Abstract
Purpose:
Paclitaxel exposure, specifically the maximum concentration (Cmax) and amount of time the concentration remains above 0.05 μM (Tc>0.05), have been associated with the occurrence of paclitaxel-induced peripheral neuropathy (PN). The objective of this study was to validate the relationship between paclitaxel exposure and PN.
Experimental Design:
Patients with breast cancer receiving paclitaxel 80 mg/m2 × 12 weekly doses were enrolled in an observational clinical study (NCT02338115). Paclitaxel plasma concentration was measured at the end of, and 16–26 hours after, the first infusion to estimate Cmax and Tc>0.05. Patient-reported PN was collected via CIPN20 at each dose, and an 8-item sensory subscale (CIPN8) was used in the primary analysis to test for an association with Tc>0.05. Secondary analyses were conducted using Cmax as an alternative exposure parameter and testing either parameter with a secondary endpoint of the occurrence of PN-induced treatment disruption.
Results:
In the sixty subjects included in the analysis, the increase in CIPN8 during treatment was associated with baseline CIPN8, cumulative dose, and relative dose intensity (p<0.05), but neither Tc>0.05 (p=0.27) nor Cmax (p=0.99). In analyses of the secondary endpoint, cumulative dose (odds ratio (OR)=1.46, 95% confidence interval (CI): 1.18–1.80, p=0.0008) and Tc>0.05 (OR=1.79, 95% CI: 1.06–3.01, p=0.029) or Cmax (OR=2.74, 95% CI: 1.45–5.20, p=0.002) were associated with PN-induced treatment disruption.
Conclusions:
Paclitaxel exposure is predictive of the occurrence of treatment-limiting PN in patients receiving weekly paclitaxel for breast cancer. Studies are warranted to determine whether exposure-guided dosing enhances treatment effectiveness and/or prevents PN in these patients.
Keywords: paclitaxel, peripheral neuropathy, patient-reported outcomes, therapeutic drug monitoring, pharmacokinetics
Introduction
Paclitaxel is highly effective in the treatment of breast cancer, improving overall survival in the adjuvant setting when added to anthracycline-based treatment (1). Paclitaxel can be administered in several doses and schedules, including a weekly 80 mg/m2 infusion for 12 doses that is similarly effective to the traditional four larger doses administered every two or three weeks (2, 3). Paclitaxel-induced peripheral neuropathy (PN), typically sensory dominant and characterized by numbness, tingling, or burning sensation in the fingers and toes that can progress to loss of balance or dexterity (4), is a major dose-limiting toxicity of weekly paclitaxel (5).
Within clinical trials, PN is typically graded by the treating clinician using a 0–4 point National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) grading scale (6), and the incidence of PN with weekly treatment is approximately 25% (grade 2+)(5, 7) or 8–10% (grade 3+)(3, 7). Although duloxetine can be recommended to treat established paclitaxel- and oxaliplatin-induced painful PN(8, 9), there are no effective means to prevent PN (10), or to treat non-painful sensory symptoms (numbness and tingling)(8) . Thus, PN is typically managed by delaying, reducing, and/or discontinuing neurotoxic drug treatments in patients experiencing mild-moderate PN symptoms(11). Collection of patient-reported outcomes (PRO) for PN and other subjective treatment-related adverse effects has been gaining acceptance in clinical trials and patient care (12–14), based on their improved sensitivity and accuracy compared with clinician-graded CTCAE.
In retrospective analyses, patients who experienced PN had higher systemic paclitaxel concentrations and longer durations of systemic exposure, collectively referred to as greater drug exposure (15, 16). The exposure parameter that has been most consistently associated with PN is the time above threshold (Tc>0.05), which is the amount of time (in hours) the patient’s systemic concentration remains above 0.05 μM (15), though other exposure parameters including the maximum concentration (Cmax) have yielded similar associations (16). Confirmation of the relationship between exposure and PN, and identification of the exposure at which 25% of patients experience treatment-limiting PN, would provide an evidence-based exposure target for prospective clinical trials of personalized dosing to determine whether this approach improves treatment efficacy or decreases PN with equivalent efficacy. The objective of this prospective study was to confirm the retrospective data that paclitaxel exposure, as measured by (Cmax) and (Tc>0.05), is predictive of PN development and to define a paclitaxel therapeutic exposure target to be tested in a prospective clinical trial of exposure-guided paclitaxel treatment.
Methods
Patient Enrollment and Collection of Baseline Samples and Clinical Data
Female patients >18 years old diagnosed with stage I-III or oligometastatic breast cancer receiving treatment at the University of Michigan Comprehensive Cancer Center (UMCCC) who were scheduled to receive 12 weekly doses of 80 mg/m2 paclitaxel infused over 1 hour, as per decision with their medical oncologist, were eligible for this observational clinical trial (NCT02338115). Patients were excluded if they received any prior treatment with a neurotoxic chemotherapeutic agent (i.e., taxanes, vinca alkoloids, bortezomib), had existing neuropathy that interfered with activities of daily living, had a known history of hereditary neuropathy including Charcot-Marie-Tooth disease, or were receiving treatment with duloxetine or enrolled in a clinical trial of an agent for neuropathy protection or treatment. Enrolled subjects who withdrew from the study or discontinued paclitaxel treatment for any reason prior to receiving five paclitaxel doses were excluded from analyses and replaced. All patients signed written informed consent and the study was approved by the University of Michigan IRBMed and conducted in accordance with recognized ethical guidelines including the Declaration of Helsinki and Belmont Report.
Eligible patients were enrolled into the observational clinical trial prior to their first dose of paclitaxel. The morning of their first infusion, prior to treatment, patients completed a baseline survey that collected demographic information (i.e. age, self-reported race) and neuropathy-relevant medical information such as diagnosis of diabetes mellitus (self-reported), current alcohol consumption (no or yes [and approximate number of drinks per week]), and pain medications taken regularly. Prior to treatment, blood samples were collected for future pharmacogenetic and pharmacometabolomic analyses and measurement of neuropathy-associated nutrients including hemoglobin A1c (HbA1c), vitamins B12 and D, homocysteine, and folate.
Paclitaxel doses and dates of dosing were prospectively entered into Michart, the medical record used within UMCCC, as standard clinical practice and then retrospectively abstracted by a study coordinator blinded to all other data. Actual dosing information was collected and used to quantify the relative dose intensity, defined by the amount of paclitaxel administered relative to the proportion of the planned cumulative dose. All instances of decreases in the weekly paclitaxel dose (≥10 % in mg/m2), delays between paclitaxel doses (≥ 13 days between doses), or discontinuations of paclitaxel treatment, collectively referred to as treatment disruptions, were documented in the study database. The cause of treatment disruption (neuropathy, other toxicity, scheduling, patient request, other/unknown) was determined by the blinded study coordinator based on manual review of MiChart relying primarily on the clinical notes written at each treatment visit (typically just prior to infusion on treatment weeks 4, 7, and 10). The study coordinator also reviewed all e-mail correspondence and summaries of telephonic communications between the patient and their care team or UMCCC nursing staff, all of which is documented within MiChart.
Neuropathy Data Collection
Neuropathy data were collected using paper copies of the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Chemotherapy-Induced Peripheral Neuropathy (CIPN20). The CIPN20 is a PRO questionnaire that includes 20 questions about symptoms of sensory, motor, and autonomic neuropathy, each of which is graded based on “the extent to which you have experienced these symptoms or problems during the past week” on a scale of 1 (“Not at All”) to 4 (“Very Much”) (17). Each patient completed the CIPN20 the morning of their first paclitaxel dose and received 16 blank paper copies of the CIPN20 with instructions to complete one each week prior to infusion. Extra forms were supplied in case of treatment delays; patients were instructed to complete the CIPN20 regardless of whether they received treatment that week or the prior week, therefore, some patients have multiple CIPN20 forms for a given treatment dose. Completed CIPN20 forms were most often collected by the study team at the patient’s last paclitaxel infusion. Treatment decisions were not influenced by the study procedures and the treating clinician did not have access to the CIPN20 data at any point during treatment.
Paclitaxel is known to cause a primarily sensory neuropathy; therefore, the primary endpoint for this analysis was the sum score of eight sensory items (CIPN8) that quantify numbness, tingling, and burning/shooting pain in the upper and lower extremities, difficulty standing or walking due to loss of feeling in the feet, and difficulty distinguishing between hot and cold water. The ninth item in the sensory subscale, which asks about difficulty hearing, was not included in this analysis as this item has been previously demonstrated to behave distinctly from the rest of the scale in patients treated with paclitaxel and other non-ototoxic neurotoxic chemotherapeutic agents (18, 19).
Pharmacokinetic Data Collection
Blood specimens for pharmacokinetic (PK) data were collected during the first paclitaxel infusion using 6 mL sodium heparin collection tubes. The first specimen was obtained within the last 10 minutes of paclitaxel infusion via peripheral blood draw from the contralateral arm. This sample was used to estimate Cmax. Subjects returned to UMCCC the following day for a second specimen collection 16–26 hours after the start of the paclitaxel infusion via peripheral draw or from a properly flushed port. This sample was used to estimate Tc>0.05. All samples were immediately placed on ice then centrifuged within 10 minutes of collection for 10 minutes at 2,000 x g for fractionation. The plasma was then transferred to a secondary cryotube and stored at −20°C until pharmacokinetic analysis.
Measurement of plasma paclitaxel concentration for all samples in a single batch was conducted by the University of Michigan College of Pharmacy Pharmacokinetics Core using a liquid chromatography/mass spectroscopy assay. Briefly, using a Shimadzu HPLC system, chromatographic separation of tested compound was achieved using a Waters XBridge-C18 column (5 cm × 2.1 mm, 3.5 μm). An AB Sciex QTrap 5500 mass spectrometer equipped with an electrospray ionization source (Applied Biosystems, Toronto, Canada) in the positive-ion multiple reaction monitoring (MRM) mode was used for detection. This assay is highly sensitive (lower limit of quantification=5 ng/mL), with a dynamic range of 5–5000 ng/mL and inter-batch coefficient of variation <15%. Briefly, proteins were removed from plasma samples by precipitation with acetonitrile and centrifugation for 10 minutes at 14,000 rpm. Supernatants were collected and further separated using liquid chromatography with gradient elution containing mobile phases of water and acetonitrile ACN with 0.1% formic acid. Paclitaxel and internal standard docetaxel were detected using multiple reaction monitoring transitions from 854.4 to 286.1 m/z and 808.0 to 226.0 m/z, respectively. Mass spectrometry parameter r optimization was performed using an automated quantitative method provided by the manufacturer. The highest signal intensities were obtained using a declustering potential of 190 V, entrance potential of 14.00 V, collision energy of 21.00 V, and collision cell exit potential of 13 V. Optimized parameters enable quantitation of paclitaxel concentrations over the linear range of 5 – 5000 ng/ml.
Two exposure parameters were used in this analysis. The end of infusion sample concentration is an approximate maximum concentration (Cmax). The next-day concentration, and the amount of time since the beginning of infusion, were used to estimate the time above threshold (Tc>0.05) using a previously published population-pharmacokinetics model (20, 21).
Statistical Analyses
CIPN8 scores were linearly converted to a 0–100 scale with higher scores denoting worse neuropathy, as recommended by the EORTC (17). The a priori defined primary endpoint was the CIPN8 score (0–100) and statistical analyses were conducted using linear mixed effects models. The base model included baseline CIPN8 (0–100), cumulative dose (mg/m2, actual-weight body-surface area [BSA] adjusted), relative dose intensity (proportion of cumulative planned dose received to expected cumulative dose, to account for delays and decreases), and either PK parameter (Cmax or Tc>0.05). Interactions between the PK parameter and cumulative dose were explored and kept in the model if statistically significant. The a priori defined primary analysis was the contribution of Tc>0.05 to the CIPN8 model. The square root of CIPN8 score was used as the outcome to satisfy model assumptions, a random slope was used for cumulative dose, and an unstructured covariance matrix was assumed to model the correlation within participant. A secondary analysis was conducted using Cmax as the independent variable. To compare the magnitude of effect for each exposure parameter, the increase in odds of treatment disruption associated with a one standard-deviation increase in each parameter was estimated.
The occurrence of PN-induced treatment disruption, defined previously as any dose decrease, delay, or discontinuation attributed to peripheral neuropathy identified through blind abstraction from the medical record, was analyzed as a secondary endpoint. Generalized mixed effects models of PN-induced treatment disruption were built including baseline CIPN8 (0–100), cumulative dose (mg/m2), and either PK parameter (Cmax or Tc>0.05). Again interactions between PK parameter and cumulative dose were explored. This base model was then used to estimate the paclitaxel exposure (Cmax or Tc>0.05) that would cause a patient with no baseline neuropathy (CIPN8=0) receiving the standard planned dose (80 mg/m2 × 12 weekly doses) to have a 25% chance of PN-induced treatment disruption by their last dose.
Clinical variables including age (continuous), race (white vs. other), diabetes mellitus (self-reported diagnosis, diagnosis in the medical record, or baseline HbA1c>6.5 vs. no), and any self-reported alcohol intake at baseline (yes vs. no) were each analyzed for univariate associations with CIPN8 and PN-induced treatment disruption, and significant clinical covariates at the p<0.10 level were retained in multivariable models. All analyses were conducted in SAS v9.4 (SAS Institute, Cary, NC).
Results
Patient Demographic Information and Paclitaxel Pharmacokinetics
Sixty-five patients were enrolled onto the clinical trial. Sixty eligible subjects were included in the analysis (Supplementary Figure 1). One patient who withdrew, one who was excluded prior to starting paclitaxel treatment, and three who were excluded after initiating treatment but prior to completing five paclitaxel doses due to protocol violations were excluded. Ninety-three percent of included subjects were Caucasian with an average age of 52 (Table 1). At baseline, 22% (n=13) of patients had diabetes mellitus and 55% (n=33) reported drinking alcohol currently, with a mean of 5.3 drinks per month in these 33 patients. Patients received an average of 11 (SD: 1.96) out of 12 planned paclitaxel doses and the overall relative dose intensity was 95% (standard deviation (SD): 7%)).
Table 1:
Clinical and Pharmacokinetic Data for 60 Patients Included in the Analysis
| N or Mean | % or SD | ||
|---|---|---|---|
| Age | Years | 52.30 | 10.63 |
| Self-Reported Race | Caucasian | 56 | 93.3% |
| Other | 4 | 6.7% | |
| Treatment Regimen* | Prior AC | 56 | 93.3% |
| Concurrent H and/or P | 29 | 48.3% | |
| Current Alcohol Consumption | Yes | 33 | 55.0% |
| Drinks per month (n=33) | 5.33 | 7.11 | |
| Diabetes Mellitus | Prior diagnosis (self-report) | 3 | 5.0% |
| HbA1c (n=51) | 5.95 | 0.74 | |
| Prior diagnosis or HbA1c≥6.5 | 13 | 21.7% | |
| Paclitaxel Exposure | Cmax (n=57, units: ng/mL) | 2364.16 | 664.79 |
| Tc>0.05 (n=59, units; hours) | 10.71 | 2.70 | |
| Paclitaxel Doses | Number of doses received | 11.00 | 1.96 |
| Relative dose intensity | 0.95 | 0.07 | |
| Neuropathy PRO | Number of CIPN20 forms completed | 10.72 | 1.67 |
| % of doses with CIPN20 completed | 99.76% | 18.01% | |
| Baseline CIPN8 (scale: 0–100, n=60) | 3.25 | 6.34 | |
| Maximum CIPN8 during treatment (0–100) | 26.39 | 22.28 | |
| PN-Induced Treatment Disruption** | Paclitaxel dose decrease | 6 | 10.0% |
| Paclitaxel dose delay | 5 | 8.3% | |
| Paclitaxel discontinuation | 8 | 13.3% | |
| Total | 19 | 31.7% | |
| Doses received at time of first disruption | 8.26 | 2.31 |
Abbreviations: A: doxorubicin, C: cyclophosphamide, H: trastuzumab, P: pertuzumab, HbA1c: hemoglobin A1c, PRO: patient-reported outcome, SD: standard deviation, PN: peripheral neuropathy
Prior treatment with doxorubicin/cyclophosphamide (AC) and concurrent treatment with trastuzumab (H) or pertuzumab (P) are not mutually exclusive
These are the first events in each patient, these 19 patients experienced 54 total events
Paclitaxel concentration data were available for 57 subjects at the end of infusion and 59 subjects at 18–26 hours after infusion; missing samples were due to missed sample collection (n=3) or PK assay failure (n=1) (Supplementary Figure 1). The mean Tc>0.05 was 10.71 hours (SD=2.70) and the mean Cmax was 2364.16 ng/mL (SD=664.79). Actual paclitaxel dose (in mg) was not significantly associated with Tc>0.05 (r2=0.03, p=0.16) or Cmax (r2=0.01, p=0.81) and the two exposure parameters were not meaningfully correlated (r2=0.04). Predictors of paclitaxel pharmacokinetic variability including clinical, genomic, and metabolomic factors will be analyzed and reported separately.
Neuropathy at Baseline and during Treatment
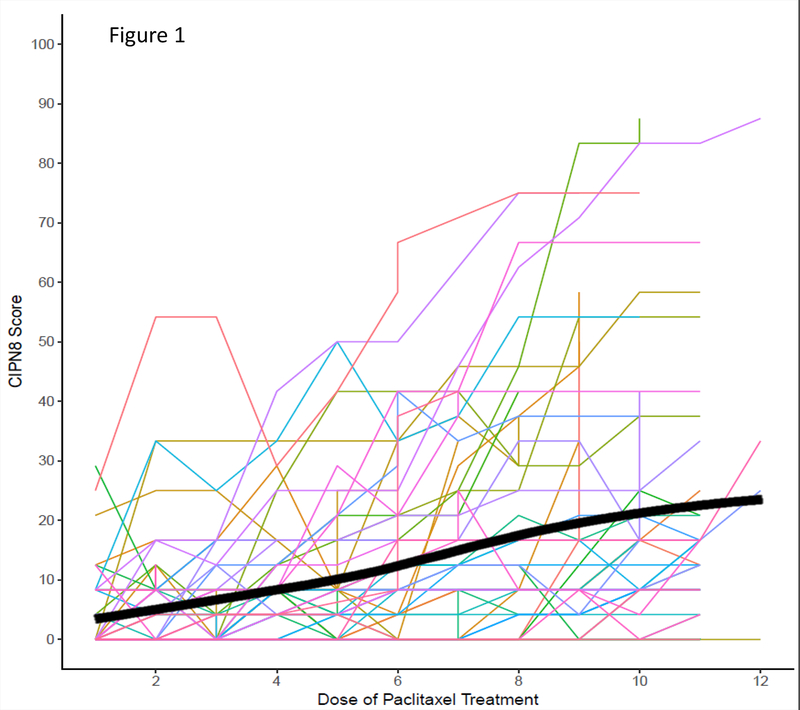
All patients completed the CIPN20 questionnaire at baseline and completed forms were available for the first 11 paclitaxel doses for most patients, since forms were collected during the 12th paclitaxel infusion. Greater than 99% of received paclitaxel doses had a corresponding CIPN20 completed. At baseline, patients reported extremely low levels of neuropathy (mean CIPN8=3.25 [scale 0–100], SD: 6.34), with 69.5% reporting a baseline CIPN8 score of 0. The mean CIPN8 rose gradually with continued treatment, as shown in Figure 1. The maximum CIPN8 reported at any point in treatment ranged from 0–87.50 (mean=26.39, SD: 22.28).
Figure 1: Increase in Self-Reported Neuropathy during Paclitaxel Treatment:
Self-reported sensory peripheral neuropathy (CIPN8) gradually increased from baseline to the end of treatment in the overall patient cohort, as expected. The thick black line represents the smoothed average of CIPN8 at that dose of treatment. Note that patients who discontinued treatment were assigned their final CIPN8 score for the remaining doses of treatment when estimating the average CIPN8 curve within this figure and that CIPN20 forms were collected at dose 12 from most patients, therefore, few patients have CIPN8 data for dose 12.
Nineteen patients (31.7%) experienced any paclitaxel treatment disruption (dose delay, decrease, or discontinuation) due to peripheral neuropathy, and there were a total 54 PN-induced treatment disruptions in these 19 patients. In these patients, the first PN-induced treatment disruption occurred on average after 8 doses (SD: 2.31 doses) and the earliest was a 1-week delay at their second dose. The mean CIPN8 score at the time of a PN-induced treatment disruption was 30.36 (range: 8.33–83.33, 95% confidence interval (CI): 21.26–39.46).
Neuropathy Modeling
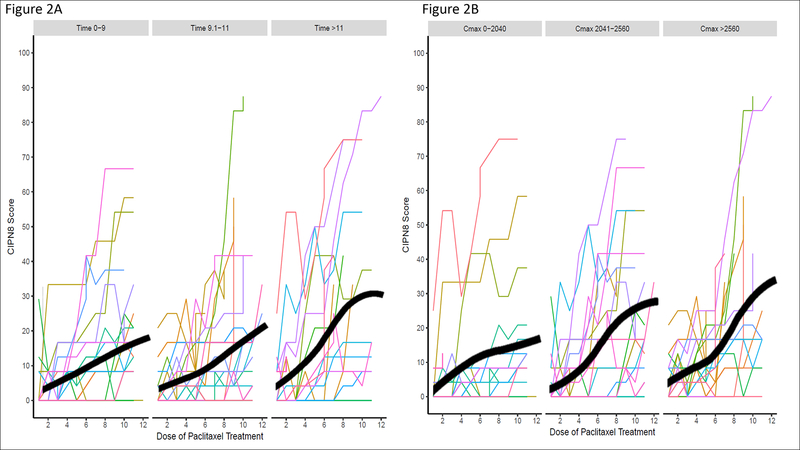
In the primary analysis, the increase in CIPN8 during treatment was associated with baseline CIPN8, cumulative dose, and relative dose intensity (all p<0.05, Table 2). Time above threshold (Tc>0.05) did not significantly contribute to this model (β=−0.11 (standard error (SE)=0.10), p=0.27). For visualization, patients were grouped into tertiles of Tc>0.05 and the mean CIPN8 throughout treatment was calculated for these groups (Figure 2, left). Upon visual inspection, it was noted that the slope of the CIPN8 curves increased more rapidly at higher doses in patients with higher Tc>0.05. In an exploratory model there was a significant interaction between cumulative dose and Tc>0.05, wherein the increase in CIPN8 at higher cumulative dose was more pronounced in patients with greater Tc>0.05 (β=0.14 (SE=0.05), p=0.009). In the secondary analysis using Cmax instead of Tc>0.05, baseline CIPN8, cumulative dose, and relative dose intensity were again significantly associated with CIPN8 score and Cmax was not (β=−0.002 (SE=0.10), p=0.99). Patients were again grouped into tertiles of Cmax to visualize the mean CIPN8 throughout treatment (Figure 2, right), though no significant interaction between cumulative dose and Cmax was identified (p=0.70). In multivariable analyses, patients who were younger, had diabetes, and drank alcohol self-reported greater increases in CIPN8 during treatment (p<0.10, Table 2).
Table 2:
Models of Increase in Self-Reported Sensory Peripheral Neuropathy
| Base Model and Univariate Associations of Clinical Variables | Multivariable Model Including Significant Clinical Variables | |||||
|---|---|---|---|---|---|---|
| B Coefficient | Standard Error | p-value | B Coefficient | Standard Error | p-value | |
| Baseline CIPN8 | 0.20 | 0.03 | <0.0001 | 0.21 | 0.04 | <0.0001 |
| Cumulative Dose | 0.42 | 0.05 | <0.0001 | 0.41 | 0.05 | <0.0001 |
| Relative Dose Intensity | −1.84 | 0.74 | 0.013 | −1.37 | 0.74 | 0.07 |
| Tc>0.05 | −0.11 | 0.10 | 0.27 | 0.01 | 0.10 | 0.93 |
| Cmax* | −0.002 | 0.10 | 0.99 | NA | NA | NA |
| Age | −0.02 | 0.01 | 0.015 | −0.02 | 0.01 | 0.020 |
| Nonwhite vs. White Race | 0.43 | 0.41 | 0.30 | NA | NA | NA |
| Alcohol vs. None | 0.84 | 0.19 | <0.0001 | 0.60 | 0.20 | 0.003 |
| Diabetes vs. No Diabetes | 0.41 | 0.23 | 0.08 | 0.42 | 0.25 | 0.09 |
Notes: The above estimates and p-values are for the base model with baseline CIPN8, cumulative dose, relative dose intensity and Tc>0.05. Both Tc>0.05 and Cmax are in standard deviation units. The clinical variables (age, race, alcohol, and diabetes) were first tested individually for their contribution to the base model, and if p<0.10 were included in the multivariable model. The outcome of the model is the square root of the CIPN8 score. These models included an intercept estimate with no meaningful interpretation, thus the intercept data are not shown. Significant p-values (<0.05) are bolded
Figure 2: Increase in Self-Reported Neuropathy During Treatment, Stratified by Paclitaxel Exposure.
Self-reported sensory peripheral neuropathy (CIPN8) gradually increased from baseline to the end of treatment. The cohort was stratified by tertiles of Tc>0.05 (Figures 2A) or Cmax (Figures 2B). There was no significant difference in increase in CIPN8 in the cohort stratified by either paclitaxel exposure parameter. The thick black line represents the smoothed average of CIPN8 at that treatment dose. Note that patients who discontinued treatment were assigned their final CIPN8 score for the remaining doses of treatment in order to estimate the average CIPN8 curve within these figures and that CIPN20 forms were collected at dose 12 from most patients, therefore, few patients have CIPN8 data for dose 12.
Similar models were built for the secondary endpoint, the occurrence of PN-induced treatment disruption (Table 3). Cumulative dose (odds ratio (OR)=1.46, 95% CI: 1.18–1.80, p=0.0008) and Tc>0.05 (OR=1.79, 95% CI: 1.06–3.01, p=0.029) were significantly associated with treatment disruption. Again, there was a significant interaction between cumulative dose and Tc>0.05, such that the risk of treatment disruption as treatment continued was greater with higher Tc>0.05 (p=0.004). Similar main effects results were obtained when replacing Tc>0.05 with Cmax (OR=2.74, 95% CI: 1.45–5.20, p=0.002), but the interaction between cumulative dose and Cmax was not significant (p=0.41). Next, because each of the exposure parameters was significantly associated with PN-induced treatment disruption, and the two parameters were not highly correlated, both parameters were included in a model simultaneously. In this model, only Cmax (p=0.009) maintained significance (Tc>0.05 p=0.14, data not shown). Age, alcohol, and diabetes were not significantly associated with PN-induced treatment disruption (all p>0.30, Table 3). For visualization, the Tc>0.05 and Cmax were compared in patients who did (n=19) and did not (n=41) experience PN-induced treatment disruption (Supplementary Figure 2).
Table 3:
Base Model of Risk of Peripheral Neuropathy-Induced Treatment Disruption
| Odds Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Baseline CIPN8 | 0.95 | 0.76–1.19 | 0.64 |
| Cumulative Dose | 1.46 | 1.18–1.80 | 0.0008 |
| Tc>0.05 | 1.79 | 1.06–3.01 | 0.029 |
| Cmax* | 2.74 | 1.45–5.20 | 0.002 |
| Age | 0.98 | 0.91–1.05 | 0.50 |
| Alcohol vs. No Alcohol | 1.60 | 0.42–6.17 | 0.49 |
| Diabetes vs. No Diabetes | 1.94 | 0.41–9.13 | 0.40 |
Notes: The above estimates and p-values are for the base model with baseline CIPN8, cumulative dose, and Tc>0.05. The model with Cmax only was not meaningfully different (Baseline CIPN 8 OR=0.93, 95% CI: 0.75–1.17, p=0.54, Cumulative dose OR=1.49, 95% CI: 1.19–1.87, p=0.0009). The odds ratios were calculated as one unit offsets from the mean with other variables set at the mean. Both Tc>0.05 and Cmax are in standard deviation units. Model with race does not converge due to low variability in race and having events. Since none of the clinical variables were associated with neuropathy, a multivariable model including clinical variables was not created. These models included an intercept estimate with no meaningful interpretation, thus the intercept data are not shown. Significant p-values (<0.05) are bolded.
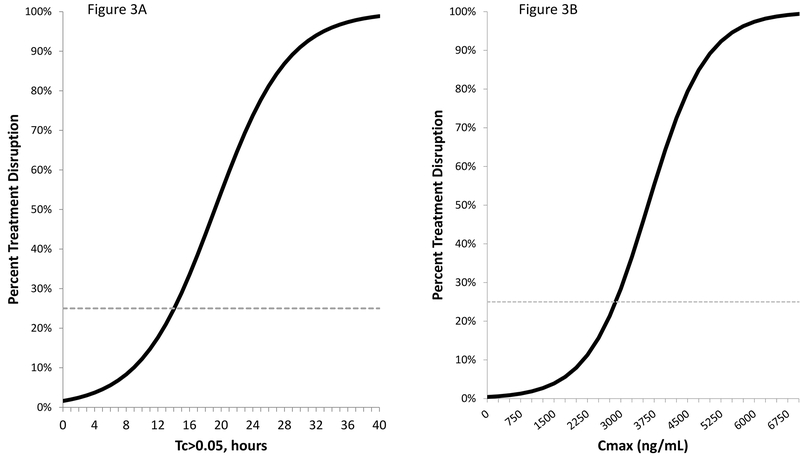
Based on the main effects models, increases in one standard deviation of Tc>0.05 (2.7 hours) and Cmax (664.8 ng/mL) were associated with 79% and 174% increases in the odds of PN-induced treatment disruption, respectively. Using these models, the risk of experiencing a PN-induced treatment disruption at any exposure for a patient with no neuropathy at baseline (CIPN8=0) who receives the standard planned treatment (80 mg/m2 × 12 weekly doses) is illustrated in Figure 3. The paclitaxel exposure corresponding with a 25% risk of PN-induced treatment disruption is Tc>0.05=14.06 hours or Cmax=2,885 ng/mL.
Figure 3: Probability of Treatment Disruption by Paclitaxel Exposure.
The probability of experiencing PN-Induced Treatment Disruption Increases as exposure (Tc>0.05 or Cmax) during the first dose increases. Assuming a patient has a baseline CIPN8=0 and receives the full dose (80 mg/m2) for 12 scheduled weekly doses, she would have a 25% risk of treatment disruption if her Tc>0.05=14.06 hours (Figure 3A) or Cmax=2,885 ng/mL (Figure 3B)).
Discussion
PN is the dose-limiting toxicity of weekly paclitaxel administered to patients with breast cancer. This debilitating toxicity decreases quality of life (22) and continues to affect approximately 40% of patients for at least two years after treatment discontinuation(23) (24, 25). Discovery of predictive biomarkers of chemotherapy-induced neuropathy is a high priority in translational cancer research(26), as these could be used to identify patients who should not be treated with neuropathic chemotherapy or could enable personalized dose adjustment to optimize therapeutic outcomes. In this prospective observational study, cumulative dose, baseline CIPN8, age, and alcohol intake were associated with patient-reported sensory neuropathy (CIPN8), but paclitaxel exposure as measured by time above threshold (Tc>0.05) and maximum concentration (Cmax) was not. In secondary analyses, both paclitaxel exposure parameters were significantly associated with a composite endpoint (i.e. dose decrease, delay, or treatment discontinuation) of PN-induced treatment disruption. These models were used to estimate the maximum paclitaxel exposure (Tc>0.05=14.06 hours or Cmax=2885 ng/mL) corresponding with a 25% risk of treatment-limiting PN.
Paclitaxel exposure has previously been associated with PN (15, 16) and other clinically relevant endpoints including neutropenia (27, 28) and treatment efficacy (29, 30). Prior studies of the exposure-PN relationship conducted in patients receiving larger, less frequent doses yielded a therapeutic exposure target for personalized dosing. In a prospective clinical trial, patients with non-small cell lung cancer were randomized to standard of care paclitaxel dosing (200 mg/m2) vs. exposure-guided paclitaxel dosing (target Tc>0.05 =26–31 hours) every 3 weeks in combination with carboplatin. The exposure-guided dosing arm had significantly decreased grade 2+ neuropathy occurrence (38% vs. 23%, p<0.001) with no corresponding decrease in progression-free or overall survival (p>0.05)(31). While this previous trial provides proof-of-principle that exposure-guided paclitaxel dosing can improve clinical outcomes, it remains to be seen whether clinicians will dose-decrease patients based on an exposure target and the prediction of toxicity. Furthermore, the selected paclitaxel exposure target within the previous trial of every 3-week paclitaxel is expected to be different from the optimal exposure target using a smaller weekly dosing regimen.
The association of paclitaxel exposure with the secondary endpoint, PN-induced treatment disruption, but not the primary endpoint, CIPN8, is somewhat surprising. Both of the prior studies of the exposure-PN association used clinician-documented CTCAE data (15, 16). The substantial variability in PRO neuropathy in patients assigned similar CTCAE grades (32) and consistently greater incidence of PN in PRO data (33, 34) are assumed to mitigate the limitations of clinician-documented toxicity (35). Based on this premise, PRO PN was selected as the primary study endpoint and CTCAE data were not prospectively collected. PN-induced treatment disruption is likely to reflect clinicians’ assessment of PN severity (36, 37), not the patients’. While PRO data may be extremely useful in clinical practice (38), additional research is needed to determine whether PRO or CTCAE data more accurately reflect PN severity, and which would therefore be a superior endpoint for biomarker discovery.
Our results suggest that measurement of paclitaxel plasma concentration during the first dose of treatment could be useful to predict PN that necessitates paclitaxel treatment disruption. Based on our model, a patient with no prior neuropathy receiving 12 weekly doses of paclitaxel 80 mg/m2 with an exposure of Cmax=2885 ng/mL or Tc>0.05=14.06 hours would have an approximately 25% risk of PN-induced treatment disruption during treatment. These maximum tolerated exposure estimates are somewhat higher than the mean exposures within our study cohort (Cmax=2,364 ng/mL and Tc>0.05=10.71 hours), suggesting that patients overall are being slightly under-dosed. The chosen threshold of 25% risk is similar to the rates of grade 2+ PN in clinical trials of weekly paclitaxel (5, 7), which is typically the threshold at which treatment disruption is mandated by the trial protocol. This exposure target, or any other selected by the investigator, could be used to conduct a prospective clinical trial of exposure-guided weekly paclitaxel dosing. Prospective demonstration that exposure-guided dosing improves treatment effectiveness, by safely enabling dose escalation to an exposure target, and/or diminishes treatment toxicity, by identifying patients for pre-emptive dose de-escalation, compared with empiric dosing could warrant translation into clinical practice. Additionally, our model can be used to estimate the target exposure associated with any risk of treatment-limiting PN, perhaps to select appropriate thresholds for patients based on disease prognosis or individualized exposure targets for patients based on their personal preference of acceptable PN risk (39).
Much of the previous work has focused on time above threshold (Tc>0.05) as the exposure parameter of interest(31, 40), though some studies have reported similar associations with paclitaxel area under the curve or maximum concentration (Cmax) (16, 41). Importantly, in our cohort Cmax was a stronger predictor of PN than Tc>0.05. A Cmax sample collected at the end of infusion is more convenient than requiring a next-day sample for Tc>0.05, improving the potential for clinical translation of exposure-guided treatment. Combined with the commercial availability of paclitaxel measurement(42), most of the logistical and analytical barriers to therapeutic drug monitoring (TDM) have been overcome (43). Prospective studies are needed to determine whether TDM of weekly paclitaxel can enhance treatment effectiveness and/or limit PN occurrence without affecting efficacy.
There are several limitations of this analysis that warrant consideration. Although a significant association was detected between paclitaxel exposure and treatment-limiting PN, the attribution of treatment disruption to PN was collected retrospectively and only on-treatment PN was considered, whereas post-treatment PN is most important for patient’s long-term quality of life(23, 25). Additionally, exposure only explained a portion of the occurrence of treatment-limiting PN. None of the clinical variables tested contributed to the overall model; however, analyses of genetics, metabolomics, or vitamin levels that are ongoing and will be reported separately in the future may identify biomarkers of PN susceptibility. An additional consideration is the relatively small size and a narrowly defined patient cohort, which precludes generalizing these findings to patients receiving paclitaxel via other dosing regimens or for other tumor types. Finally, while defining a therapeutic exposure target represents an important first step toward TDM, randomized prospective trials are necessary to demonstrate that TDM of weekly paclitaxel improves clinically important treatment outcomes, and is more informative than merely using early changes in patient-reported neuropathy, prior to translation into clinical practice.
In conclusion, a single blood sample collected at the end of, or the day after, the first paclitaxel infusion is predictive of treatment-limiting PN. This study identified a paclitaxel exposure target associated with an acceptable risk of treatment limiting PN (25%). This exposure target, or any other of the investigator’s choosing, can be tested in a prospective clinical trial of TDM to determine whether this approach can increase treatment effectiveness while maintaining acceptable toxicity risk for patients receiving weekly paclitaxel, which is the goal of treatment in the curative setting. Prospective demonstration of the benefit of this personalized approach could be practice-changing for breast cancer patients, as it could allow for optimization of the benefit-risk ratio for individual patients, and could lead to continued expansion of paclitaxel TDM to other regimens and tumor types.
Supplementary Material
Supplementary Figure 1: Consort Diagram. Of the sixty-five patients enrolled on the prospective observational clinical trial (UMCC2014.002), 60 were eligible for analysis. Analyses of each exposure metric used a subset of these patients due to missed sample collection or assay failure. Abbreviations; D/C: discontinued paclitaxel
Supplementary Figure 2: Paclitaxel Exposure in Patients Who Did or Did Not Experience Peripheral Neuropathy-Induced Treatment Disruption. Patients who experienced PN-induced treatment disruption had greater median paclitaxel Tc>0.05 than patients who did not (11 hours (IQR 10–15) vs. 9.5 hours (IQR= 9–11), p=0.001) (left panel). The mean Cmax for patients who experienced treatment disruption was not statistically different from those who did not (2591.8 ng/mL (SD 897.9) vs. 2267.4 ng/mL (SD 521.2), p=0.18) (right panel). The line in the box is the median. The lower and upper hinges correspond to the first and third quartiles and the lines extend 1.5 × IQR, representing an approximate 95% confidence interval.
Statement of Translational Relevance:
This prospective, observational clinical study of patients with breast cancer receiving weekly paclitaxel treatment demonstrated that paclitaxel exposure during the first dose is predictive of treatment-limiting peripheral neuropathy (PN). A maximum concentration (Cmax) measured at the end of infusion was similarly predictive of PN and is more convenient to collect than “time above threshold” (Tc>0.05), which requires a sample collected 16–26 hours after infusion. This study identified the maximum exposure (Cmax=2,885 ng/mL or Tc>0.05=14.06 hours) associated with <25% risk of treatment-limiting PN. This is a critical step toward exposure-guided paclitaxel treatment, by providing an evidence-based exposure target for prospective clinical trials of individualized dosing. Prospective studies are warranted to determine whether dose escalating patients with exposure below this target enhances treatment effectiveness, and/or dose decreasing patients with exposure above this target prevents PN, for potential translation into clinical practice.
Statement of Significance.
Paclitaxel maximum concentration, measured at the end of the first infusion, is predictive of the occurrence of treatment-limiting peripheral neuropathy in patients receiving weekly infusions for breast cancer. We identified the exposure level associated with a 25% risk of treatment-limiting peripheral neuropathy, which can be used as a target exposure in a prospective trial to assess whether this personalized treatment approach improves outcomes.
Acknowledgments
Research reported in this publication was supported by the National Center for Advancing Translational Sciences under award number KL2TR000434 and 2UL1TR000433 (D. Hertz) and the National Cancer Institutes of Health under Award Number P30CA046592 (K. Kidwell). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support was provided by the Fashion Footwear Charitable Foundation of New York/QVC Presents Shoes on Sale ™ (D. Hayes).
Abbreviations
- PN
peripheral neuropathy
- OR
odds ratio
- CI
confidence interval
- NCI
National Cancer Institute
- CTCAE
Common Terminology Criteria for Adverse Events
- PRO
Patient-reported outcomes
- UMCCC
University of Michigan Comprehensive Cancer Center
- HbA1c
hemoglobin A1c
- EORTC
European Organisation for Research and Treatment of Cancer
- CIPN20
Quality of Life Questionnaire Chemotherapy-Induced Peripheral Neuropathy
- PK
pharmacokinetic
- MRM
multiple reaction monitoring
- SD
standard deviation
- SE
standard error
- TDM
therapeutic drug monitoring
- A
doxorubicin
- C
cyclophosphamide
- H
trastuzumab
- P
pertuzumab
Footnotes
This work was presented in part at the 2017 San Antonio Breast Cancer Symposium.
Conflict of Interest: DLH has an informal, unpaid collaborative relationship with Saladax Biomedical Inc., a company that offers CLIA-approved paclitaxel measurement. Saladax was not involved in the design, conduct, analysis, or sponsorship of this trial, and had no contribution to the writing of this manuscript.
References
- 1.Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sparano JA, Zhao F, Martino S, Ligibel JA, Perez EA, Saphner T, et al. Long-Term Follow-Up of the E1199 Phase III Trial Evaluating the Role of Taxane and Schedule in Operable Breast Cancer. J Clin Oncol. 2015;33:2353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budd GT, Barlow WE, Moore HC, Hobday TJ, Stewart JA, Isaacs C, et al. SWOG S0221: a phase III trial comparing chemotherapy schedules in high-risk early-stage breast cancer. J Clin Oncol. 2015;33:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mielke S, Sparreboom A, Mross K. Peripheral neuropathy: A persisting challenge in paclitaxel-based regimes. European journal of cancer. 2006;42:24–30. [DOI] [PubMed] [Google Scholar]
- 5.Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, et al. Randomized Phase III Trial of Weekly Compared With Every-3-Weeks Paclitaxel for Metastatic Breast Cancer, With Trastuzumab for all HER-2 Overexpressors and Random Assignment to Trastuzumab or Not in HER-2 Nonoverexpressors: Final Results of Cancer and Leukemia Group B Protocol 9840. Journal of Clinical Oncology. 2008;26:1642–9. [DOI] [PubMed] [Google Scholar]
- 6.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Insitute; 2010. [Google Scholar]
- 7.Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. The New England journal of medicine. 2008;358:1663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. Jama. 2013;309:1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:1941–67. [DOI] [PubMed] [Google Scholar]
- 10.Hershman DL, Till C, Wright JD, Awad D, Ramsey SD, Barlow WE, et al. Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants in SWOG clinical trials. ASCO Meeting Abstracts. 2016;34:10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speck RM, Sammel MD, Farrar JT, Hennessy S, Mao JJ, Stineman MG, et al. Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. Journal of oncology practice. 2013;9:e234–40. [DOI] [PubMed] [Google Scholar]
- 12.Basch E, Iasonos A, McDonough T, Barz A, Culkin A, Kris MG, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. The Lancet Oncology. 2006;7:903–9. [DOI] [PubMed] [Google Scholar]
- 13.Basch E, Jia X, Heller G, Barz A, Sit L, Fruscione M, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101:1624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kluetz PG, Chingos DT, Basch EM, Mitchell SA. Patient-Reported Outcomes in Cancer Clinical Trials: Measuring Symptomatic Adverse Events With the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). American Society of Clinical Oncology educational book American Society of Clinical Oncology Meeting. 2016;35:67–73. [DOI] [PubMed] [Google Scholar]
- 15.Mielke S, Sparreboom A, Steinberg SM, Gelderblom H, Unger C, Behringer D, et al. Association of Paclitaxel Pharmacokinetics with the Development of Peripheral Neuropathy in Patients with Advanced Cancer. Clinical Cancer Research. 2005;11:4843–50. [DOI] [PubMed] [Google Scholar]
- 16.de Graan A-JM, Elens L, Sprowl JA, Sparreboom A, Friberg LE, van der Holt B, et al. CYP3A4*22 Genotype and Systemic Exposure Affect Paclitaxel-Induced Neurotoxicity. Clinical Cancer Research. 2013;19:3316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. European journal of cancer. 2005;41:1135–9. [DOI] [PubMed] [Google Scholar]
- 18.Lavoie Smith EM, Barton DL, Qin R, Steen PD, Aaronson NK, Loprinzi CL. Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2013;22:2787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kieffer JM, Postma TJ, van de Poll-Franse L, Mols F, Heimans JJ, Cavaletti G, et al. Evaluation of the psychometric properties of the EORTC chemotherapy-induced peripheral neuropathy questionnaire (QLQ-CIPN20). Qual Life Res. 2017;26:2999–3010. [DOI] [PubMed] [Google Scholar]
- 20.Joerger M, Huitema AD, van den Bongard DH, Schellens JH, Beijnen JH. Quantitative effect of gender, age, liver function, and body size on the population pharmacokinetics of Paclitaxel in patients with solid tumors. Clin Cancer Res. 2006;12:2150–7. [DOI] [PubMed] [Google Scholar]
- 21.Kraff S, Lindauer A, Joerger M, Salamone SJ, Jaehde U. Excel-Based Tool for Pharmacokinetically Guided Dose Adjustment of Paclitaxel. Ther Drug Monit. 2015;37:725–32. [DOI] [PubMed] [Google Scholar]
- 22.Shimozuma K, Ohashi Y, Takeuchi A, Aranishi T, Morita S, Kuroi K, et al. Taxane-induced peripheral neuropathy and health-related quality of life in postoperative breast cancer patients undergoing adjuvant chemotherapy: N-SAS BC 02, a randomized clinical trial. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2012;20:3355–64. [DOI] [PubMed] [Google Scholar]
- 23.Hershman DL, Unger JM, Crew KD, Till C, Greenlee H, Minasian LM, et al. Two-Year Trends of Taxane-Induced Neuropathy in Women Enrolled in a Randomized Trial of Acetyl-L-Carnitine (SWOG S0715). J Natl Cancer Inst. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mustafa Ali M, Moeller M, Rybicki L, Moore HCF. Long-term peripheral neuropathy symptoms in breast cancer survivors. Breast Cancer Res Treat. 2017;166:519–26. [DOI] [PubMed] [Google Scholar]
- 25.Bandos H, Melnikow J, Rivera DR, Swain SM, Sturtz K, Fehrenbacher L, et al. Long-term Peripheral Neuropathy in Breast Cancer Patients Treated With Adjuvant Chemotherapy: NRG Oncology/NSABP B-30. J Natl Cancer Inst. 2018;110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Travis LB, Fossa SD, Sesso HD, Frisina RD, Herrmann DN, Beard CJ, et al. Chemotherapy-induced peripheral neurotoxicity and ototoxicity: new paradigms for translational genomics. J Natl Cancer Inst. 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gianni L, Kearns CM, Giani A, Capri G, Vigano L, Lacatelli A, et al. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. Journal of Clinical Oncology. 1995;13:180–90. [DOI] [PubMed] [Google Scholar]
- 28.Miller AA, Rosner GL, Egorin MJ, Hollis D, Lichtman SM, Ratain MJ. Prospective evaluation of body surface area as a determinant of paclitaxel pharmacokinetics and pharmacodynamics in women with solid tumors: Cancer and Leukemia Group B Study 9763. Clin Cancer Res. 2004;10:8325–31. [DOI] [PubMed] [Google Scholar]
- 29.Mielke S, Sparreboom A, Behringer D, Mross K. Paclitaxel pharmacokinetics and response to chemotherapy in patients with advanced cancer treated with a weekly regimen. Anticancer Research. 2005;25:4423–7. [PubMed] [Google Scholar]
- 30.Joerger M, Huitema ADR, Richel DJ, Dittrich C, Pavlidis N, Briasoulis E, et al. Population Pharmacokinetics and Pharmacodynamics of Paclitaxel and Carboplatin in Ovarian Cancer Patients: A Study by the European Organization for Research and Treatment of Cancer-Pharmacology and Molecular Mechanisms Group and New Drug Development Group. Clinical Cancer Research. 2007;13:6410–8. [DOI] [PubMed] [Google Scholar]
- 31.Joerger M, von Pawel J, Kraff S, Fischer JR, Eberhardt W, Gauler TC, et al. Open-label, randomized study of individualized, pharmacokinetically (PK)-guided dosing of paclitaxel combined with carboplatin or cisplatin in patients with advanced non-small-cell lung cancer (NSCLC). Ann Oncol. 2016;27:1895–902. [DOI] [PubMed] [Google Scholar]
- 32.Le-Rademacher J, Kanwar R, Seisler D, Pachman DR, Qin R, Abyzov A, et al. Patient-reported (EORTC QLQ-CIPN20) versus physician-reported (CTCAE) quantification of oxaliplatin- and paclitaxel/carboplatin-induced peripheral neuropathy in NCCTG/Alliance clinical trials. Support Care Cancer. 2017;25:3537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett BK, Park SB, Lin CS, Friedlander ML, Kiernan MC, Goldstein D. Impact of oxaliplatin-induced neuropathy: a patient perspective. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2012;20:2959–67. [DOI] [PubMed] [Google Scholar]
- 34.Cirillo M, Venturini M, Ciccarelli L, Coati F, Bortolami O, Verlato G. Clinician versus nurse symptom reporting using the National Cancer Institute-Common Terminology Criteria for Adverse Events during chemotherapy: results of a comparison based on patient’s self-reported questionnaire. Annals of Oncology : Official Journal of the European Society for Medical Oncology / ESMO. 2009;20:1929–35. [DOI] [PubMed] [Google Scholar]
- 35.Park SB, Kwok JB, Asher R, Lee CK, Beale P, Selle F, et al. Clinical and genetic predictors of paclitaxel neurotoxicity based on patient- versus clinician-reported incidence and severity of neurotoxicity in the ICON7 trial. Ann Oncol. 2017;28:2733–40. [DOI] [PubMed] [Google Scholar]
- 36.Beutler AS, Majithia N, Loprinzi CL. The past and future of ‘reported outcomes’ in studies on chemotherapy neuropathy. Ann Oncol. 2017;28:2631–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuroi K, Shimozuma K, Ohashi Y, Takeuchi A, Aranishi T, Morita S, et al. A questionnaire survey of physicians’ perspectives regarding the assessment of chemotherapy-induced peripheral neuropathy in patients with breast cancer. Jpn J Clin Oncol. 2008;38:748–54. [DOI] [PubMed] [Google Scholar]
- 38.Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA. 2017;318:197–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Paolo A, Sarkozy F, Ryll B, Siebert U. Personalized medicine in Europe: not yet personal enough? BMC health services research. 2017;17:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraff S, Nieuweboer AJ, Mathijssen RH, Baty F, de Graan AJ, van Schaik RH, et al. Pharmacokinetically based dosing of weekly paclitaxel to reduce drug-related neurotoxicity based on a single sample strategy. Cancer Chemother Pharmacol. 2015;75:975–83. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi M, Oba K, Sakamoto J, Kondo K, Nagata N, Okabayashi T, et al. Pharmacokinetic study of weekly administration dose of paclitaxel in patients with advanced or recurrent gastric cancer in Japan. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2007;10:52–7. [DOI] [PubMed] [Google Scholar]
- 42.Joerger M, Kraff S, Jaehde U, Hilger RA, Courtney JB, Cline DJ, et al. Validation of a Commercial Assay and Decision Support Tool for Routine Paclitaxel Therapeutic Drug Monitoring (TDM). Ther Drug Monit. 2017;39:617–24. [DOI] [PubMed] [Google Scholar]
- 43.Krens SD, McLeod HL, Hertz DL. Pharmacogenetics, enzyme probes and therapeutic drug monitoring as potential tools for individualizing taxane therapy. Pharmacogenomics. 2013;14:555–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Consort Diagram. Of the sixty-five patients enrolled on the prospective observational clinical trial (UMCC2014.002), 60 were eligible for analysis. Analyses of each exposure metric used a subset of these patients due to missed sample collection or assay failure. Abbreviations; D/C: discontinued paclitaxel
Supplementary Figure 2: Paclitaxel Exposure in Patients Who Did or Did Not Experience Peripheral Neuropathy-Induced Treatment Disruption. Patients who experienced PN-induced treatment disruption had greater median paclitaxel Tc>0.05 than patients who did not (11 hours (IQR 10–15) vs. 9.5 hours (IQR= 9–11), p=0.001) (left panel). The mean Cmax for patients who experienced treatment disruption was not statistically different from those who did not (2591.8 ng/mL (SD 897.9) vs. 2267.4 ng/mL (SD 521.2), p=0.18) (right panel). The line in the box is the median. The lower and upper hinges correspond to the first and third quartiles and the lines extend 1.5 × IQR, representing an approximate 95% confidence interval.