Abstract
Alcohol intoxication causes serious diseases, whereas current treatments are mostly supportive and unable to remove alcohol efficiently. Upon alcohol consumption, alcohol is sequentially oxidized to acetaldehyde and acetate by the endogenous alcohol dehydrogenase and aldehyde dehydrogenase, respectively. Inspired by the metabolism of alcohol, we develop a hepatocyte–mimicking antidote for alcohol intoxication through the co–delivery of the nanocapsules of alcohol oxidase (AOx), catalase (CAT), and aldehyde dehydrogenase (ALDH) to the liver, where AOx and CAT catalyze the oxidation of alcohol to acetaldehyde, while ALDH catalyzes the oxidation of acetaldehyde to acetate. Administered to alcohol–intoxicated mice, the antidote rapidly accumulates in the liver and enables a significant reduction of the blood alcohol concentration. Moreover, blood acetaldehyde concentration is maintained at an extremely low level, significantly contributing to liver protection. Such an antidote, which can eliminate alcohol and acetaldehyde simultaneously, holds great promise for the treatment of alcohol intoxication and poisoning and can provide therapeutic benefits.
Keywords: enzyme nanocapsules, biomimetic antidotes, alcohol detoxification, nanomedicine
Alcohol consumption is a millennium–old fashion of human civilization, while excessive use of alcohol causes serious diseases and health problems, such as gastrointestinal and hepatic diseases, cancer, and cardiovascular disease[1–6]. Among people aged 15–49 years, alcohol consumption is the leading risk factor for premature mortality and disability[3]. Although acute alcohol intoxication takes up 8–10% of emergency room administrations[7], current treatments (e.g., homeostasis management and prevention of complications) are mostly supportive and still rely on the endogenous enzymes to eliminate alcohol[8–11]. Despite the development of colloidal antidotes[9], small molecule drugs[10,12–14], and inorganic nanoparticles[15,16] for alcohol detoxification, their inability to actively eliminate alcohol limits their therapeutic efficacy. To date, there are no effective antidotes for alcohol intoxication yet.
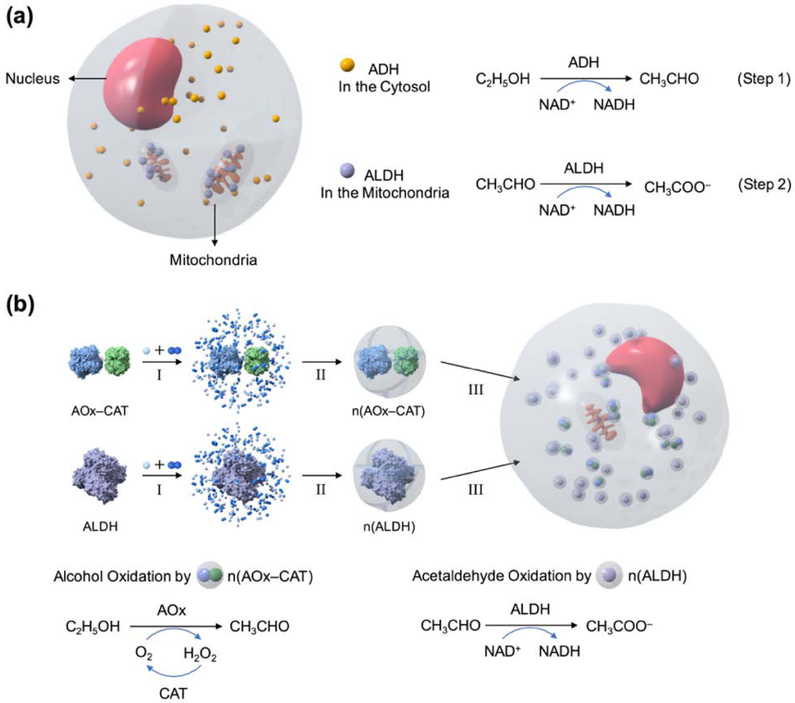
The metabolism of alcohol mainly relies on cytosolic alcohol dehydrogenase (ADH) and mitochondrial aldehyde dehydrogenase (ALDH) in the hepatocytes[17,18]. Cytochrome P450 2E1 in the microsomes only becomes active after a significant amount of alcohol is consumed. ADH and ALDH convert alcohol to acetaldehyde and then to acetate with the help of nicotinamide adenine dinucleotide (NAD+) (Figure 1a). We envision that the effective removal of alcohol and acetaldehyde could be achieved by the co–delivery of alcohol oxidase (AOx), catalase (CAT), and ALDH to the liver. As illustrated in Figure 1b, AOx and CAT in the form of an enzyme complex, as well as ALDH, are encapsulated within a cationic polymer shell through in situ polymerization[19,20], which forms enzyme nanocapsules denoted as n(AOx–CAT) and n(ALDH), respectively. The polymer shells stabilize the enzymes while allowing fast transport of the substrates, rendering the enzyme nanocapsules with highly retained activity and enhanced stability[21,22]. Similar to other positively–charged nanoparticles, such nanocapsules can be effectively delivered to the liver through intravenous administration[23–25], where n(AOx–CAT) converts alcohol to acetaldehyde and hydrogen peroxide (H2O2), with the latter removed by the CAT. As–generated acetaldehyde is then converted to acetate by n(ALDH) with the help of NAD+.
Figure 1.
Design of a hepatocyte–mimicking antidote for alcohol intoxication. (a) Alcohol metabolism in hepatocytes. Cytosolic ADH converts alcohol to acetaldehyde with the cofactor NAD+ (Step 1). Then, ALDH in the mitochondria converts acetaldehyde to acetate with NAD+ (Step 2). (b) Schematic illustration of the synthesis of n(AOx–CAT) and n(ALDH) through in situ polymerization. • and •• represent monomers and crosslinkers. Then, n(AOx–CAT) and n(ALDH) are co–delivered to the liver cells, where they catalyze the consecutive oxidation of alcohol to acetaldehyde, then to acetate.
Note that ADH and ALDH have been encapsulated within erythrocytes by electroporation[26–28]. Such–enzyme loaded erythrocytes were intravenously administered to alcohol–intoxicated mice, exhibiting a circulation half–life of 4.5 days and leading to a significant decrease in the blood alcohol concentration (BAC)[28]. However, due to the low loading efficiency, it requires the administration of a large number of enzyme–loaded erythrocytes in order to achieve a reasonable reduction in BAC. For instance, given an enzyme loading efficiency of 2.1 × 10−9 U ADH or 5.4 × 10−11 U ALDH per erythrocyte[28], it would take ~ 4.8 × 108 or 1.9 × 1010 enzyme–loaded erythrocytes to deliver 1U of ADH or ALDH. This quantity approximates to the number of erythrocytes in 100 or 4000 mL blood of human. In addition, the short shelf–life of erythrocytes (up to 42 days)[29,30] and the biosafety concerns[31] over the blood specimens further preclude its use for therapeutic purposes.
Our antidote strategy mimics the function of hepatocytes by co–delivering n(AOx–CAT) and n(ALDH) to the liver, where these enzymes are located in close proximity within the cells, enabling the simultaneous and effective breakdown of alcohol and the toxic intermediates (H2O2 and acetaldehyde). Furthermore, alcohol oxidation by ADH and ALDH in the liver consumes a substantial amount of NAD+, which may result in NAD+ deficiency that hinders continuous elimination of alcohol and acetaldehyde. Despite the regeneration of NAD+ through mitochondrial respiration, the insufficient availability of NAD+ remains as the rate–limiting step in alcohol metabolism[32]. In our biomimetic strategy, in contrast, the majority of NAD+ could be used by n(ALDH) for efficient acetaldehyde oxidation, given that n(AOx–CAT) does not require this cofactor.
Results and Discussion
Synthesis and characterization of the enzyme nanocapsules.
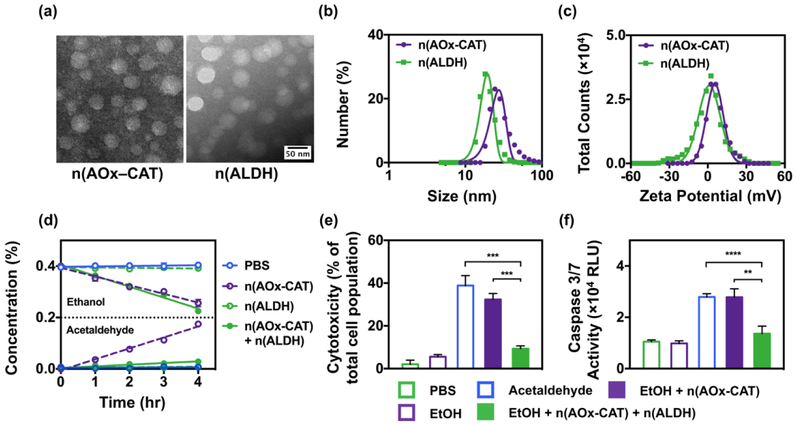
Spherical and monodispersed n(AOx–CAT) and n(ALDH) averaging 32.8±4.0 nm and 34.3±3.9 nm were observed with transmission electron microscopy and dynamic light scattering (Figure 2a, b). Meanwhile, n(AOx–CAT) and n(ALDH) showed zeta potentials of ~4 mV and ~2 mV, respectively (Figure 2c). The positive zeta potentials would allow their rapid accumulation in the liver after administration[23,24,33,34]. While the native enzymes are found to be unstable under physiological temperature or in the presence of proteases, the polymer shells also enhance the thermal and proteolytic stability of the enzymes. For instance, when incubated at 37 ˚C for 2 hr, especially in the presence of protease, the native enzymes quickly lost their activity (Figure S1, Supporting Information). On the contrary, both n(AOx–CAT) and n(ALDH) could maintain over 75% of their activity under the same conditions. In addition, the solution of n(AOx–CAT) and n(ALDH) remained stable and free of aggregation in 2 weeks (Figure S1, Supporting Information). The increased stability would warrant the use of nanocapsules in vivo.
Figure 2.
Characterization of the nanocapsules. (a) Transmission electron microscopy images of n(AOx–CAT) and n(ALDH) with uniform diameters of 32.8±4.0 nm and 34.3±3.9 nm, respectively. (b) Size and (c) Zeta potentials of n(AOx–CAT) and n(ALDH) measured by dynamic light scattering. (d) The kinetics of the removal of alcohol and acetaldehyde in a closed system containing alcohol (0.4%, w/v), after incubating with PBS, or n(AOx–CAT) (0.8 U/mL), or n(ALDH) (6.0 U/mL), or the mixture of n(AOx–CAT) and n(ALDH) for 4 hr. (e) Reduced cytotoxicity in primary mouse hepatocytes (PMH) after the simultaneous removal of alcohol and acetaldehyde. Cytotoxicity was assessed by measuring the release of lactate dehydrogenase. (f) Reduced apoptosis in PMH after the simultaneous removal of alcohol and acetaldehyde. Apoptosis was indicated by the relative luminescent unit (RLU) of Caspase 3/7 activity. Data are presented as mean ± SEM (n=3~6). **P < 0.01, ***P < 0.005 and ****P < 0.0001.
The close proximity of AOx and CAT within a nanocapsule was demonstrated using Förster resonance energy transfer (FRET), in which AOx and CAT were conjugated with fluorescein (FL) and tetramethylrhodamine (TAMRA), respectively (Figure S2, Supporting Information). Under 450 nm excitation, the mixture of AOx and CAT only exhibited an emission peak of FL at ~520 nm. In contrast, n(AOx–CAT) showed emission peaks from both FL (520 nm) and TAMRA (580 nm), confirming the close association of the two enzymes in the nanocapsules. The close proximity of the AOx and CAT also enabled the efficient removal of the toxic H2O2 generated during the process of alcohol oxidation (Figure S3, Supporting Information). The effective breakdown of alcohol and acetaldehyde by the nanocapsules were confirmed by adding the two nanocapsules to an alcohol–containing solution (0.4%, w/v) (Figure 2d). The concentration of ethanol continuously decreased (0.05% per hour), with only a small amount of acetaldehyde accumulated in the solution (0.006% per hour). Although n(AOx–CAT) and n(ALDH) were biocompatible, the acetaldehyde produced by n(AOx–CAT) during alcohol oxidation could induce severe cell injuries and apoptosis in primary mouse hepatocytes (PMH). The acetaldehyde produced by n(AOx–CAT) induced injuries among ~36% of the cell population, while the addition of n(ALDH) substantially reduced the injury population to < 6% (Figure 2e). Furthermore, the cells treated with alcohol and n(AOx–CAT) showed a high–level of Caspase activity (3.0×104 RLU), whereas adding n(ALDH) significantly decreased the level of Caspase (1.2×104 RLU) (Figure 2f, Figure S4, Supporting Information). The efficient and simultaneous breakdown of alcohol and acetaldehyde highlights the potential of co–delivering the two nanocapsules as an effective antidote for alcohol intoxication.
Delivery and efficacy of the antidote.
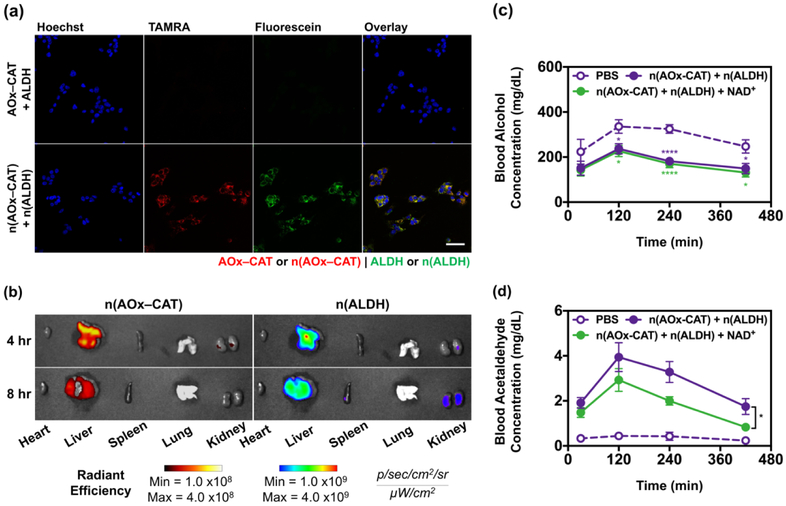
Similar to other positively–charged nanoparticles, intravenous administration of the nanocapsules enables their accumulation in the liver[23,24,33], the major organ for alcohol metabolism. To confirm their effective delivery to the liver, we first examined the uptake of n(AOx–CAT) and n(ALDH) by hepatocytes (Figure 3a, Figure S5, Supporting Information). Herein, the native AOx–CAT and n(AOx–CAT) were conjugated with TAMRA, and the native ALDH and n(ALDH) were conjugated with FL. After incubation with mouse hepatocytes (AML12) for 4 hr, the cells treated with the native AOx–CAT and ALDH exhibited little fluorescence, whereas intense fluorescence signals were observed from the cells incubated with n(AOx–CAT) and n(ALDH). Moreover, the fluorescence signals from n(AOx–CAT) and n(ALDH) overlapped in the cytosol of the hepatocytes[19,35], indicating the co–delivery of the two nanocapsules to the same cells (Figure S6, Supporting Information). Similar results were also observed in mouse macrophages (J774A.1), which could transport the nanocapsules from the circulation to the liver (Figure S7, Supporting Information). With both n(AOx–CAT) and n(ALDH) internalized in the cytosol through endocytosis (Figure S8, Supporting Information), these cells can function as mini–reactors to eliminate alcohol and acetaldehyde simultaneously. The biodistribution of the nanocapsules in mice was further investigated with n(AOx–CAT) and n(ALDH) conjugated with TAMRA and Alexa Fluor 680 (AF680), respectively. The nanocapsules were intravenously administered to the mice, and the organs were imaged 4 and 8 hr post–injection (Figure 3b, Figure S9, Supporting Information). High TAMRA and AF680 intensities were observed predominantly in the liver, indicating the efficient delivery of both nanocapsules to the liver. The rapid accumulation of n(AOx–CAT) and n(ALDH) would potentially aid in the consecutive breakdown of alcohol and acetaldehyde. To investigate the potential secondary poisoning that may be caused by the degradation of the nanocapsules, we administered the n(AOx–CAT) (as an example of nanocapsules) to the mice to study their biodistribution. From fluorescence imaging, we observed that most of the nanocapsules rapidly accumulated in the liver and the fluorescence intensity gradually decreased in the next 3 days. Only slight increases in the ALT levels during the first 48 hr after the administration of the nanocapsules were observed. (Figure S10, Supporting Information).
Figure 3.
Delivery and therapeutic efficacy of n(AOx–CAT) and n(ALDH) as the antidote. (a) Confocal laser scanning microscopy (CLSM) images of mouse hepatocytes (AML12) after 4 hr incubation with the native AOx–CAT and ALDH, or n(AOx–CAT) and n(ALDH). Hoechst 33342 was used to stain the nuclei. The native AOx–CAT and n(AOx–CAT) were labeled with TAMRA; the native ALDH and n(ALDH) were labeled with FL. Scale bar, 50 μm. (b) Fluorescence imaging of the major organs after intravenous administration of n(AOx–CAT) and n(ALDH). For imaging purpose, n(AOx–CAT) and n(ALDH) were labeled with TAMRA and AF680, respectively. (c), (d) Blood alcohol concentrations (BAC) (c) and blood acetaldehyde concentrations (BAchC) (d) of alcohol–intoxicated mice treated with PBS, n(AOx–CAT) and n(ALDH), or n(AOx–CAT) and n(ALDH) with NAD+. Mice were gavaged with alcohol at 5 mg/g body weight, and BAC were measured at 30, 120, 240, and 420 min. Data are presented as mean ± SEM (n=6~9). *P < 0.05, **P < 0.01, and ****P < 0.0001.
To study the efficacy of the nanocapsules as an antidote, we intravenously administered n(AOx–CAT) and n(ALDH) with or without additional NAD+ to the alcohol–intoxicated mice (5 mg alcohol per gram of mouse body weight). Additional NAD+ was used to evaluate if acetaldehyde oxidation by n(ALDH) could be enhanced. The blood samples were taken at different time after the administration (30, 120, 240, and 420 min) to determine the BAC and blood acetaldehyde concentrations (BAchC). Compared to the PBS–treated group that showed a BAC of ~335, ~325, and ~250 mg/dL at 120, 240, and 420 min, the group treated with nanocapsules (without NAD+) showed a BAC of ~236, ~182, and ~127 mg/dL, respectively (Figure 3c). The group given the nanocapsules with NAD+ exhibited a similar BAC to the group given nanocapsules alone, suggesting that the alcohol oxidation by n(AOx–CAT) was independent of the level of NAD+. The substantial decrease in BAC demonstrates the efficacy of the nanocapsules as an antidote and results in a faster restoration of consciousness (Figure S11, Supporting Information).
More importantly, the acetaldehyde generated from alcohol oxidation by n(AOx–CAT) could be rapidly eliminated by n(ALDH). In the group given nanocapsules (without NAD+), the BAchC remained at ~4.0, ~3.3, and ~1.9 mg/dL at 120, 240, and 420 min (Figure 3d). Moreover, the additional NAD+ could help further decrease the BAchC to ~3.0, ~2.0, and ~0.8 mg/dL at 120, 240, and 420 min. The extremely low BAchC would significantly contribute to the liver protection, given that the accumulation of acetaldehyde could induce liver cirrhosis and hepatocellular carcinoma[17,36–39]. The simultaneous and efficient removal of both alcohol and acetaldehyde highlighted the feasibility of using n(AOx–CAT) and n(ALDH) as an antidote toward alcohol intoxication or poisoning.
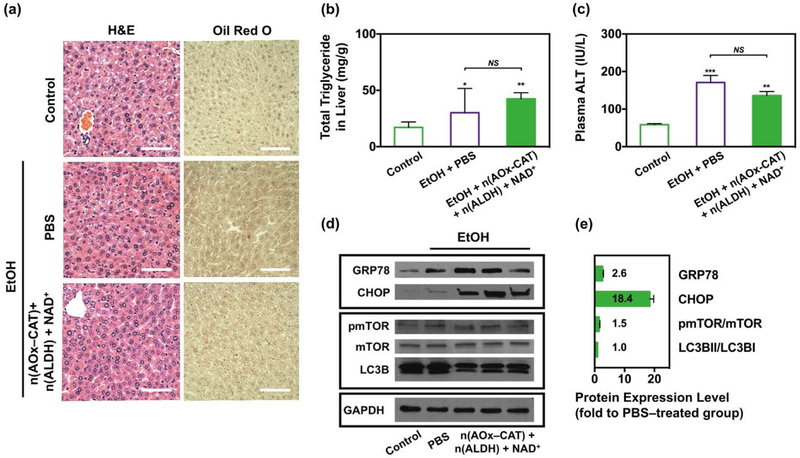
While acute alcohol intoxication causes mild elevation of ALT and steatosis, liver injury becomes more evident with chronic high–fat diet (HFD) plus a single binge[40]. Thus, we studied the alcohol–induced liver injury and organelle stress response in mice given HFD for 3 weeks, followed by acute alcohol intoxication. The mice were then treated with PBS, or n(AOx–CAT) and n(ALDH) with NAD+ as the antidote, and their liver samples were analyzed. Compared with the healthy liver, the formation of lipid droplets (LD) was slightly increased in alcohol–intoxicated mice given PBS or the antidote (Figure 4a). Consistent with the histology, the liver triglyceride content was 30 and 42 mg/g in the group treated with PBS and the antidote, respectively (Figure 4b). While the accumulation of acetaldehyde in the liver of mice treated only with n(AOx–CAT) could substantially increase LD formation (Figure S12, Supporting Information), the efficient removal of acetaldehyde by the antidote reduced it remarkably. Moreover, the plasma ALT level was increased 170 IU/L after alcohol intake, whereas the antidote brought the level down to 135 IU/L (Figure 4c). Although the administration of the antidote exhibited a higher level of liver triglyceride and ALT than those of the healthy mice, BAC and BAchC were significantly decreased, and sufficient liver protection was achieved.
Figure 4.
Biocompatibility of the antidote after HFD and acute alcohol intoxication. (a) Representative H&E and Oil Red O staining of the liver tissues in alcohol–intoxicated mice treated with PBS, or n(AOx–CAT) and n(ALDH) with NAD+ as the antidote. Liver tissue from healthy mice was used as the control. Scale bar, 50 μm. (b) Total liver triglycerides in healthy mice (n=5) and alcohol–intoxicated mice treated with PBS (n=5) or the antidote (n=7). (c) Plasma ALT level in healthy mice (n=5) and alcohol–intoxicated mice treated with PBS (n=5) or the antidote (n=7). (d) Protein expression levels of the ER stress markers (GRP78, CHOP), and autophagy markers including the mechanistic target of rapamycin (mTOR), phosphorylated mTOR (pmTOR) and microtubule–associated protein 1A/1B–light chain 3 (LC3B). (e) Quantification of protein expression levels of the ER stress and autophagy markers, normalized with glyceraldehyde–3–phosphate dehydrogenase (GAPDH). Data are presented as mean ± SEM (n=5~7).
To evaluate the organelle stress responses in the liver, we investigated the expression levels of ER stress markers (GRP78, CHOP)[36,41,42] and autophagy markers (pmTOR, mTOR, LC3B)[43] (Figure 4d). Compared with the PBS–treated group, the expression levels of GRP78, CHOP, pmTOR/mTOR, and LC3BII/LC3BI in the antidote–treated group were upregulated 2.6, 18.4, 1.5, and 1.0–fold, respectively. All these markers but CHOP indicated negligible organelle stress responses and autophagy disruptions. With regards to CHOP in this chronic experimental system, the complete elimination of alcohol and acetaldehyde with even faster kinetics would potentially reduce its expression level and achieve complete liver protection. Collectively, the antidote allows the efficient removal of both alcohol and acetaldehyde, without significant disruption to the liver health.
Conclusion
In summary, we have designed a hepatocyte–mimicking antidote for alcohol intoxication by the co–delivery of n(AOx–CAT) and n(ALDH) to the liver. While n(AOx–CAT) enables rapid alcohol removal, as–generated acetaldehyde could be efficiently removed by n(ALDH). Administration of the antidote to alcohol–intoxicated mice resulted in significant reduction in BAC without the accumulation of acetaldehyde. Such an antidote could provide profound therapeutic benefits to alcohol–intoxicated patients, and rescue lives in emergency rooms.
Supplementary Material
Acknowledgements
D. X. and H. H. contributed equally to this work. This work is supported by the U.S. National Institute of Health under Grant Number R21AA023952 and R01DA042632. We would like to thank the USC Liver Research Center and California NanoSystem Institute for technical assistance.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Duo Xu, Department of Chemical and Biomolecular Engineering, University of California, Los Angeles, CA 90095, USA, luucla@ucla.edu.
Dr. Hui Han, Department of Medicine, Keck School of Medicine of USC, University of Southern California, Los Angeles, CA 90033, USA, chengji@usc.edu
Yuxin He, Department of Medicine, Keck School of Medicine of USC, University of Southern California, Los Angeles, CA 90033, USA, chengji@usc.edu.
Harrison Lee, Department of Medicine, Keck School of Medicine of USC, University of Southern California, Los Angeles, CA 90033, USA, chengji@usc.edu.
Di Wu, Department of Chemical and Biomolecular Engineering, University of California, Los Angeles, CA 90095, USA, luucla@ucla.edu.
Fang Liu, Department of Chemical and Biomolecular Engineering, University of California, Los Angeles, CA 90095, USA, luucla@ucla.edu.
Dr. Xiangsheng Liu, California NanoSystem Institute, Los Angeles, CA 90095, USA
Prof. Yang Liu, State Key Laboratory of Medicinal Chemical Biology, Institute of Polymer Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China
Prof. Yunfeng Lu, Department of Chemical and Biomolecular Engineering, University of California, Los Angeles, CA 90095, USA, luucla@ucla.edu
Prof. Cheng Ji, Department of Medicine, Keck School of Medicine of USC, University of Southern California, Los Angeles, CA 90033, USA, chengji@usc.edu
Reference
- [1].Gunzerath L, Hewitt BG, Li T-K, Warren KR, Ann. N. Y. Acad. Sci 2011, 1216, 1. [DOI] [PubMed] [Google Scholar]
- [2].Friedmann PD, Engl N. J. Med 2013, 368, 365. [DOI] [PubMed] [Google Scholar]
- [3].Gilmore W, Chikritzhs T, Stockwell T, Jernigan D, Naimi T, Gilmore I, Nat. Rev. Gastroenterol. Hepatol 2016, 13, 426. [DOI] [PubMed] [Google Scholar]
- [4].Lee K, Møller L, Hardt F, Haubek A, Jensen E, The Lancet 1979, 2, 759. [DOI] [PubMed] [Google Scholar]
- [5].Fernández-Solà J, Nat. Rev. Cardiol 2015, 12, 576. [DOI] [PubMed] [Google Scholar]
- [6].Garaycoechea JI, Crossan GP, Langevin F, Mulderrig L, Louzada S, Yang F, Guilbaud G, Park N, Roerink S, Nik-Zainal S, Stratton MR, Patel KJ, Nature 2018, 553, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moss M, Burnham EL, The Lancet 2006, 368, 2231. [DOI] [PubMed] [Google Scholar]
- [8].Jamaty C, Bailey B, Larocque A, Notebaert E, Sanogo K, Chauny J-M, Clin. Toxicol 2010, 48, 1. [DOI] [PubMed] [Google Scholar]
- [9].Bertrand N, Bouvet C, Moreau P, Leroux J-C, ACS Nano 2010, 4, 7552. [DOI] [PubMed] [Google Scholar]
- [10].Shen Y, Lindemeyer AK, Gonzalez C, Shao XM, Spigelman I, Olsen RW, Liang J, J. Neurosci 2012, 32, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Connor JP, Haber PS, Hall WD, The Lancet 2016, 387, 988. [DOI] [PubMed] [Google Scholar]
- [12].Wang F, Li Y, Zhang Y-J, Zhou Y, Li S, Li H-B, Molecules 2016, 21, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW, Proc. Natl. Acad. Sci. USA 2006, 103, 8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liang J, Olsen RW, Acta Pharmacol Sin 2014, 35, 981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sun A, Mu L, Hu X, ACS Appl. Mater. Interfaces 2017, 9, 12241. [DOI] [PubMed] [Google Scholar]
- [16].Qu X, Gou M, Zaidan J, Zhang K, Chen S, Nanomedicine. 2014, 9, 2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lieber CS, Drug Alcohol Rev. 1991, 10, 175. [DOI] [PubMed] [Google Scholar]
- [18].Zakhari S, Alcohol Res. Health 2006, 29, 245. [PMC free article] [PubMed] [Google Scholar]
- [19].Yan M, Du J, Gu Z, Liang M, Hu Y, Zhang W, Priceman S, Wu L, Zhou ZH, Liu Z, Segura T, Tang Y, Lu Y, Nat. Nanotechnol 2010, 5, 48. [DOI] [PubMed] [Google Scholar]
- [20].Liu Y, Du J, Yan M, Lau MY, Hu J, Han H, Yang OO, Liang S, Wei W, Wang H, Li J, Zhu X, Shi L, Chen W, Ji C, Lu Y, Nat. Nanotechnol 2013, 8, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liang S, Liu Y, Jin X, Liu G, Wen J, Zhang L, Li J, Yuan X, Chen ISY, Chen W, Wang H, Shi L, Zhu X, Lu Y, Nano Res. 2016, 9, 1022. [Google Scholar]
- [22].Zhang X, Xu D, Jin X, Liu G, Liang S, Wang H, Chen W, Zhu X, Lu Y, Control J. Release 2017, 255, 54. [DOI] [PubMed] [Google Scholar]
- [23].Tsoi KM, MacParland SA, Ma X-Z, Spetzler VN, Echeverri J, Ouyang B, Fadel SM, Sykes EA, Goldaracena N, Kaths JM, Conneely JB, Alman BA, Selzner M, Ostrowski MA, Adeyi OA, Zilman A, McGilvray ID, Chan WCW, Nat. Mater 2016, 15, 1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Blanco E, Shen H, Ferrari M, Nat. Biotechnol 2015, 33, 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lu Y, Aimetti AA, Langer R, Gu Z, Nat. Rev. Mater 2016, 1, 16075. [Google Scholar]
- [26].Magnani M, Laguerre M, Rossi L, Bianchi M, Ninfali P, Mangani F, Ropars C, Alcohol Alcohol. 1990, 25, 627. [DOI] [PubMed] [Google Scholar]
- [27].Lizano C, Sanz S, Luque J, Pinilla M, Biochim. Biophys. Acta 1998, 1425, 328. [DOI] [PubMed] [Google Scholar]
- [28].Lizano C, Pérez MT, Pinilla M, Life Sci. 2001, 68, 2001. [DOI] [PubMed] [Google Scholar]
- [29].D’Alessandro A, Liumbruno G, Grazzini G, Zolla L, Blood Transfus. 2010, 8, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].García-Roa M, Del Carmen Vicente-Ayuso M, Bobes AM, Pedraza AC, González-Fernández A, Martín MP, Sáez I, Seghatchian J, Gutiérrez L, Blood Transfus. 2017, 15, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hart S, Cserti-Gazdewich CM, McCluskey SA, Anaesthesia 2015, 70, 38. [DOI] [PubMed] [Google Scholar]
- [32].Cederbaum AI, Clin. Liver Dis 2012, 16, 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Elci SG, Jiang Y, Yan B, Kim ST, Saha K, Moyano DF, Yesilbag Tonga G, Jackson LC, Rotello VM, Vachet RW, ACS Nano 2016, 10, 5536. [DOI] [PubMed] [Google Scholar]
- [34].Alexis F, Pridgen E, Molnar LK, Farokhzad OC, Mol. Pharm. 2008, 5, 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sun W, Jiang T, Lu Y, Reiff M, Mo R, Gu Z, J. Am. Chem. Soc 2014, 136, 14722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Seitz HK, Stickel F, Nat. Rev. Cancer 2007, 7, 599. [DOI] [PubMed] [Google Scholar]
- [37].Setshedi M, Wands JR, de la Monte SM, Oxid. Med. Cell. Longev 2010, 3, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Brooks PJ, Theruvathu JA, Alcohol 2005, 35, 187. [DOI] [PubMed] [Google Scholar]
- [39].Parker R, Kim SJ, Gao B. Nat. Rev. Gastroenterol. Hepatol 2018, 15, 50. [DOI] [PubMed] [Google Scholar]
- [40].Chang B, Xu M-J, Zhou Z, Cai Y, Li M, Wang W, Feng D, Bertola A, Wang H, Kunos G, Gao B, Hepatology 2015, 62, 1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ji C, Biomolecules 2015, 5, 1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ji C, Kaplowitz N, Lau MY, Kao E, Petrovic LM, Lee AS, Hepatology 2011, 54, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ding W-X, Manley S, Ni H-M, Exp. Biol. Med 2011, 236, 546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.