Figure 4: A hypothesized model for the conformational and energetic coupling between enzyme and solvent.
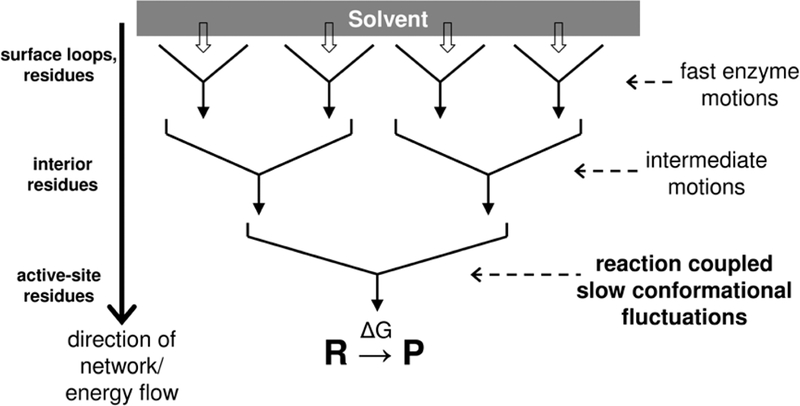
Random motions of solvent molecules slave the motions of surface residues through collisions. These fast motions drive the conformational fluctuations of the interior regions of the protein. These intermediate motions eventually drive the slow conformational fluctuations, some of which promote the catalytic reaction. The hierarchy of protein motions enables the transfer of thermo-dynamical energy from the surface regions to the active-site.
