Figure 1: The inter-connection between protein structure, dynamics and function has significance for improving catalytic efficiency of enzymes.
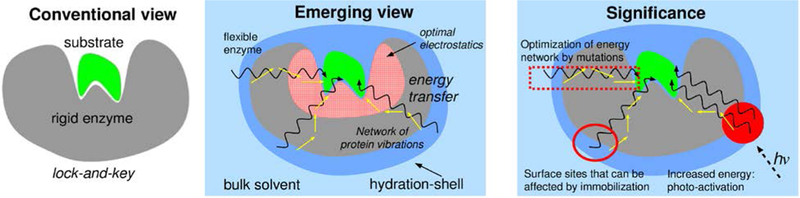
The active-site region provides environment with optimal electrostatics to promote the catalyzed reaction. Several enzymes contain conserved networks of residues that connect surface regions to the active-site. These networks provide thermo-dynamical coupling between the hydration-shell and bulk solvent, and the catalyzed reaction. The discovery of these enzyme energy networks allows new strategies for increasing the catalytic efficiency through enhancing the energy flow through these networks and optimization through mutations.
