or older. CNS Spectr. 2017;22(4):348–362. doi: 10.1017/S1092852916000626. [DOI] [PubMed] [Google Scholar]
- 40.Baune BT, Sluth LB, Olsen CK. The effects of vortioxetine on cognitive performance in working patients with major depressive disorder: A short–term, randomized, double-blind, exploratory study. J Affect Disord. 2018;229:421–428. doi: 10.1016/j.jad.2017.12.056. [DOI] [PubMed] [Google Scholar]
- 41.Christensen MC, Loft H, McIntyre RS. Vortioxetine improves symptomatic and functional outcomes in major depressive disorder: A novel dual outcome measure in depressive disorders. Journal of Affective Disorders. 2018;227:787–794. doi: 10.1016/j.jad.2017.11.081. [DOI] [PubMed] [Google Scholar]
- 42.Vieta E, Sluth LB, Olsen CK. The effects of vortioxetine on cognitive dysfunction in patients with inadequate response to current antidepressants in major depressive disorder: A short-term, randomized, double-blind, exploratory study versus escitalopram. J Affect Disord. 2018;227:803–809. doi: 10.1016/j.jad.2017.11.053. [DOI] [PubMed] [Google Scholar]
- 43.Freeman MP, Cheng LJ, Moustafa D, Davies A, Sosinsky AZ, Wang B, Petrillo LF, Hogan C, Cohen LS. Vortioxetine for major depressive disorder, vasomotor, and cognitive symptoms associated with the menopausal transition. Ann Clin Psychiatry. 2017;29(4):249–257. [PubMed] [Google Scholar]
- 44.Callegari C, Ielmini M, Bianchi L, Lucano M, Bertù L, Vender S. Antiepileptic drug use in a nursing home setting: a retrospective study in older adults. Funct Neurol. 2016;31(2):87–93. doi: 10.11138/FNeur/2016.31.2.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brignone M, Diamand F, Painchault C, Takyar S. Efficacy and tolerability of switching therapy to vortioxetine versus other antidepressants in patients with major depressive disorder. Curr Med Res Opin. 2016;32(2):351–366. doi: 10.1185/03007995.2015.1128404. [DOI] [PubMed] [Google Scholar]
- 46.Connolly KR, Thase M. Vortioxetine: a New Treatment for Major Depressive Disorder. Expert Opin Pharmacother. 2016;17(3):421–431. doi: 10.1517/14656566.2016.1133588. [DOI] [PubMed] [Google Scholar]
- 47.Koesters M, Ostuzzi G, Guaiana G, Breilmann J, Barbui C. Vortioxetine for depression in adults. Cochrane Database Syst Rev. 2017;7 doi: 10.1002/14651858.CD011520.pub2. CD011520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thase ME, Danchenko N, Brigone M, Florea I, Diamand F, Jacobsen PL, Vieta E. Comparative evaluation of vortioxetine as a switch therapy in patients with major depressive disorder. Eur Neuropsychopharmacol. 2017;27(8):773–781. doi: 10.1016/j.euroneuro.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Dell’Osso B, Albert U, Atti AR, Carmassi C, Carrà G, Cosci F, Del Vecchio V, Di Nicola M, Ferrari S, Goracci A, Iasevoli F, Luciano M, Martinotti G, Nanni MG, Nivoli A, Pinna F, Poloni N, Pompili M, Sampogna G, Tarricone I, Tosato S, Volpe U, Fiorillo A. Bridging the gap between education and appropriate use of benzodiazepines in psychiatric clinical practice. Neuropsychiatr Dis Treat. 2015;11:1885–1909. doi: 10.2147/NDT.S83130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albert U, Carmassi C, Cosci F, De Cori D, Di Nicola M, Ferrari S, Poloni N, Tarricone I, Fiorillo A. Role and clinical implications of atypical antipsychotics in anxiety disorders, obsessive-compulsive disorder, trauma-related, and somatic symptom disorders: a systematized review. Int Clin Psychopharmacol. 2016;31(5):249–258. doi: 10.1097/YIC.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 51.Diurni M, Baranzini F, Costantini C, Poloni N, Vender S, Callegari C. Metabolic side effects of second generation antipsychotics in drug-naïve patients: a preliminary study. Riv Psichiatr. 2009;44(3):176–178. [PubMed] [Google Scholar]
- 52.Baldwin DS, Florea I, Jacobsen PL, Zhong W, Nomikos GG. A meta–analysis of the efficacy of vortioxetine in patients with major depressive disorder (MDD) and high levels of anxiety symptoms. J Affect Disord. 2016;206:140–150. doi: 10.1016/j.jad.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 53.De Bartolomeis A, Fagiolini A, Maina G. Vortioxetina nel trattamento della depressione maggiore. Riv Psichiatr. 2016;51(6):215–230. doi: 10.1708/2596.26720. [DOI] [PubMed] [Google Scholar]
- 54.Cuadros JL, Fernandez-Alonso AM, Cuadros AM, Fernandez-Luzòn N, Guadix-Peinado MJ, del Cid-Martìn N, Chedraui P, Perez-Lopez P. Perceived stress, insomnia and related factors in women around the menopause. Maturitas. 2012;72(4):367–372. doi: 10.1016/j.maturitas.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 55.DiBonaventura MD, Wagner JS, Alvir J, Whiteley J. Depression, quality of life, work productivity, resource use, and costs among women experiencing menopause and hot flashes: a cross–sectional study. Prim Care Companion CNS Disord. 2012;14(6) doi: 10.4088/PCC.12m01410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng LJ, Choen LS, Davies A, Freeman MP, Hogan C, Moustafa D, Petrillo LF, Sosinsky AZ, Wang B. Vortioxetine for major depressive disorder, vasomotor, and cognitive symptoms associated with the menopausal transition. Ann Clin Psychiatry. 2017;29(4):249–257. [PubMed] [Google Scholar]
- 57.Singh AB. Improved Antidepressant Remission in Major Depression via a Pharmacokinetic Pathway Polygene Pharmacogenetic Report. Clin Psychopharmacol Neurosci. 2015;13(2):150–156. doi: 10.9758/cpn.2015.13.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poloni N, Callegari C, Buzzi A, Aletti F, Baranzini F, Vecchi F, Vender S. The Italian version of ISOS and RSQ, two suitable scales for investigating recovery style from psychosis. Epidemiol Psichiatr Soc. 2010;19(4):352–359. [PubMed] [Google Scholar]
- 59.Poloni N, Diurni M, Buzzi A, Cazzamalli S, Aletti F, Baranzini F. Recovery style, symptoms and psychosocial functioning in psychotic patients: a preliminary study. Rivista di Psichiatria. 2013;48(5):386–392. doi: 10.1708/1356.15065. [DOI] [PubMed] [Google Scholar]
- 60.Vender S, Poloni N, Aletti F, Bonalumi C, Callegari C. Service Engagement: Psychopathology, Recovery Style and Tratments. Psychiatry J. 2014 doi: 10.1155/2014/249852. 249852:6 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Callegari C, Bertù L, Caselli I, Isella C, Ielmini M, Bonalumi C, Ferrario F, Vender S. Resilience in older adults: influence of the admission in nursing home and psychopathology. Neuropsychiatry (London) 2016;6(4):117–123. [Google Scholar]
- 62.Callegari C, Bertù L, Lucano M, Ielmini M, Braggio E, Vender S. Reliability and validity of the Italian version of the 14-item Resilience Scale. Psychol Res Behav Manag. 2016;3(9):277–284. doi: 10.2147/PRBM.S115657. [DOI] [PMC free article] [PubMed] [Google Scholar]


