Abstract
The use of CuAAC chemistry to crosslink and stabilize oligonucleotides has been limited by the incompatibility of azides with the phosphoramidites used in automated oligonucleotide synthesis. Herein we report optimized reaction conditions to synthesize azide derivatives of thymidine and cytidine phosphoramidites. Investigation of the stability of the novel phosphoramidites using 31P NMR at room temperature showed less than 10% degradation after 6 hours. The azide modified thymidine was successfully utilized as an internal modifier in the standard phosphoramidite synthesis of a DNA sequence. The synthesized azide and alkyne derivatives of pyrimidines will allow efficient incorporation of azide and alkyne click pairs into nucleic acids, thus widening the applicability of click chemistry in investigating the chemistry of nucleic acids.
Keywords: Phosphoramidites, Oligonucleotide synthesis, Click Chemistry, CuAAC, Chemical biology
Graphical Abstract
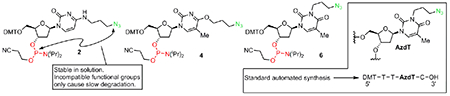
The cycloaddition of an alkyne and an azide catalyzed by Cu(I) (CuAAC),1,2 is a widely used example of click chemistry3,4 utilized in chemical biology as a tool for elucidating biological interactions.5 The applicability of click chemistry in chemical biology has been facilitated by its compatibility with aqueous solvents, rapid reaction rates, and feasibility with bioorthogonal functional groups. The applicability of click chemistry on nucleic acids and nucleoside analogues has been investigated.6 For example, click chemistry has been used to ligate two oligonucleotides, to immobilize DNA on solid surfaces7 decorated by either azide or alkyl moieties, and to conjugate large molecules to DNA to generate functional nucleic acid polymers.8 Despite the success of CuAAC in nucleic acid manipulation, the application of CuAAC click reactions in nucleic acid research remains elusive. This is due to the incompatibility of the azide functional group and P(III). Typically, automated DNA synthesis is based on phosphoramidite chemistry; however, azides readily undergo the Staudinger reaction with P(III), preventing the use of azide and P(III).9 Alternative methods to incorporate azides into oligonucleotides are predominately based on azide compatible P(V) reagents, such as H-phosphonates10 and phosphotriesters.11,12 In addition, azides have also been introduced into oligonucleotides post-synthesis by substitution of alkyl (pseudo)halides by NaN3.13,14 Recently, two important observations have been made in two independent studies, demonstrating the feasibility of synthesizing azide modified nucleic acid phosphoramidites. First, Kiviniemi and co-workers showed that azide containing thymidine analogs can be incorporated into an oligonucleotide (using H-phosphonates), which could subsequently undergo elongation using phosphoramidites without disrupting the thymidine azides.15 Second, Shmanai and co-workers synthesized hydroxypyrrolidines containing azides and phosphoramidites (Fig. 1A),16 and further demonstrated the successful incorporation of the azide phosphoramidite building blocks into oligonucleotides utilizing standard automated synthesis. While pyrrolidine mimics the ribose unit of nucleosides, it lacks nucleobases, which hinders the application of azide derivatives in nucleic acid research. Herein, we report the synthesis of azide modified thymidine and 2-deoxycytidine phosphoramidites, and demonstrate that the undesired Staudinger reaction can be suppressed under optimized conditions.
Figure 1.
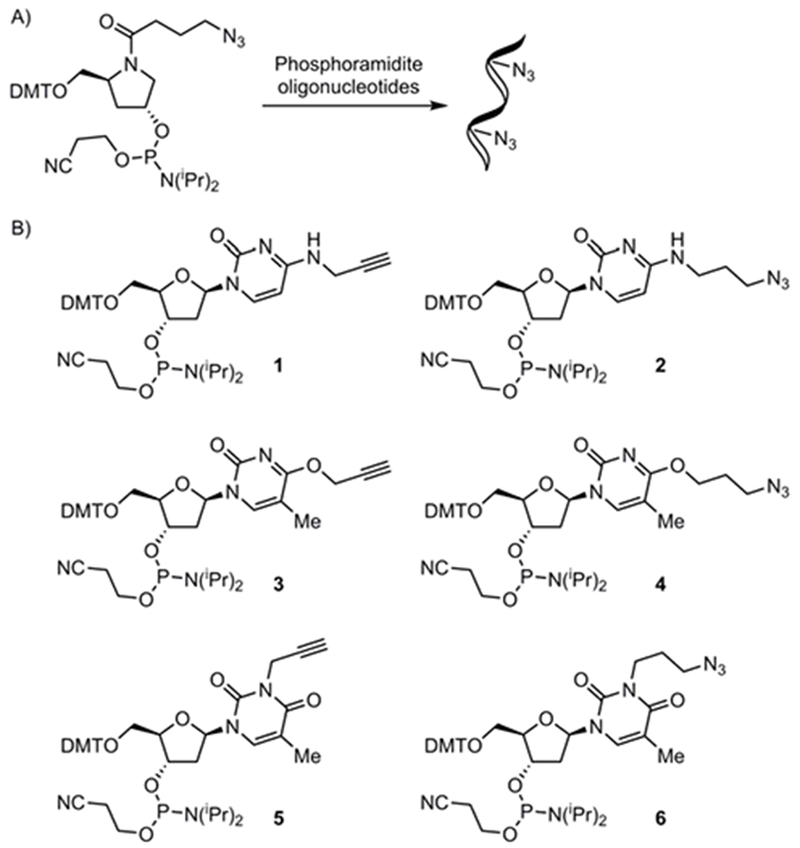
A) stable azide containing nucleoside analogs used in phophoramidtite-based synthesis.13 B) Targets for new cytidine and thymidine phosphoramidite click pairs.
We chose propargyl and 3-azidopropyl substituents in position 4 of the pyrimidine bases for our proof-of-principle-study to incorporate azide and alkyne moieties to generate phosphoramidite derivatives of T and dC. Thus, we targeted N4 alkylated dC analogs 1 and 2 (Fig. 1B). For the thymidine analogs we targeted the homologous O4-alkylated compounds (3 and 4). Furthermore, we investigated the possibility of adding an azide or alkyne at the readily accessible N2-position (5 and 6). The synthesis of the thymidine derivatives (Scheme 1) started with dimethoxytrityl (DMT) protection of the 5’ position to give 7,17 followed by alkylation18 of N2 using propargyl bromide or N3(CH2)3OTs to generate 8 and 9, respectively, in moderate yields (52% for 8; 60% for 9). Addition of the phosphoramidite unit to 8 (CEP-Cl, DIPEA, CH2Cl2) gave alkyne 5 in 53% yield. The azide containing secondary alcohol 9 rapidly reacted with CEP-Cl, and TLC analysis showed formation of the expected diastereomers of the product. Several minor components were also observed by TLC. Interestingly, the TLC profile of the reaction mixture did not change significantly with prolonged reaction time at room temperature. While this observation indicated that the azide containing phosphoramidites are relatively stable in solution. Initial attempts to isolate the product resulted in significant decomposition. In particular, high concentration or exposure to polar solvents (for example MeCN) was found to accelerate the decomposition. We noted that performing the reaction in benzene led to a much cleaner reaction profile by TLC analysis. Therefore, the reaction was repeated in C6D6. Following aqueous work-up, 1H NMR showed the desired phosphoramidite 6 with only minor impurities. The 31P NMR showed the expected signals at 148.92 and 148.44 ppm, but one P(V)-derived impurity (13.26 ppm), was also observed, which is most likely the iminophosphorane intermediate of the Staudinger reaction.19 This impurity was removed by silica gel column chromatography with acetone in hexanes as eluent. The removal of hexanes and acetone gave 6 in C6D6 in good purity (1H and 31P NMR and TLC). The yield was measured as 54% by qNMR (triphenyl phosphate as an internal standard).
Scheme 1.
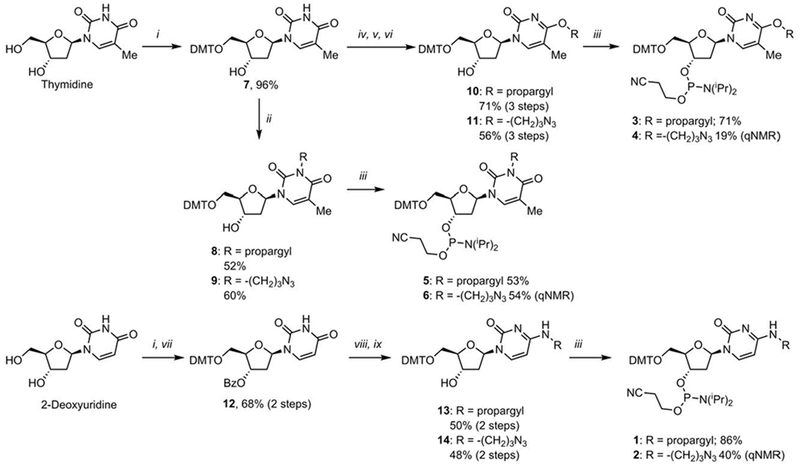
Synthesis of azide and alkyne functionalized phosphoramidites of cytidine and thymidine. Reagents and conditions: (i) DMTCl, Pyr; (ii) K2CO3, MeOH, Δ, propargyl bromide (8) or N3(CH2)3OTs (9); (iii) CEP-Cl, DIPEA (iv) TBSCl, Imid. DMF; (v) BOP, DBU, THF then propargyl alcohol (10) or HO(CH2)3N3 (11); (vi) TBAF, THF (vii) BzCl, DMAP, Pyr.; (viii) BOP, DBU, THF then propargylamine (16) or H2N(CH2)3N3 (17); (ix) K2CO3, MeOH.
Having established conditions to generate azide-containing phosphoramidites with minimum decomposition, we proceeded to synthesize additional azide/alkyne pairs. Thus, O4-substituted thymidines 3 and 4 were synthesized. The secondary alcohol (7) was TBS-protected15 and the required C4 substituents were then introduced by activation with BOP followed by addition of the appropriate alcohols utilizing to Lakshman’s procedure.20 TBAF mediated deprotection gave secondary alcohols 10 and 11 with overall yields (3 steps) of 71% for 10 and 56% for 11. Reaction with CEP-Cl gave phosphoramidites 3 (71%) and 4 (19% by qNMR).
2-Deoxycytidine analogs 1 and 2 were synthesized in a similar fashion. First 2-deoxyuridine was protected by DMT17 followed by benzoyl protection of the secondary alcohol giving 12 in 90% yield over two steps. The required C4 substituents were then introduced (BOP, then primary amine). Debenzoylation (K2CO3, MeOH) gave N4-alkylated cytidines 12 and 13. Slightly higher yields were achieved by replacing the benzoyl-protecting groups with TBS, but the purification of the intermediates was challenging. Thus, we utilized benzoyl as the preferred protecting group, which led to easier purification of the secondary alcohols. Finally, treatment with CEP-Cl provided the phosphoramidites 1 (49%) and 2 (40% by qNMR).
The stability of phosphoramidite 6 in solution was studied using 31P NMR in C6D6 using triphenyl phosphate as an internal standard. Interestingly, we found that at room temperature, 6 was relatively stable and underwent slow decomposition. After six hours, 91% of the phosphoramidite remained. However, prolonged storage at room temperature led to significant decomposition. After 27 hours at room temperature 58% remained, while only 30% and 8% was left after 4 and 9 days, respectively. Storing a sample of 6 at −20 °C in C6D6 for a period of 9 days showed no decomposition. Typically in automated oligonucleotide synthesis, acetonitrile is used as the main solvent. Due to the initial observation of the instability of azide containing phosphoramidites in acetonitrile, we first investigated the stability of azide 6 in a 1:1 mixture of C6D6/CD3CN. The 31P NMR spectrum did not change significantly over the course of 4 hours at room temperature, demonstrating the stability of azide containing phosphoramidites in relatively polar solvent mixtures, which are similar to the conditions used in automated synthesis (ESI, Fig. SI1).
Based on the stability studies, we chose benzene as the optimal solvent for the synthesis and storage of phosphoramidites 2, 4, and 6. These benzene solutions can be used directly in automated oligonucleotide synthesis. In order to validate the applicability of azide containing phosphoramidites in the automated synthesis of oligonucleotide containing azide modifiers, we designed a sequence with AzdT as an internal modifier in the sequence 5’-TTAzdTC-3’ (Fig. 2). We found that 6 was coupled in high yield, and the azide can be incorporated internally in the sequence. We used the standard procedure for DNA synthesis on an ABI 394 DNA synthesizer with 10 minute coupling time for AzdT and the delivery time was increased to 5 seconds. The deprotection was performed with NH4OH at 37 °C for 24 hours. The deprotected oligonucleotide was dried and reconstituted in phosphate buffered saline and analyzed by mass spectrometry (calculated MW 1524.4077 and found MW 1524.415), indicating that AzdT can be successfully utilized in synthesizing azide modified DNA molecules.
Figure 2.
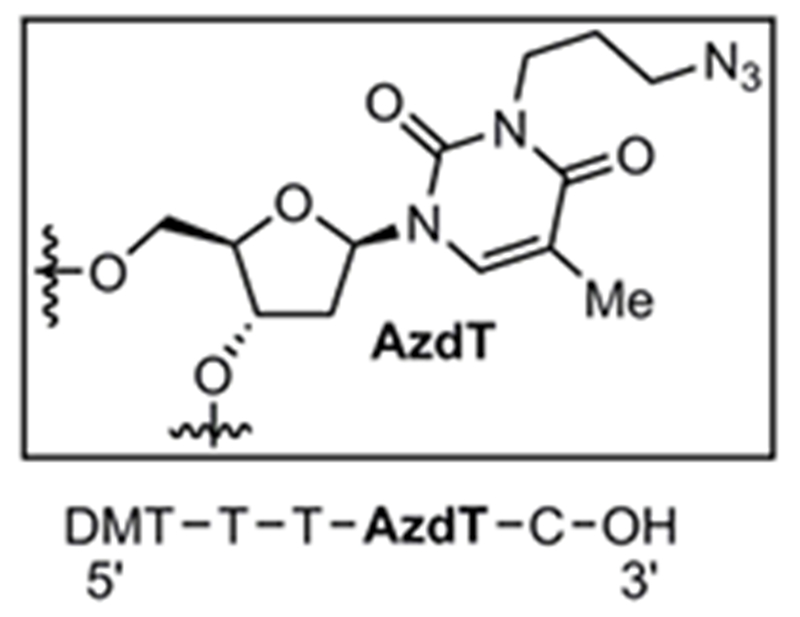
Synthetic sequence incorporating the azide-modified deoxythymidine (AzdT).
In summary, we have successfully identified conditions that allow the synthesis of stable azide containing phosphoramidites with no significant decomposition. The availability of these building blocks will allow the routine synthesis of azide and alkyne containing oligonucleotides. With the successful synthesis of an AzdT containing DNA oligomer, currently, our lab is focused on incorporating the azide and alkyne building blocks into DNA sequences to cross-link DNA stands using CuAAC chemistry.
Supplementary Material
Highlights.
-
1)
Azide containing phosphoramidites
-
2)
Clickable DNA base-pairs
-
3)
Azide containing nucleosides for automated synthesis
Acknowledgments
We thank Dr. Sean Cahill from the Structural Biology Core Facility (Albert Einstein College of Medicine) for help with recording the qNMR data. The authors wish to acknowledge the financial support from NIGMS (SC1 GM122648-02).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary data associated with this article can be found, in the online version.
References and notes
- 1.Rostovtsev VV; Green LG; Fokin VV; Sharpless KB Angew. Chem. Int Ed 2002, 41, 2596. [DOI] [PubMed] [Google Scholar]
- 2.Tornøe CW; Christensen C; Meldal M J. Org. Chem 2002, 67, 3057. [DOI] [PubMed] [Google Scholar]
- 3.Kolb HC; Finn MG; Sharpless KB Angew. Chem. Int. Ed 2001, 40, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Thirumurugan P; Matosiuk D; Jozwiak K Chem. Rev 2013, 113, 4905. [DOI] [PubMed] [Google Scholar]
- 5.McKay CS; Finn MG Chem. Biol 2014, 21, 1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amblard F; Cho JH; Schinazi RF Chem. Rev 2009, 109, 4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efthymiou T; Gong W; Desaulniers J-P Molecules 2012, 17, 12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Sagheer AH; Brown T Acc. Chem. Res 2012, 45, 1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uzagare MC; Claußnitzer I; Gerrits M; Bannwarth W ChemBioChem 2012, 13, 2204. [DOI] [PubMed] [Google Scholar]
- 10.Ötvös L; Bajor Z; Kraicsovits F; Sági G; Tegyey Z Nucleosides, Nucleotides and Nucl. Acids 2002, 21, 79. [DOI] [PubMed] [Google Scholar]
- 11.El-Sagheer AH; Brown TJ Am. Chem. Soc 2009, 131, 3958. [DOI] [PubMed] [Google Scholar]
- 12.Efimov VA; Aralov AV; Klykov VN; Chakhmakhcheva OG Nucleosides, Nucleotides and Nucl. Acids 2009, 28, 846. [DOI] [PubMed] [Google Scholar]
- 13.Dallmann A; El-Sagheer AH; Dehmel L; Mügge C; Griesinger C; Ernsting NP; Brown T Chem. Eur. J 2011, 17, 14714. [DOI] [PubMed] [Google Scholar]
- 14.Gerowska M; Hall L; Richardson J; Shelbourne M; Brown T Tetrahedron 2012, 68, 857–864. [Google Scholar]
- 15.Kiviniemi A; Virta P; Lönnberg H Bioconjugate Chem 2008, 19, 1726. [DOI] [PubMed] [Google Scholar]
- 16.Fomich MA; Kvach MV; Navakouski MJ; Weise C; Baranovsky AV; Korshun VA; Shmanai VV Org. Lett 2014, 16, 4590. [DOI] [PubMed] [Google Scholar]
- 17.Domingo O; Hellmuth I; Jäschke A Kreutz C; Helm M Nucleic Acids Res 2015, 43, 5275–5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen N; Wang P; Wang Y; Chem. Res. Toxicol 2013, 26, 1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wada T; Mochizuki A; Higashiya S; Tsuruoka H; Kawahara S.-i.; Ishikawa M; Sekine M Tetrahedron Lett 2011, 42, 9215. [Google Scholar]
- 20.Akula HK; Kokatla H; Andrei G; Snoeck R; Schols D; Balzarini J; Yang L; Lakshman MK Org. Biomol. Chem 2017, 15, 1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


